Abstract
EBV-positive nasopharyngeal carcinoma and Hodgkin, T, and natural killer (NK) lymphomas express EBNA-1 and the latent membrane proteins (LMP1-2; type II latency). In contrast to type III EBV-transformed lymphoblastoid cell lines, in these cells the LMPs are expressed in the absence of EBNA-2. We have previously reported that exposure to CD40 ligand and IL-4 could induce LMP-1 in an in vitro EBV-infected Hodgkin lymphoma-derived cell line, which expressed only EBNA-1. We show now that both human and EBV-encoded IL-10 can induce LMP-1 in the absence of EBNA-2 in the Daudi, P3HR1, and other BL cell lines. Interestingly, induction of LMP-1 was not accompanied by the downregulation of BCL-6. IL-10 could also induce LMP-1 in the conditional lymphoblastoid cell line ER/EB2-5 where EBNA-2 was downregulated in the absence of estrogen. Moreover, IL-10 could induce the expression of LMP-1 in tonsillar B cells infected with the nontransforming, EBNA-2-deficient EBV strain P3HR1 and enhance LMP-1 expression in 2 EBV-positive NK lymphoma lines. The demonstration that IL-10 can induce the expression of LMP-1 in an EBNA-2-independent manner shows that the major transforming EBV gene LMP-1 can be induced by extracellular signals in lymphoid cells, and IL-10 might contribute to the establishment of type II EBV latency.
Introduction
The human gammaherpesvirus Epstein-Barr virus (EBV) is ubiquitous and it persists for the lifetime of the individual after the first encounter. In spite of the efficient transforming potential of the virus for B lymphocytes in vitro, the infection with EBV is largely harmless in vivo due to the vigorous immune response directed against the virus-encoded proteins expressed in proliferating cells,1 combined with the viral strategy to downregulate the expression of the immunogenic viral proteins in the infected memory B cells.2 A variety of lymphomas and carcinomas were found to carry EBV genome and to express virally encoded proteins.1 Except for the lymphomas in immunosuppressed individuals, the role of the virus in the genesis of the tumors is unknown.
EBV readily infects B-lymphocyte cultures in vitro and transforms them into proliferating lymphoblastoid cell lines (LCLs). These cells carry the virus in a latent form and express a set of viral genes that in concert with cellular genes induce the immunoblastic transformation and proliferation of the B cells.1 The emerging lymphoblastoid cell lines (LCLs) express 6 nuclear (EBNA1-6) and 3 membrane viral proteins (latent membrane protein 1 [LMP-1], -2A, -2B). This expression pattern is termed type III latency or growth program.1,2 In this program the expression of LMPs is driven by the trans-activator EBNA-2 together with EBNA-5 (EBNA-2-dependent expression of LMPs).1
In EBV-positive malignant cells the virus expresses only a few viral genes, such as only EBNA-1 in Burkitt lymphoma (BL), whereas in nasopharyngeal carcinoma,3 Hodgkin lymphoma,4 and T and NK lymphomas5 EBNA-1 is expressed together with LMP-1 and LMP-2 (EBNA-2-independent LMP expression).1 EBER and BART RNAs are expressed in all latency forms.1
LMP-1 is required for in vitro transformation and proliferation of B cells in vitro.6,7 Acting as a constitutively active receptor,8 LMP-1 activates similar pathways as the triggering of the CD40 receptor and is therefore regarded as a functional homologue of CD40.
B cells with all 3 EBV latency types have been detected in the lymphoid tissues of patients with clinically manifest primary EBV infection (infectious mononucleosis [IM]) and also in healthy EBV carriers.2 In healthy individuals, transcripts of type III latency-associated genes were found exclusively in naive B cells, whereas germinal center (GC) and memory B cells expressed the restricted type II and type I latency, respectively.2 It has been proposed that EBV exploits the normal B-cell differentiation pathway to get access to the memory compartment. LMP-1 and LMP-2A are believed to provide survival signals for the EBV-infected GC B lymphocytes by mimicking the externally activated CD40 and B-cell receptor (BCR) pathways, respectively.
Type II latent B cells were also found by immunohistochemical analysis of tonsillar sections of healthy EBV carriers9 and IM patients.10 Furthermore, rare B cells expressing type II EBV latency were detected in several lymphoid malignancies, such as Burkitt lymphomas11-15 and posttransplantation lymphoproliferative disease,16,17 and in B cells of angioimmunoblastic T-cell lymphomas,18 pleural effusion lymphomas,19 and plasmablastic lymphomas.20 In these conditions LMP-1 is expressed in the absence of the trans-activators EBNA-2 and EBNA-5, implying that yet unknown cellular or viral factors are responsible for its expression. Induction of LMP-1 in the absence of EBNA-2 and lytic cycle antigens in B-derived cell lines was detected earlier following immunoglobulin cross-linking in the Eli- BL line21 and exposure to CD40 ligand (CD40L) together with IL-4 in an in vitro EBV-converted subline of the Hodgkin lymphoma line, KMH-2, that expresses a type I EBV latency pattern.22
Superinfection of the EBNA-2-deficient BL line Daudi with EBV-containing culture supernatants was shown to induce the early expression of the endogenous LMP-1 before EBNA-2 of the incoming B95-8 virus was expressed.23 Both infectious and UV-exposed B95-8 and P3HR1 virus-containing supernatants were effective.23
Since EBV-carrying supernatants contained both human and viral IL-10,24 we have examined the possibility that IL-10 could be responsible for the induction of LMP-1 in the superinfected Daudi cells. We found that both human and viral IL-10 could induce LMP-1 expression in Daudi cells. Furthermore, IL-10 induced the expression of LMP-1 in normal B cells infected with the EBNA-2-defective EBV strain P3HR1 and it upregulated LMP-1 in 2 NK lymphoma cell lines. IL-10 may be responsible for the establishment of type II latency in EBV-infected cells in vivo, since it may be provided by cells of the GC microenvironment and of the granulation tissue in Hodgkin lymphomas (HLs) and NK lymphomas.
Materials and methods
Cell lines
The cell lines used in this study are listed in Table 1. The conditional LCL ER/EB2-534 and its c-myc-transfected derivates A-1 and P493-635 were kindly provided by Dr G. W. Bornkamm (Inst of Clinical Molecular Biology and Tumor Genetics, GSF-National Research Center for Environment Health, Munich, Germany). ER/EB2-5 cells were maintained in RPMI medium supplemented with 2 μM β-estradiol (Sigma-Aldrich, Stockholm, Sweden). The EBV-positive IL-2-dependent NK-cell lymphomas SNK-636 and KAI-337 were provided by Dr N. Shimizu (Dept of Virology, Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan) and from Dr Y. Harabuchi (Dept of Otolaryngology-Head and Neck Surgery, Asahikawa Medical College, Asahikawa, Japan), respectively. The cells were grown in RPMI medium supplemented with 10% inactivate fetal calf serum (FCS) and 100 U/mL penicillin and 100 μg/mL streptomycin, except for the NK lymphoma lines that were cultured in IMDM medium supplemented with 15% FCS, 25 U/mL human recombinant IL-2 (gift from Ajinomoto, Tokyo, Japan), and antibiotics. The B95-8 LCL and Akata LCL were generated in our laboratory from tonsillar B cells.
Flow cytometric analysis of cell-surface antigens
The surface antigens were stained by indirect immunofluorescence with the following mouse monoclonal antibodies: CD80, CD83 (BD Pharmingen, Heidelberg, Germany); J5 (anti-CD10), gift from L. Nadler (Dana Farber Cancer Institute, Boston, MA); IPO-3 (anti-SLAM) from S. Sidorenko (Academy of Science of Ukraine, Kiev, Ukraine); LB-2 (anti-ICAM-1) from E. A. Clark (University of Washington, Seattle, WA); HB-5 (anti-CD21), HB202 (anti-LFA-1) purchased from ATCC (Manassas, VA); R1/69 (anti-IgM), CR3/43 (anti-HLA-DP, -DQ, -DR; DakoCytomation, Glostrup, Denmark). RPE-conjugated rabbit anti-mouse Ig F(ab)2 (DakoCytomation) was used as secondary antibody.
IL-10 treatment of cell lines and immunoblotting
Daudi, P3HR1, or DG75 cells (0.5 million of each) were plated in 2 mL RPMI-10% FCS medium supplemented with the indicated concentrations of recombinant human IL-10 (hIL-10; PeproTech, London, United Kingdom), recombinant EBV viral IL-10, or recombinant viral HCMV IL-10 (both from R&D Systems, Minneapolis, MN). For the pSTAT3 immunoblotting, the cell extracts were prepared 30 minutes after the addition of IL-10.
ER/EB2-5 cells were cultured in medium with or without estrogen in the presence of 50 ng/mL hIL-10 (1 million cells/2 mL medium). IL-10 was added either at the time of estrogen removal or after 3 days of estrogen starvation. For the IL-10 treatment of SNK-6 and KAI-3 cells, IL-2 was removed from the culture medium and the next day 1 million cells were plated in 2 mL medium with the indicated concentrations of hIL-10 for 48 hours. For the IL-10R-blocking experiments, the rat anti-human IL-10R monoclonal antibody (Ab; clone 3F9; BD Pharmingen) was added 3 hours prior to the addition of different cytokines.
After the indicated time, total cell lysates were prepared and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting, as previously described,22 with the following antibodies: S12 supernatant (mouse anti-LMP-1); OT1x (mouse anti-EBNA-1; gift from J. M. Middeldorp, Dept of Pathology, Vrije Universiteit Medical Center, Amsterdam, The Netherlands); E3CA10 supernatant (mouse anti-EBNA-6); CS1-4 (mouse anti-LMP-1) and PE-2 (mouse anti-EBNA-2; DAKO, Copenhagen, Denmark); AC-15 (mouse anti-human β-actin; Sigma-Aldrich, Stockholm, Sweden); rabbit anti-STAT3 and anti-phospho-STAT3 (Tyr705; Cell Signaling Technology, Beverly, MA); D-8 (mouse anti-BCL-6), N-262 (rabbit anti-c-myc), and C-20 (rabbit anti-human IL-10Ralpha; Santa Cruz Biotechnology, Santa Cruz, CA); 13A3 (mouse anti-human cyclin E; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom).
Immunofluorescence staining
The cells were deposited on glass slides in cytospin cytocentrifuge at 161g (1200 rpm) for 5 minutes and fixed in methanol-acetone (1:1). After rehydration in PBS, the slides were incubated with the anti-LMP-1 monoclonal Ab mixture CS1-4 (DAKO; dilution 1:200) for 1 hour at room temperature. After washing, the slides were incubated with FITC-conjugated rabbit antimouse Ab (DAKO; dilution 1:200) for 30 minutes at room temperature. The nuclei were visualized with 0.5 g/mL Hoechst 33258 that was added to the conjugated antibodies. The slides were mounted with 70% glycerol, 2.5% DABCO (Sigma; pH 8.5) in PBS. Images were generated with a Leitz DM RB microscope (Leica Microsystems, Wetzlar, Germany) using a 63 ×/1.32 NA oil immersion objective lens. Images were captured with a Hamamatsu dual-mode cooled charged coupled device camera (C4880) and Hipic 6.4.0 software (Hamamatsu Photonics Deutschland, Herrsching am Ammersee, Germany) as 8-bit uncompressed TIFF files. Pictures were edited for optimal color contrast with Adobe Photoshop 7 (Adobe Systems, San Jose, CA).
Thymidine incorporation
For assessment of cell proliferation, 5 × 104 SNK-6 and KAI-3 cells were cultured in a 96-well plate in 200 μL IMDM medium with 10% FCS in the presence or absence of the indicated cytokines. After 3 days of estrogen starvation, ER/EB2-5 cells were plated at 105 cells/200 μL medium with or without 50 ng/mL IL-10. Twenty-four and 48 hours later, 1 μCi (0.037 MBq) 3H-thymidine was added to each well for the following 12 hours. The cells were harvested on a glass fiber filter and radioactivity was measured in a liquid scintillation counter. The results represent the mean counts per minute (CPM) of 10 parallel wells of 3 independent experiments.
Production of P3HR1 viral supernatant and infection of B cells
For the induction of lytic replication, P3HR1 cells were treated for 5 days with phorbol myristate acetate (PMA; 20 ng/mL) and sodium butyrate (2.5 mM). The culture supernatant was filtered through a sterile 0.8-μm pore filter and was concentrated 100 times through centrifugation at 16 000g for 90 minutes at 4°C. The concentrated virus stock was tested for the induction of early antigen (EA) on Raji cells. Five million Raji cells were infected with 200 μL of concentrated virus. Twelve hours later the cells were cytospun and stained with a previously characterized EA-reactive antiserum conjugated with FITC (F6-esther; dilution 1:60). The concentrated virus induced EA in 20% of the Raji cells (not shown). Tonsillar B cells were purified from tonsils of healthy individuals by 2 subsequent sheep red blood cell rosettings and plastic adherence. Five million B cells were infected with 200 μL of concentrated virus and plated in 3 mL RPMI 1640 medium in the presence of 50 ng/mL of hIL-10 or 20 ng/mL of the phorbol ester PMA.
cDNA synthesis and reverse transcriptase-polymerase chain reaction (RT-PCR)
cDNAs were synthesized from poly (A)+RNA. The details of the cDNA synthesis, the sequences of the primers, and the PCR conditions used were similar to the ones reported in our previous study.22
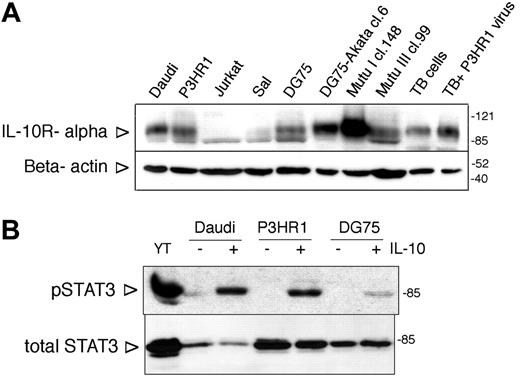
Expression and functionality of IL-10R in BL lines. (A) IL-10R alpha expression of BL lines assessed by SDS-PAGE and immunoblotting. Jurkat cells were used as negative control. TB indicates tonsillar B cells. The membrane was stripped and reprobed with anti-β-actin antibody to serve as loading control. On all immunoblots the arrowheads on the left side indicate the location of the specific band, whereas the numbers on the right side denote the apparent molecular weight (kDa) of the prestained protein ladder. (B) Immunoblot analysis of total cell extracts of IL-10-treated (100 ng/mL, 30 minutes) BL lines with phospho-STAT3 (Tyr705)- and total STAT3-specific antibodies. YT NK lymphoma cells were used as positive control.
Expression and functionality of IL-10R in BL lines. (A) IL-10R alpha expression of BL lines assessed by SDS-PAGE and immunoblotting. Jurkat cells were used as negative control. TB indicates tonsillar B cells. The membrane was stripped and reprobed with anti-β-actin antibody to serve as loading control. On all immunoblots the arrowheads on the left side indicate the location of the specific band, whereas the numbers on the right side denote the apparent molecular weight (kDa) of the prestained protein ladder. (B) Immunoblot analysis of total cell extracts of IL-10-treated (100 ng/mL, 30 minutes) BL lines with phospho-STAT3 (Tyr705)- and total STAT3-specific antibodies. YT NK lymphoma cells were used as positive control.
Results
Expression and functionality of the IL-10 receptor (IL-10R) in BL lines
The IL-10R α expression of the cell lines was investigated by immunoblotting. All BL lines expressed the IL-10R α (Figure 1A; not shown). The IL-10R expression varied among the lines, Mutu I cl.148 cells showing the highest expression level. One of the downstream signaling intermediates of the IL-10R, STAT3, was phosphorylated on Tyr705 upon IL-10 treatment in all the lines, as assessed in immunoblotting with the anti-phospho-STAT3 (Tyr705)-specific antibody (Figure 1B; not shown).
Induction of LMP-1 by IL-10 in Daudi cells
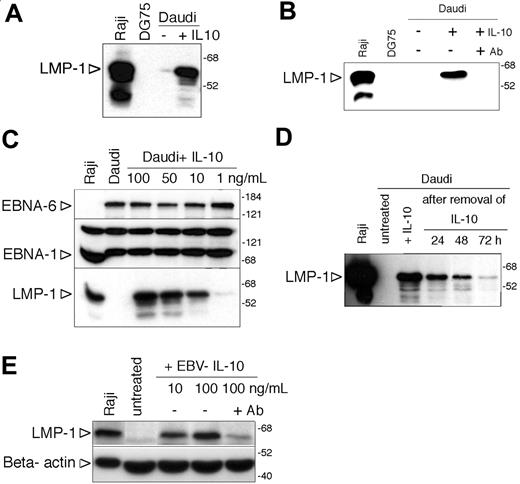
Motivated by the earlier findings and our hypothesis, we tested whether recombinant hIL-10 can induce the expression of LMP-1. Indeed we found that LMP-1 was induced in Daudi cells after incubation with 100 ng/mL hIL-10 for 48 hours (Figure 2A). Preincubation of the cells with an IL-10 receptor-blocking antibody prevented the induction of LMP-1 (Figure 2B), providing further evidence for the specific effect of the cytokine. The effect of hIL-10 was dose dependent (range, 1-100 ng/mL; Figure 2C). Expression of EBNA-6 and EBNA-1 did not change in these cells (Figure 2C). LMP-1 induction by hIL-10 was transient (Figure 2D).
Since the culture supernatants used for infection contain both human and viral IL-10 (vIL-10),24 we tested the effect of EBV vIL-10 as well. We found that recombinant EBV IL-10 induced LMP-1 expression (Figure 2E) but it was about 50 times less efficient. The effect was prevented by IL-10R-blocking antibody (Figure 2E). The lower efficiency of the recombinant EBV IL-10 is in line with the observations that EBV-IL-10 has an approximately 1000-fold lower affinity for recombinant IL-10R.39 The vIL-10 encoded by the human cytomegalovirus also induced the expression of LMP-1 and IL-10R-blocking antibody prevented its effect (not shown). However, the IL-10R-blocking antibody did not prevent the induction of LMP-1 by the supernatant of B95-8 cell cultures when tested 6 hours after the exposure of Daudi cells to the supernatant (not shown).
Induction of LMP-1 by IL-10 in Daudi cells. (A) Immunoblot analysis of LMP-1 expression of Daudi cells 48 hours after incubation with 100 ng/mL hIL-10. Total cell extracts of Raji and DG75 cells were used as positive and negative controls, respectively. The numbers on the right side denote the molecular weight in kDa. (B) Immunoblot analysis of LMP-1 expression of hL-10-treated (100 ng/mL) Daudi cells in the presence or absence of IL-10 receptor-blocking antibody (2 μg/mL). (C) EBNA-6, EBNA-1, and LMP-1 immunoblot of Daudi cells treated with 100, 50, 10, or 1 ng/mL hIL-10 for 48 hours. (D) LMP-1 immunoblot of IL-10-treated Daudi cells after the removal of the cytokine. Forty-eight hours after of incubation with 100 ng/mL hIL-10 the cells were washed and replated in the absence of IL-10. Total protein extracts were prepared 24, 48, and 72 hours after the removal of hIL-10 and expression of LMP-1 was monitored by immunoblotting. (E) Total cell extracts of Daudi cells incubated with 10 or 100 ng/mL EBV IL-10 for 48 hours were analyzed for LMP-1 and β-actin expression by SDS-PAGE and immunoblotting. In parallel cultures, Daudi cells were preincubated with 2 μg/mL anti-IL-10R-blocking antibody before the addition of 100 ng/mL cytokine. Of note: because of the low expression of LMP-1, the loading of the cell extracts and the exposure time were optimized, and therefore the immunoblot is not comparable with the other LMP-1 immunoblots.
Induction of LMP-1 by IL-10 in Daudi cells. (A) Immunoblot analysis of LMP-1 expression of Daudi cells 48 hours after incubation with 100 ng/mL hIL-10. Total cell extracts of Raji and DG75 cells were used as positive and negative controls, respectively. The numbers on the right side denote the molecular weight in kDa. (B) Immunoblot analysis of LMP-1 expression of hL-10-treated (100 ng/mL) Daudi cells in the presence or absence of IL-10 receptor-blocking antibody (2 μg/mL). (C) EBNA-6, EBNA-1, and LMP-1 immunoblot of Daudi cells treated with 100, 50, 10, or 1 ng/mL hIL-10 for 48 hours. (D) LMP-1 immunoblot of IL-10-treated Daudi cells after the removal of the cytokine. Forty-eight hours after of incubation with 100 ng/mL hIL-10 the cells were washed and replated in the absence of IL-10. Total protein extracts were prepared 24, 48, and 72 hours after the removal of hIL-10 and expression of LMP-1 was monitored by immunoblotting. (E) Total cell extracts of Daudi cells incubated with 10 or 100 ng/mL EBV IL-10 for 48 hours were analyzed for LMP-1 and β-actin expression by SDS-PAGE and immunoblotting. In parallel cultures, Daudi cells were preincubated with 2 μg/mL anti-IL-10R-blocking antibody before the addition of 100 ng/mL cytokine. Of note: because of the low expression of LMP-1, the loading of the cell extracts and the exposure time were optimized, and therefore the immunoblot is not comparable with the other LMP-1 immunoblots.
Induction of LMP-1 by hIL-10 in other BL lines
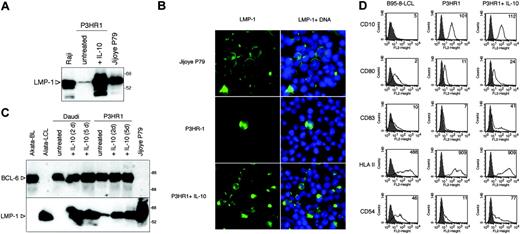
We found that P3HR1 cells, which lack EBNA-2, responded strongly to the LMP-1-inducing effect of hIL-10. The treated cells expressed LMP-1 at a higher level than the EBNA-2-positive type III parental cell line Jijoye (Figure 3A). The majority of the cells became LMP-1 positive in the IL-10-treated P3HR1 cultures as shown by immunofluorescence stainings with anti-LMP-1 antibody (Figure 3B). LMP-1 expression was heterogeneous, 30% of the cells being strongly positive (Figure 3B). BCL-6 is known to be downregulated in BL lines that have drifted from type I to LMP-1-positive type III cells in the course of serial culturing.40 However, BCL-6 was expressed in hIL-10-treated Daudi or P3HR1 cells, even after 5 days of continuous exposure to the cytokine (Figure 3C).
Induction of LMP-1 by hIL-10 in P3HR1 cells was correlated with the upregulation of CD80, CD83, HLA II, and CD54 (ICAM-1), as assessed by fluorescence-activated cell sorter (FACS) analysis (Figure 3D). CD10 did not change in these cells. DG75 cells were treated with hIL-10 in parallel, but only slight downregulation of CD10 and upregulation of ICAM-1 was observed (not shown). Daudi cells exposed to hIL-10 showed only upregulation of ICAM-1 (not shown). SLAM (CD150) expression was not influenced by hIL-10 in either Daudi or DG75 cells (not shown).
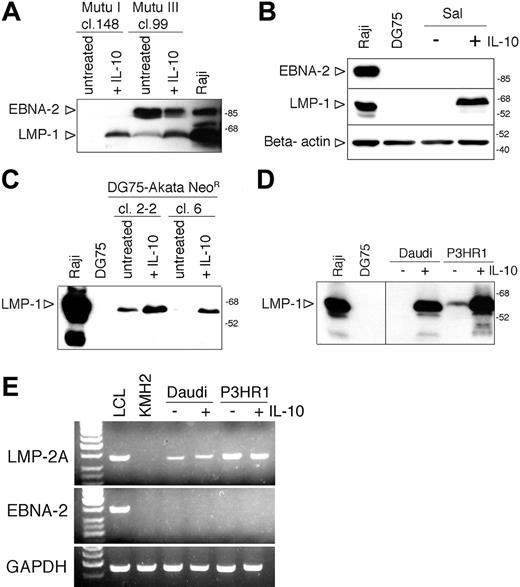
In the 2 Mutu BL sublines, hIL-10 induced LMP-1 in the type I (cl. 148) cells in the absence of EBNA-2 expression and upregulated the expression of LMP-1 in the type III Mutu (cl. 99) cells (Figure 4A). Furthermore, hIL-10 treatment induced LMP-1 expression in the Sal BL cells in the absence of EBNA-2 expression (Figure 4B).
Induction of LMP-1 by hIL-10 in P3HR1 BL cells. (A) Total cell extracts of P3HR1 cells incubated with 100 ng/mL hIL-10 for 48 hours were analyzed for LMP-1 expression by SDS-PAGE and immunoblotting. The type III Raji and Jijoye P79 lines were included for comparison. (B) LMP-1 immunofluorescence staining of the IL-10-treated P3HR1 cells (50 ng/mL, 48 hours). The nuclei were stained with Hoechst 33258. (C) LMP-1 and BCL-6 expression of hIL-10-treated Daudi and P3HR1 cells. The cells were cultured in the presence of 100 ng/mL hIL-10 for 2 or 5 days. The membrane was first developed with anti-BCL-6 antibody then stripped and reprobed with LMP-1-specific antibody. The Akata BL/LCL pair was included as controls. (D) Flow cytometric analysis of CD10, CD80, CD83, HLA-II, and ICAM-1 (CD54) antigens in the hIL-10-treated P3HR1 cells. The cells were treated with 100 ng/mL hIL-10 for 48 hours before the staining. The fluorescence intensity in the FL2 channel was measured (logarithmic amplification) and analyzed with the CellQuest Pro program (BD Biosciences, San Jose, CA). On the histogram plots the shaded areas represent the background fluorescence of the secondary Ab-stained cells, whereas the solid black lines denote the specific staining of the untreated and hIL-10-treated cells. The mean fluorescence intensity is indicated. B958-LCL was used as positive staining control.
Induction of LMP-1 by hIL-10 in P3HR1 BL cells. (A) Total cell extracts of P3HR1 cells incubated with 100 ng/mL hIL-10 for 48 hours were analyzed for LMP-1 expression by SDS-PAGE and immunoblotting. The type III Raji and Jijoye P79 lines were included for comparison. (B) LMP-1 immunofluorescence staining of the IL-10-treated P3HR1 cells (50 ng/mL, 48 hours). The nuclei were stained with Hoechst 33258. (C) LMP-1 and BCL-6 expression of hIL-10-treated Daudi and P3HR1 cells. The cells were cultured in the presence of 100 ng/mL hIL-10 for 2 or 5 days. The membrane was first developed with anti-BCL-6 antibody then stripped and reprobed with LMP-1-specific antibody. The Akata BL/LCL pair was included as controls. (D) Flow cytometric analysis of CD10, CD80, CD83, HLA-II, and ICAM-1 (CD54) antigens in the hIL-10-treated P3HR1 cells. The cells were treated with 100 ng/mL hIL-10 for 48 hours before the staining. The fluorescence intensity in the FL2 channel was measured (logarithmic amplification) and analyzed with the CellQuest Pro program (BD Biosciences, San Jose, CA). On the histogram plots the shaded areas represent the background fluorescence of the secondary Ab-stained cells, whereas the solid black lines denote the specific staining of the untreated and hIL-10-treated cells. The mean fluorescence intensity is indicated. B958-LCL was used as positive staining control.
Induction of LMP-1 by IL-10 in other BL lines. (A) Total cell extracts of hIL-10-treated (50 ng/mL, 48 hours) Mutu I cl.148 and Mutu III cl.99 were analyzed for EBNA-2 and LMP-1 expression by SDS-PAGE and immunoblotting, developed on the same membrane. (B) Immunoblot analysis of LMP-1, EBNA-2, and β-actin expression in total cell extracts of IL-10-treated Sal BL cells (50 ng/mL, 48 hours). (C) Total cell extracts of hIL-10-treated (50 ng/mL, 48 hours) DG75-Akata NeoR clones 2-2 and 6 were analyzed for EBNA-2 and LMP-1 expression by SDS-PAGE and immunoblotting. (D) Immunoblot analysis of LMP-1 expression in total cell lysates of IL-10-treated Daudi and P3HR1 cells (50 ng/mL, 6 hours). (E) RT-PCR results of LMP-2A, EBNA-2, and GAPDH expression of untreated and IL-10-treated (50 ng/mL, 24 hours) Daudi and P3HR1 cells. cDNAs prepared from the LCL B95-8 and the EBV-negative Hodgkin lymphoma cell line KMH2 were used as positive and negative controls, respectively. The PCR products were visualized in ethidiumbromide-stained agarose gels. The first lane contains 3 μL of GeneRuler 50-bp DNA ladder (MBI, Fermentas, Vilnius, Lithuania).
Induction of LMP-1 by IL-10 in other BL lines. (A) Total cell extracts of hIL-10-treated (50 ng/mL, 48 hours) Mutu I cl.148 and Mutu III cl.99 were analyzed for EBNA-2 and LMP-1 expression by SDS-PAGE and immunoblotting, developed on the same membrane. (B) Immunoblot analysis of LMP-1, EBNA-2, and β-actin expression in total cell extracts of IL-10-treated Sal BL cells (50 ng/mL, 48 hours). (C) Total cell extracts of hIL-10-treated (50 ng/mL, 48 hours) DG75-Akata NeoR clones 2-2 and 6 were analyzed for EBNA-2 and LMP-1 expression by SDS-PAGE and immunoblotting. (D) Immunoblot analysis of LMP-1 expression in total cell lysates of IL-10-treated Daudi and P3HR1 cells (50 ng/mL, 6 hours). (E) RT-PCR results of LMP-2A, EBNA-2, and GAPDH expression of untreated and IL-10-treated (50 ng/mL, 24 hours) Daudi and P3HR1 cells. cDNAs prepared from the LCL B95-8 and the EBV-negative Hodgkin lymphoma cell line KMH2 were used as positive and negative controls, respectively. The PCR products were visualized in ethidiumbromide-stained agarose gels. The first lane contains 3 μL of GeneRuler 50-bp DNA ladder (MBI, Fermentas, Vilnius, Lithuania).
In contrast, IL-10 did not induce LMP-1 in the stable type I lines Rael and Akata or in the c-myc-driven BL-like variants of ER/EB2-5, A1 and P493-6 (not shown). IL-10 did not enhance the induction of LMP-1 in the 5-azacytidine-treated Rael cells (not shown). The lack of LMP-1 inducibility by IL-10 in Akata cells was not due to the characteristic of the resident viral strain because IL-10 could upregulate the expression of LMP-1 in 2 DG75 clones carrying the Akata-NeoR virus, designated DG75-Akata cl. 2-2 and DG75-Akata cl. 6 (Figure 4C). Induction of LMP-1 protein by hIL-10 was rapid and could be detected in Daudi and P3HR1 cells after 6 hours of exposure to the cytokine (Figure 4D).
We also examined the expression of LMP-2A and LMP-2B mRNAs by RT-PCR. Interestingly, LMP-2A but not LMP-2B transcripts could be detected in the untreated Daudi and P3HR1 cells (Figure 4E; not shown). There was no difference in the expression of LMP-2A when the cDNAs were prepared from untreated or IL-10-treated cells (50 ng/mL IL-10 for 24 hours). The control LCLs expressed both LMP-2A and EBNA-2 mRNAs, whereas Daudi and P3HR1 cells did not express EBNA-2 (Figure 4E) because of the EBNA-2 deletion in their viral genome.
Effect of hIL-10 on LMP-1 expression in EBV-transformed B cells
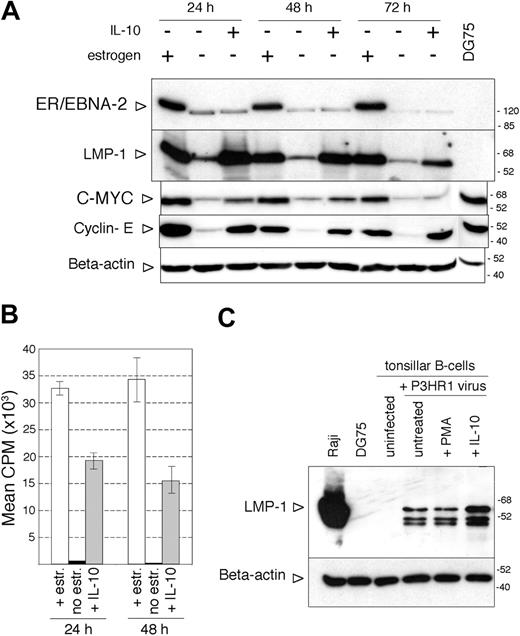
IL-10 did not influence the LMP-1 expression of 2 LCLs carrying B95-8 or Akata virus, respectively, or in the estrogen-treated conditional LCL ER/EB2-5 (not shown). In the absence of estrogen, the ER/EB2-5 cells stop proliferating and gradually downregulate EBNA-2 and LMP-1.34 They can survive up to 4 days after the removal of estrogen.34 We found that IL-10 could maintain the expression of LMP-1 for 3 days when added in a single dose at the time of estrogen removal (Figure 5A). LMP-1 was expressed independently of EBNA-2, since by day 3 the ER/EBNA-2 fusion protein was significantly downregulated in the absence of estrogen, seen as a doublet of about 120 kDa. EBNA-1 expression did not change under these conditions (Figure 5A). In line with the maintenance of LMP-1 expression, the estrogen-starved, IL-10-containing ER/EB2-5 cultures displayed larger cell aggregates than the untreated cultures (not shown).
In the absence of estrogen for 72 hours, CD21and CD80 were downregulated, whereas IgM, HLA-II, and LFA-1 expression did not change (Figure 5B; not shown). IL-10 prevented the downregulation of CD21 and CD80 and induced a marked downregulation of HLA-II but it did not influence the expression of IgM and LFA-1 (Figure 5B; not shown).
IL-10 not only maintained the expression of LMP-1 after estrogen removal but also reinduced LMP-1 when added to ER/EB2-5 cells starved of estrogen for 3 days (Figure 6A). At this time point, ER/EBNA-2 expression in the non-estrogen-treated cells was very low and appeared as a single faster migrating band (Figure 6A). Interestingly, IL-10 treatment induced the proliferation of the estrogen-starved cells, as indicated by the expression of c-myc and cyclin E (Figure 6A) and by the incorporation of thymidine (Figure 6B). The hIL-10-treated, estrogen-starved ER/EB2-5 cells incorporated 33 times more thymidine than the untreated control cells, assessed 24 hours after the addition of 50 ng/mL cytokine to 3-day-estrogen-starved cultures (Figure 6B). There was no difference in survival in the presence or absence of IL-10 at 3 days after estrogen removal when the cytokine was added in a single dose, but twice as many cells survived in the IL-10-treated cultures tested at 6 days after estrogen starvation (not shown).
Maintenance of LMP-1 expression by hIL-10 in ER/EB2-5 cells after estrogen removal. (A) ER/EB2-5 cells were cultured in the presence or absence of estrogen or in the presence of 50 ng/mL hIL-10 for 3 days. EBNA-2, EBNA-1, and LMP-1 expression were assayed in the same total cell extracts by SDS-PAGE and immunoblotting. The lower expression of EBNA-1 in the estrogen-treated ER/EB2-5 cells was not reproducible and was probably due to difference in loading of the sample. (B) Flow cytometric analysis of the CD21, CD80, and HLA-II antigens in the estrogen-starved, hIL-10-treated (50 ng/mL, 3 days) ER/EB2-5 cells. The fluorescence intensity in the FL2 channel was measured (logarithmic amplification) and analyzed with the CellQuest Pro program. On the histogram plots the shaded areas represent the background fluorescence of the secondary Ab-stained cells, whereas the solid black lines denote the specific staining of the untreated and hIL-10-treated cells. The mean fluorescence intensity is indicated. B958 LCL and Jurkat cells were used as positive and negative staining controls, respectively (not shown).
Maintenance of LMP-1 expression by hIL-10 in ER/EB2-5 cells after estrogen removal. (A) ER/EB2-5 cells were cultured in the presence or absence of estrogen or in the presence of 50 ng/mL hIL-10 for 3 days. EBNA-2, EBNA-1, and LMP-1 expression were assayed in the same total cell extracts by SDS-PAGE and immunoblotting. The lower expression of EBNA-1 in the estrogen-treated ER/EB2-5 cells was not reproducible and was probably due to difference in loading of the sample. (B) Flow cytometric analysis of the CD21, CD80, and HLA-II antigens in the estrogen-starved, hIL-10-treated (50 ng/mL, 3 days) ER/EB2-5 cells. The fluorescence intensity in the FL2 channel was measured (logarithmic amplification) and analyzed with the CellQuest Pro program. On the histogram plots the shaded areas represent the background fluorescence of the secondary Ab-stained cells, whereas the solid black lines denote the specific staining of the untreated and hIL-10-treated cells. The mean fluorescence intensity is indicated. B958 LCL and Jurkat cells were used as positive and negative staining controls, respectively (not shown).
Induction of proliferation in the ER/EB2-5 cells by hIL-10 in the absence of EBNA-2 expression. (A) Immunoblot analysis of LMP-1, EBNA-2, c-myc, cyclin E, and β-actin expression in total ER/EB2-5 cell lysates. The cells were starved of estrogen for 3 days before the addition of hIL-10 and then harvested at the indicated time points. Total cell extracts of DG75 cells were analyzed as controls for c-myc and cyclin E expression. (B) Proliferation of the estrogen-starved ER/EB2-5 cells in the presence of hIL-10 as measured by 12-hour incorporation of radioactive thymidine. □ indicates estrogen-treated cells; ▪, nontreated cells; and  , IL-10-treated cells. The mean CPM was calculated from 3 independent experiments. The error bars indicate ± SD. (C) Immunoblot analysis of LMP-1 and β-actin expression in P3HR1 virus-infected tonsillar B cells. Total cell lysates were prepared 24 hours after infection and treatment with 50 ng/mL hIL-10 or 20 ng/mL PMA.
, IL-10-treated cells. The mean CPM was calculated from 3 independent experiments. The error bars indicate ± SD. (C) Immunoblot analysis of LMP-1 and β-actin expression in P3HR1 virus-infected tonsillar B cells. Total cell lysates were prepared 24 hours after infection and treatment with 50 ng/mL hIL-10 or 20 ng/mL PMA.
Induction of proliferation in the ER/EB2-5 cells by hIL-10 in the absence of EBNA-2 expression. (A) Immunoblot analysis of LMP-1, EBNA-2, c-myc, cyclin E, and β-actin expression in total ER/EB2-5 cell lysates. The cells were starved of estrogen for 3 days before the addition of hIL-10 and then harvested at the indicated time points. Total cell extracts of DG75 cells were analyzed as controls for c-myc and cyclin E expression. (B) Proliferation of the estrogen-starved ER/EB2-5 cells in the presence of hIL-10 as measured by 12-hour incorporation of radioactive thymidine. □ indicates estrogen-treated cells; ▪, nontreated cells; and  , IL-10-treated cells. The mean CPM was calculated from 3 independent experiments. The error bars indicate ± SD. (C) Immunoblot analysis of LMP-1 and β-actin expression in P3HR1 virus-infected tonsillar B cells. Total cell lysates were prepared 24 hours after infection and treatment with 50 ng/mL hIL-10 or 20 ng/mL PMA.
, IL-10-treated cells. The mean CPM was calculated from 3 independent experiments. The error bars indicate ± SD. (C) Immunoblot analysis of LMP-1 and β-actin expression in P3HR1 virus-infected tonsillar B cells. Total cell lysates were prepared 24 hours after infection and treatment with 50 ng/mL hIL-10 or 20 ng/mL PMA.
Importantly, the effect of IL-10 was not restricted to cell lines. Tonsillar B lymphocytes infected with the nontransforming, EBNA-2-defective P3HR1 virus expressed LMP-1 when exposed to IL-10 for 24 hours (Figure 6C). The LMP-1 expression in the nontreated, P3HR1-infected cells is due to the carry over of LMP-1 by the EBV virions, as previously described.41 LMP-1 could be detected in total cell lysates prepared from tonsillar B cells immediately after incubation with the concentrated P3HR1 virus (not shown).
Induction of LMP-1 by hIL-10 in EBV-positive NK-cell lymphoma lines
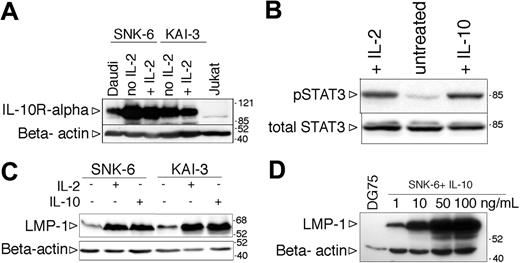
The IL-2-dependent NK lymphoma cell lines SNK-6 and KAI-3 expressed the IL-10R alpha chain (Figure 7A). To test the functionality of the IL-10R, the SNK-6 cells were starved of IL-2 for 24 hours, exposed to IL-10, and after one hour total cell lysates were prepared to assess the phosphorylation of STAT3. Similarly to the BL lines, STAT3 was phosphorylated on Tyr705 in the IL-10-treated NK cells (Figure 7B). Withdrawal of IL-2 resulted in the downregulation of LMP-1 (Figure 7B) and growth arrest (not shown). Addition of recombinant human IL-10 to the IL-2-deprived SNK-6 and KAI-3 cells induced the expression of LMP-1 (Figure 7C). The effect of IL-10 was dose dependent (Figure 7D). IL-10 treatment did not influence the expression of EBNA-1 and EBNA-2 (EBNA-1 positive, EBNA-2 negative; not shown). LMP-2 mRNA was not expressed in either cell line in the presence or absence of IL-2 or IL-10, assessed by RT-PCR with specific primers for LMP-2A and LMP-2B (not shown), similarly to the original description of the KAI-3 cell line.37
Discussion
The molecular mechanism of LMP-1 and LMP-2 expression in type II cells is not known. Here we identified IL-10 as a cytokine that could induce the expression of LMP-1 in the absence of EBNA-2 in BL and NK-lymphoma cell lines and in tonsillar B cells. The BL lines used in this study were heterogeneous with regard to their response to IL-10. This might reflect differences in the IL-10R functionality, in signaling intermediates, or in the methylation of the LMP-1 promoter.
IL-10 is a potent anti-inflammatory and immunosuppressive cytokine.42 It is produced by a variety of cells, such as CD4+ T cells, macrophages/monocytes, dendritic cells, and NK cells.42 IL-10 promotes the survival and plasma-cell differentiation of human B cells.42
There are many connections between EBV and IL-10. EBV-infected B cells are known to produce hIL-10,43 and LMP-1 was identified as the gene responsible for its induction.44 Taken together with our finding that IL-10 can induce the expression of LMP-1, this suggests the existence of an autocrine regulatory loop between IL-10 and LMP-1. A similar regulatory loop was defined in epithelial cells for LMP-1 and IL-6.45 The rapid induction of LMP-1 by IL-10 argues for a direct relationship between IL-10 receptor signaling and LMP-1 transcriptional regulation. This is in line with the activation of STAT3 and STAT5 by IL-10 and with the existence of 2 functional STAT-binding sites in the LMP-1 promoters.46
Induction of LMP-1 by hIL-10 in NK-cell lymphoma lines. (A) Expression of IL-10R assessed by SDS-PAGE and immunoblotting in total cell lysates of SNK-6 and KAI-3 cells cultured in the presence or absence of hIL-2. The membrane was stripped and reprobed with anti-β-actin antibody to serve as loading control. (B) Immunoblot analysis of total cell extracts of IL-2- or IL-10-treated SNK-6 with phospho-STAT3 (Tyr705)- and total STAT3-specific antibodies. (C) LMP-1 and β-actin expression in IL-2- or IL-10-treated (48 hours) SNK-6 and KAI-3 cells assessed by SDS-PAGE and immunoblotting. Of note: all immunoblots of NK lymphoma line extracts were probed with the less sensitive anti-LMP-1 monoclonal Ab mixture CS1-4 (in comparison with the anti-LMP-1 S12 monoclonal Ab used in all the previous figures). (D) LMP-1 and β-actin immunoblot of SNK-6 cells treated with 1, 10, 50, or 100 ng/mL hIL-10 for 48 hours.
Induction of LMP-1 by hIL-10 in NK-cell lymphoma lines. (A) Expression of IL-10R assessed by SDS-PAGE and immunoblotting in total cell lysates of SNK-6 and KAI-3 cells cultured in the presence or absence of hIL-2. The membrane was stripped and reprobed with anti-β-actin antibody to serve as loading control. (B) Immunoblot analysis of total cell extracts of IL-2- or IL-10-treated SNK-6 with phospho-STAT3 (Tyr705)- and total STAT3-specific antibodies. (C) LMP-1 and β-actin expression in IL-2- or IL-10-treated (48 hours) SNK-6 and KAI-3 cells assessed by SDS-PAGE and immunoblotting. Of note: all immunoblots of NK lymphoma line extracts were probed with the less sensitive anti-LMP-1 monoclonal Ab mixture CS1-4 (in comparison with the anti-LMP-1 S12 monoclonal Ab used in all the previous figures). (D) LMP-1 and β-actin immunoblot of SNK-6 cells treated with 1, 10, 50, or 100 ng/mL hIL-10 for 48 hours.
IL-10 is involved in the proliferation and autonomous growth of EBV-transformed B cells.47,48 Our results suggest that IL-10 can contribute to the survival and proliferation of EBV-infected B cells through the induction of LMP-1. The proliferation induced by IL-10 in the absence of EBNA-2 in the ER/EB2-5 cells is intriguing since ectopic expression of LMP-1 in the ER/EB2-5 cells could not maintain the proliferation of these cells.49 Furthermore IL-10 alone did not induce the proliferation of normal B cells but strongly enhanced proliferation when combined with CD40 or BCR activation.50 Since LMP-1 mimics a constitutively active CD40 receptor, IL-10 together with LMP-1 might drive the cells into the cell cycle.
Interestingly, high IL-10 serum levels were found in 2 chronic infections known to be risk factors for EBV-associated lymphomas, namely infection with human immunodeficiency virus (HIV) and malaria.51,52 Several studies have provided evidence for a role of hIL-10 in the pathogenesis of B-cell lymphoproliferations and B-cell lymphomas.53 Human IL-10 was found not only to be produced by BLs, HLs, AIDS-associated lymphomas, and posttransplantation lymphoproliferative diseases (PTLDs) but also to be an autocrine growth factor for some of these malignancies.53
Induction of LMP-1 by external stimuli, such as IL-10, could also partly explain the heterogeneous expression of LMP-1 in some EBV-positive B-cell malignancies17 and the intriguing finding of scattered LMP-1-positive cells in BLs.11-15 IL-10 could be produced by the malignant cells or it could be provided by the surrounding, infiltrating cells.
IL-10 expression was found in NK/T-cell lymphomas as well.54,55 The in vitro findings that LMP-1 expression in the NK lymphoma lines depends on the presence of IL-2 (required for the propagation of these lines), together with the LMP-1 expression by only a fraction of the EBV-infected malignant cells in the nasal NK lymphoma tissues, argues for the possibility that LMP-1 is induced by extracellular signals in this lymphoma also.
EBV expresses different latency types in B cells, depending on their differentiation stage and/or activation. Naive B cells express type III, whereas GC B cells express type II latency.2 Our results point to the possibility that the LMP-1 expression of the EBV-infected GC B cells is not necessarily constitutive, since LMP-1 may be induced by extracellular signals in the GC environment. Because LMP-1 down-regulates the master transcription factor of GC B cells, BCL-6,40 the existence of LMP-1-positive GC B cells has been questioned.56 Here we provide in vitro evidence that there are circumstances when LMP-1 and BCL-6 are coexpressed in malignant B cells with a GC phenotype and IL-10 could be one of the factors responsible for this expression pattern. It is not known why BCL-6 was not downregulated in the IL-10-treated BL lines but one possibility is that IL-10 signaling, concomitantly with the induction of LMP-1, activates the BCL-6 promoter. This assumption is in line with the existence of 3 STAT-binding sites in the BCL-6 promoter57 and with the recent finding that BCL-6 is a direct target of STAT5B in primary B cells.58
Modulation of latent EBV gene expression by extracellular signals in EBV-infected lymphoid cells is an interesting new concept that could explain the heterogeneous LMP-1 expression within tumors, the lack of any in vitro model of type II EBV latency, and the apparent discrepancy that tonsillar memory B cells express LMP-1 whereas peripheral-blood memory B cells do not.59 Furthermore, our present and previous results22 point to a direct relationship between type I and type II EBV latency with regard to LMP-1 expression (ie, extracellular signals could readily induce LMP-1 expression in the absence of EBNA-2 in type I BL, NK, and Hodgkin lymphoma cell lines). Therefore LMP-1 expression in type II latency may not be a constitutive characteristic of the EBV-infected cell, rather under specific circumstances it may be induced by extracellular signals.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-06-2569.
Supported by funds from the Swedish Cancer Society, Sweden. L.L.K., M.T., and N.N. are recipients of Cancer Research Fellowship of Cancer Research Institute (New York)/Concern Foundation (Los Angeles).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs G. W. Bornkamm, N. Shimizu, Y. Harabuchi, and J. M. Middeldorp for generously providing cell lines and reagents and Ajionomoto company for recombinant IL-2.