Abstract
Fas ligand (FasL), a member of the TNF protein family, potently induces cell death by activating its matching receptor Fas. Fas-mediated killing plays a critical role in naturally and pathologically occurring cell death, including development and homeostasis of the immune system. In addition to its receptor-interacting and cell death–inducing extracellular domain, FasL has a well-conserved intracellular portion with a proline-rich SH3 domain–binding site probably involved in non-apoptotic functions. We report here that, as with the Fas receptor, a fraction of FasL is constitutively localized in rafts. These dynamic membrane microdomains, enriched in sphingolipids and cholesterol, are important for cell signaling and trafficking processes. We show that FasL is partially localized in rafts and that increased amounts of FasL are found in rafts after efficient FasL/Fas receptor interactions. Raft disorganization after cholesterol oxidase treatment and deletions within the intracellular FasL domain diminish raft partitioning and, most important, lead to decreased FasL killing. We conclude that FasL is recruited into lipid rafts for maximum Fas receptor contact and cell death–inducing potency. These findings raise the possibility that certain pathologic conditions may be treated by altering the cell death–inducing capability of FasL with drugs affecting its raft localization.
Introduction
The Fas ligand (FasL, CD95/Apo-1 ligand, CD178, TNFSF6) molecule belongs to the TNF family and potently induces cell death in Fas (CD95/Apo-1/TNFRSF6) receptor–expressing cells.1 Fas and FasL interact as oligomers,2 and crosslinking of Fas leads to the recruitment of the adaptor protein FADD and of caspase-8 (Casp-8) to the cytoplasmic Fas domain and to the formation of an intracellular receptor complex called death-inducing signaling complex (DISC).3 Within the DISC, Casp-8 is activated and triggers the downstream caspase cascade, which executes the apoptotic dismantling of the cell.
FasL is a type 2 transmembrane protein consisting of a receptor-interacting extracellular domain and a well-conserved 80-amino acid (aa)–long N-terminal cytoplasmic portion.1,4,5 Cell death initiated by the Fas/FasL system is important for, among others things, homeostasis of the immune system (eg, activation-induced T-cell death6 ), cytotoxic T cell (CTL)–mediated killing of virally infected or transformed cells,7 and immune privilege maintenance.8-14 The corresponding experimental studies are supported by the pathology of model systems displaying functional inactivation or aberrant activation of Fas/FasL. Mice carrying loss-of-function mutations in the Fas (lpr) or the Fasl (gld) genes develop lymphadenopathy and splenomegaly with a massive accumulation of CD4–CD8– T cells.15 In addition, depending on the genetic background, these animals spontaneously acquire various forms of autoimmune disease with high titers of autoantibodies.16 In humans, mutations in Fas and Fasl are associated with autoimmune lymphoproliferative syndrome (ALPS).17-19 In such patients, the normal homeostasis of T and B lymphocytes is disturbed, leading to hepatosplenomegaly and lymphadenopathy. As in lpr and gld mice, autoimmunity and the accumulation of CD4–CD8– T cells are observed in most patients. Other diseases involve excessive induction of Fas-mediated cell death. Autoimmune (type 1) diabetes mellitus, for example, results from the destruction of insulin-producing pancreatic β-cells by autoreactive T lymphocytes and involves Fas/FasL and perforin/granzyme–mediated cell death pathways (see Kreuwel et al20 and references therein).
The intracellular FasL domain is involved in sorting the molecule into secretory lysosomes in T and natural killer (NK) cells and is then released to the cell surface on target cell contact.21,22 The cytoplasmic FasL portion may also be responsible for the transduction of (extracellular) signals into FasL-bearing cells, also known as reverse signaling.23-27 Several signaling motifs within the FasL intracellular domain are highly conserved, including a tandem casein kinase 1 phosphorylation site (aa 17-21),28 a phosphorylatable tyrosine at position 7, and a proline-rich region (aa 40-70) with bona fide SH3/WW domain–binding sites.24 Such sequence elements are likely to contribute to FasL reverse signaling and regulation, which, however, have thus far only been described phenomenologically.
Membrane rafts are sphingolipid/cholesterol membrane domains found in all mammalian cell types.29 Sphingolipids and cholesterol not only accumulate in membrane rafts, they are essential for raft formation.30,31 Recent studies using model membranes suggest that membrane rafts correspond to a particular phase of the lipid bilayer, the liquid-ordered (lo) phase.29 This lo lipid bilayer phase displays an intermediate fluidity between those of the liquid-disordered (ld) and the gel phases. An increasing amount of data suggests that rafts play fundamental roles in diverse cellular functions, particularly in signal transduction, by promoting segregated compartmentalization of membrane proteins and lipids.31,32 Several works, including ours, have highlighted the role of membrane rafts in the initiation of Fas signal transduction.33-37 Ligation of the Fas receptor promotes rapid recruitment of FADD and Casp-8 to the rafts and leads to efficient DISC formation and cell death signaling. Disruption of rafts by membrane cholesterol depletion abolishes DISC formation and Fas-induced cell death.
In this study, we aimed to investigate whether raft localization influences the ability of FasL to initiate Fas-triggered cell death.
Materials and methods
Antibodies and reagents
The antibodies (Abs) used for Western blotting were as follows: anti-Rab5 (Santa Cruz Biotechnology, Santa Cruz, CA), anti–FasL G247 (PharMingen, San Diego, CA), antiphosphotyrosine (4G10; PharMingen), anti-Fyn (Santa Cruz Biotechnology), anti-FLAG (Sigma, St Louis, MO), and horseradish peroxidase–conjugated antirabbit or antimouse antibodies (Jackson ImmunoResearch, Bar Harbor, ME). The anti-FasL antibody NOK-1 (PharMingen) was used for expression analysis by flow cytometry. Phorbol myristate acetate (PMA) was purchased from Calbiochem (San Diego, CA), and ionomycin and phytohemagglutinin (PHA) were purchased from Sigma. FL-PC ([Bodipy-3-pentanoyl]-1-hexadecanoyl-snglycero-3-phosphocholine) and FL-GM1 (Bodipy-ganglioside GM1) were purchased from Molecular Probes (Eugene, OR).
Cell culture and transfection procedures
Human embryonic kidney cells (HEK 293; no. CRL-1573; American Type Culture Collection [ATCC], Manassas, VA) stably expressing the different FasL constructs were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Biotech Aidenbach, Germany) and G418 (1.2 mg/mL; Calbiochem) in a 5% CO2 atmosphere. COS-7 cells (CRL-1657; ATCC) were grown in DMEM supplemented with 10% fetal calf serum, glutamine, and sodium pyruvate. L1210 and L1210 Fas cells (described in Rouvier et al38 ) were grown in DMEM supplemented with 5% fetal calf serum. Fas-expressing JH6.2 cells and Fas-negative Jurkat cells (J16 rapo) stably transfected with pEFBos-hFasL, PCR33 hFasL, or PCR33 hFasL delta 1-40 were cultured in RPMI 1640 medium (Invitrogen) containing 10% fetal calf serum in a 5% CO2 atmosphere. Human T cells were isolated and cultured as described elsewhere.39 For T-cell stimulation, 100 ng/mL PMA (Sigma) and 0.5 μg/mL ionomycin were added to the medium for 3 hours before cell harvesting. The nontransformed immortalized human NK cell line NK92, originally described by Gong et al,40 was maintained in X-vivo 10 serum-free solution (Cambrex, Emcrainville, France) supplemented with 5% heat-inactivated human serum (a gift from Torsten Tonn, Institute for Transfusion Medicine and Immunohematology Red Cross Blood Donor Service [RCBDS], Frankfurt, Germany) and 100 U/mL human interleukin-2 (IL-2; Roche Diagnostics, Mannheim, Germany). To increase the extent of FasL surface expression, cells were pretreated with PMA (100 ng/mL; Sigma) and ionomycin (0.5 μg/mL; Sigma) in the presence of the matrix metalloproteinase (MMP) inhibitor GM6001 (N-[(2R)-2 hydroxyamido-carbonylmethyl)-4-methylpentanoyl]-L-tryptophan methylamide; 30 μM; Chemicon International, Temecula, CA) for 4 hours.
HEK293 cells were transiently transfected with the indicated DNA constructs using calcium phosphate precipitation in 10-cm culture dishes. Twenty-four hours after transfection, cells were harvested, washed with ice-cold phosphate-buffered saline, and used for raft preparation. Transient transfections in COS-7 cells were performed with ExGen 500 (Euromedex, Souffelweyersheim, France). Fluorescence-labeled lipid analogs GM1 and PC were incorporated in the plasma membrane at low concentrations (approximately 100-1000 molecules/μm2) by the lipid exchange method.41
Plasmids
N-terminally Flag-tagged human FasL (FASLG) was produced by polymerase chain reaction (PCR) from a plasmid template and was cloned into the mammalian expression vector pCR33 (kindly provided by Dr Hermann Eibel, Freiburg, Germany), resulting in pCR33-Flag-FASLG. In the same way, FASLG deletion mutants lacking either aa 1-40 or aa 1-80 were cloned into pCR33 (FASLG delta 1-40, FASLG delta 1-80). For N-terminal GFP-tagging, FASLG cDNA (full length or lacking the first N-terminal 40 aa) was cloned into the pEGFPC1 vector from Clontech (Palo Alto, CA). For all constructs, the correct reading frames were confirmed by DNA sequencing.
Biochemical raft separation
Rafts were isolated as described previously. Briefly, postnuclear supernatant (PNS) from HEK293 (1 × 107), Jurkat hFasL (5 × 107), or NK92 (3.5 × 107) cells was solubilized in 1 mL buffer A (25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl, 1 mM EGTA [ethyleneglycotetraacetic acid], protease inhibitors cocktail) containing 1% Brij 98 (Aldrich Chemical, Milwaukee, WI) detergent for 5 minutes at 37°C and was chilled on ice before it was placed at the bottom of a step sucrose gradient (1.33-0.9-0.867-0.833-0.8-0.767-0.733-0.7-0.6 M sucrose) in buffer A. Gradients were centrifuged at 250 000 g for 16 hours in a SW41 Beckman rotor (Beckman Instruments, Gagny, France) at 4°C. One-milliliter fractions were harvested from the top, except for the last fraction (no. 9), which contained 3 mL. The DIM fraction contained the pooled fractions 1 to 4, and the heavy fraction (HF) consisted of pooled fractions 8 and 9.
Western blot analysis
Western blot analysis of proteins was performed according to standard protocols. Briefly, cells were collected and directly resuspended in Laemmli buffer. After sonication and denaturing (95°C, 5 minutes), the solubilized proteins were resolved by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and were transferred to a PVDF membrane (Immobilon-P; Millipore, Molsheim, France) by electroblotting.
Cell death assays
The killing ability of FasL-expressing cells (HEK293 or Jurkat) was determined by coculture assays with Fas-expressing target cells (L1210 Fas; parental L1210 cells without Fas receptor served as a control; in earlier experiments, the Fas-expressing JH6.2 Jurkat cell line was used), with an effector-target ratio of 1:10. After the appropriate incubation time, the apoptotic rate in the target cells was determined by flow cytometry analysis. Cells were fixed in ice-cold 70% ethanol, washed in 38 mM sodium citrate (pH 7.4), and stained for 20 minutes at 37°C in 38 mM sodium citrate (pH 7.4) with 69 μM propidium iodide (Sigma) and 5 μg/mL RNase A (Sigma). Cells were analyzed with a flow cytometer (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ), and the proportion of apoptotic cells represented by the sub-G1 peak (after exclusion of objects with a fractional DNA content less than 10% of intact G1 cells) was determined.
Cholesterol oxidase treatment
HEK293 cells were incubated in 37°C preheated serum-free HEPES buffer (10 mM) containing 2 U/mL cholesterol oxidase (Calbiochem, La Jolla, CA) at 37°C for 2 hours. After drug treatment, cells were washed once before raft isolation or coculture experiment was performed.
Fluorescence correlation spectroscopy experiments
Fluorescence correlation spectroscopy (FCS) measurements were made on a custom apparatus, as previously described.42,43 Briefly, FCS data were collected using an 200 M microscope (Axiovert; Zeiss, Oberkochen, Germany) equipped with an Apochromat 40 × /1.2 numeric aperture water-immersion objective and with excitation from a 488-nm line of an Ar+-ion laser. The laser waist was set by selecting the lateral extension of laser beam falling into the back aperture of the objective with a diaphragm. Fluorescence was collected through the same objective, separated from the excitation light by a dichroic mirror, and sent onto avalanche photodiodes through a 525- to 565-nm bandpass filter. A confocal pinhole reduced the out-of-focus fluorescence. FCS measurements were performed in Hanks balanced salt solution with 10 mM HEPES, pH 7.4, by illuminating the sample with an excitation power of less than 4 μW at the back aperture of the objective lens. Autocorrelations were processed by a hardware correlator (ALV, Langen, Germany), and data were analyzed with built-in functions of IGOR Pro (WaveMetrics, Lake Oswego, OR).
Results
FasL localizes to lipid rafts after membrane treatment with Brij 98 detergent
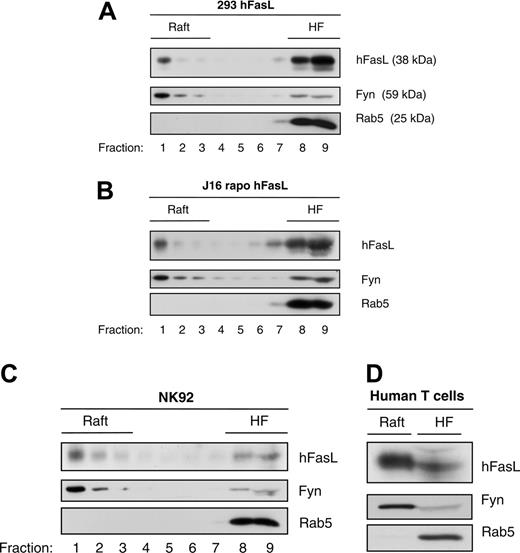
In a biochemical approach that allows the analysis of rafts at physiologic temperature, PNSs from different cells were solubilized by the polyoxyethylene ether Brij 98, and lysates were subjected to ultracentrifugation through a sucrose density gradient. Using this method, rafts, which are resistant to the detergent treatment, are separated in the light gradient fractions from other solubilized, disordered membrane structures remaining in the heavy fractions.44 Importantly, the use of Brij 98 as detergent prevents raft coalescence observed with the commonly used Triton X-100.45,46 In HEK293 cells stably expressing human FasL, a fraction of the ligand was detected by Western blot analysis within the raft fractions (Figure 1A). As a control for proper separation, the membrane was probed with an antibody recognizing the raft marker protein Fyn, which is localized in rafts because of posttranslational myristoylation and palmitoylation.47 As expected, Fyn appeared mainly in the light raft fractions, whereas the non–raft marker Rab5 was isolated in the heavy fractions.48 A similar result was obtained when the experiment was performed with Jurkat cells stably expressing hFasL (Figure 1B). We then analyzed endogenous FasL in the human NK92 cell line (which expresses relatively high endogenous amounts of the protein) (Figure 1C) and in human T cells after activation (Figure 1D). In both experiments, most of the endogenous ligand was localized in the detergent-insoluble light fractions of the sucrose gradient. Together, these biochemical data demonstrate that exogenously expressed and endogenous hFasL are partitioned to lipid membrane rafts.
Dynamic confinement of GFP-FasL into discrete domains at the plasma membrane
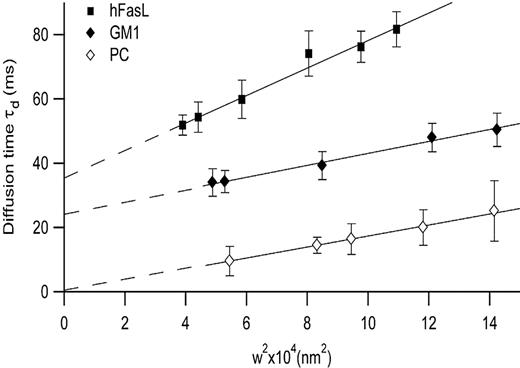
In the next experiment, we analyzed the diffusion behavior of GFP-tagged FasL protein by FCS. When transiently expressed in COS-7 cells, the recombinant molecule was efficiently expressed at the plasma membrane (data not shown). We then investigated the lateral diffusion of the GFP-FasL molecule in the cell membrane by FCS,49,50 performed at different spatial scales.42,43 This approach allows the submicron organization of the plasma membrane of living cells to be studied with minimal perturbation. If a molecule undergoes free diffusion, the average time τd a molecule stays within the illuminated area is strictly proportional to the square of the beam radius (w2). It is then possible to derive a diffusion law with t0 corresponding to the value of intercept on the y-axis and a diffusion coefficient effective, Deff, corresponding to the slope. t0 has been described as a pertinent parameter related to a confinement index for isolate corrals when it is a positive value, whereas Deff depends on the partition and the diffusion coefficient outside the corrals. GFP-FasL diffusion behavior clearly differed from that of a free diffusion mode. τd increased linearly with w2, but the intercept t0 was strictly and significantly positive, with a value of 35.4 ± 3.66 milliseconds (Figure 2). This diffusion behavior was compared with the characteristics of molecules thought to be Bodipy-C5-ganglioside-GM1– or not to be Bodipy-C5-phosphatidylcholine (PC)–enriched in raft membranes42,43 (P. F. Lenne L.W., D. Wurtz, A. Boned, H. Rigneault, and D.M., manuscript submitted) (Figure 2). This analysis suggested that GFP-FasL exhibited a lateral confinement within raft-type membrane domains, a finding further confirmed by sensitivity of this confinement to cholesterol oxidase (see Figure 5A for a description of the process) and in agreement with the biochemical data presented in Figure 1.
Ligand-independent partitioning of FasL in lipid membrane rafts. (A) PNSs from HEK293 cells stably expressing human FasL were prepared and then solubilized in Brij 98 before they were subjected to sucrose gradient separation, as described in “Materials and methods.” Sucrose fractions were analyzed by Western blot with anti-FasL, anti-Fyn, and anti-Rab5 antibodies. Detergent-insoluble membrane fractions containing isolated rafts were identified by the presence of the raft marker protein Fyn. Nonraft components of the membrane (eg, Rab5) were purified in the heavy fraction (HF). (B) An experiment similar to that described in panel A was performed with Jurkat cells (J16) stably expressing hFasL. Again, a significant fraction of FasL was detected in raft fractions. (C-D) Brij 98 solubilization and sucrose gradient separation revealed that in the natural killer cell line NK92 (C) and in activated primary human T cells (D), endogenous FasL was localized within detergent-insoluble rafts. Immunoblots were performed on pooled heavy (8-9; HF) and light (1-4; Raft) fractions.
Ligand-independent partitioning of FasL in lipid membrane rafts. (A) PNSs from HEK293 cells stably expressing human FasL were prepared and then solubilized in Brij 98 before they were subjected to sucrose gradient separation, as described in “Materials and methods.” Sucrose fractions were analyzed by Western blot with anti-FasL, anti-Fyn, and anti-Rab5 antibodies. Detergent-insoluble membrane fractions containing isolated rafts were identified by the presence of the raft marker protein Fyn. Nonraft components of the membrane (eg, Rab5) were purified in the heavy fraction (HF). (B) An experiment similar to that described in panel A was performed with Jurkat cells (J16) stably expressing hFasL. Again, a significant fraction of FasL was detected in raft fractions. (C-D) Brij 98 solubilization and sucrose gradient separation revealed that in the natural killer cell line NK92 (C) and in activated primary human T cells (D), endogenous FasL was localized within detergent-insoluble rafts. Immunoblots were performed on pooled heavy (8-9; HF) and light (1-4; Raft) fractions.
FCS spectroscopy demonstrates confinement of GFP-tagged FasL in microdomains. Diffusion behaviors of FL-PC (⋄), FL-GM1 (♦), and GFP-hFasL (▪) in COS-7 cells were measured by FCS. t0 was determined from the position at which diffusion curves intersect the time-axis (diffusion time). All FCS measurements were performed at 37°C. Error bars indicate the SD of 3 independent experiments.
FCS spectroscopy demonstrates confinement of GFP-tagged FasL in microdomains. Diffusion behaviors of FL-PC (⋄), FL-GM1 (♦), and GFP-hFasL (▪) in COS-7 cells were measured by FCS. t0 was determined from the position at which diffusion curves intersect the time-axis (diffusion time). All FCS measurements were performed at 37°C. Error bars indicate the SD of 3 independent experiments.
Intracellular FasL domain is essential for the partitioning of FasL in rafts
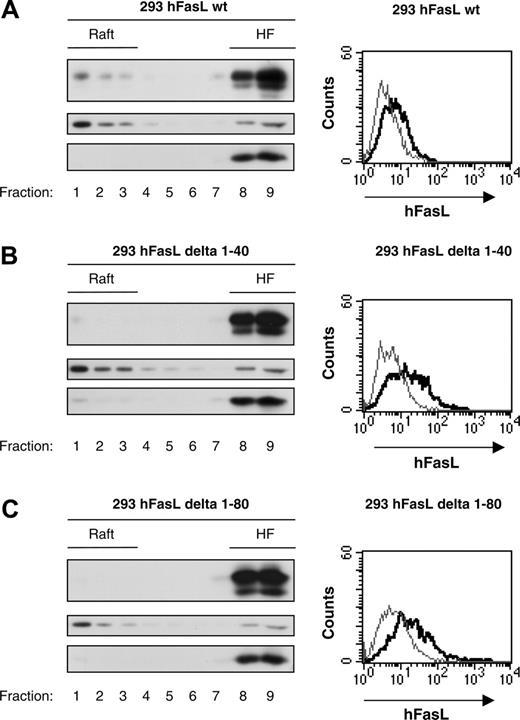
The fact that N-terminal intracellular FasL is highly conserved between species indicates an important function of this domain.13,24,28 In addition to its role in the proper transport and localization of the molecule to secretory lysosomes in hematopoietic cells (from which the ligand is released “on demand” to the cell surface21,22 ), the intracellular tail contains signaling motives in its sequence that are most likely involved in the transduction of extracellular signals to the cell.23-27 Here we tested a potential involvement of the intracellular domain in FasL partitioning into rafts. For this purpose, HEK293 cells were stably transfected with deletion mutants that lacked either the first half of the intracellular FasL domain (hFasL delta 1-40) or the complete cytoplasmic portion (hFasL delta 1-80). We checked by flow cytometry analysis that the deletion mutants were properly expressed at the cell surface. In addition, to compare the relative proportion of raft-localized FasL, we chose HEK293 cell clones with similar cell surface expression levels of full-length, wild-type FasL, FasL delta 1-40, or FasL delta 1-80 (Figure 3) because deletion of the intracellular domain has been shown to result in increased levels of cell surface FasL.51 The 293 cell clones were subjected to biochemical raft separation by Brij 98 solubilization and sucrose gradient. Figure 3 illustrates that both deletion mutants were severely impaired in their raft localization. Interestingly, though removal of the first 40 N-terminal amino acids allowed residual FasL raft partitioning, deletion of all 80 N-terminal amino acids completely abolished any localization to the light raft fractions. Therefore, we concluded that the intracellular domain of FasL was crucial for its targeting to or maintenance within the membrane rafts.
FasL protein levels increase in rafts after interaction of the ligand with the Fas receptor
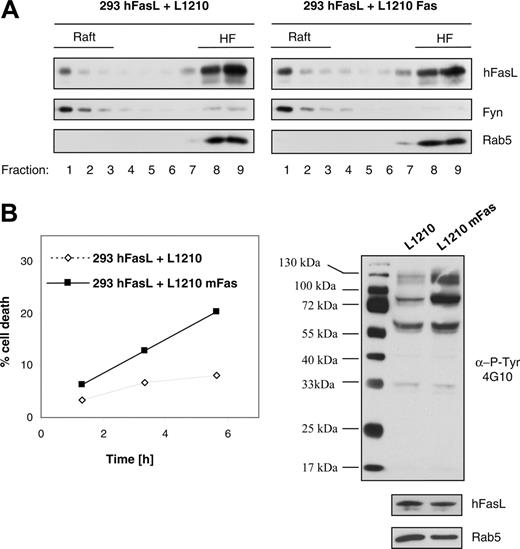
Our previous experiments showed that a significant amount of FasL is constitutively localized in rafts. To test whether binding of the ligand to its receptor changes the extent of FasL raft partitioning, we performed coculture experiments and incubated HEK293 cells stably expressing human FasL with target cells either with (L1210 Fas) or without (L1210) Fas receptor. As can be seen in Figure 4A, interaction with the Fas receptor leads to the translocation of FasL from the disordered membrane environment into the ordered membrane raft domains. We analyzed our coculture system for efficient Fas/FasL interaction by quantifying Fas-induced cell death in the target cells. Although no appreciable cell death was observed with L1210 target cells devoid of Fas receptor, incubation of HEK293T hFasL cells with the Fas-bearing L1210 Fas cells led to target cell death that increased with incubation time (Figure 4B, left panel). Further evidence for functional binding of FasL to target cell–expressed Fas receptor was obtained by Western blot analysis of total cell lysates prepared from the cocultured cells. Coincubation of ligand- and receptor-expressing cells led to elevated tyrosine phosphorylation (Figure 4B, right panel). Such increased phosphorylation as a consequence of Fas/FasL interaction has been described for receptor- and ligand-bearing cells.27,52
FasL must be localized within rafts for maximum induction of cell death
It has been shown that the signaling activity of an increasing number of transmembrane proteins is regulated by raft translocation (for reviews, see Simons and Toomre32 and He et al53 ). We wondered what functional consequences the partitioning of FasL in rafts might have for its cell death–inducing capacity. Therefore, we analyzed the effects of FasL dislocation from rafts to non–raft compartments on FasL-mediated cell death. To achieve this goal, we performed a cholesterol oxidase treatment that, by converting cholesterol to cholestenone, inhibited lipid microdomain formation. In addition, and independently, we made use of the deletion mutant Fasl delta 1-40 because removal of the intracellular first 40 aa of FasL appears essential for FasL raft localization (Figure 3).
Deletion of the intracellular FasL domain abolishes raft localization of the ligand. PNSs from HEK293 cells stably expressing full-length human FasL (A), FasL delta 1-40 (lacking the N-terminal aa 1-40 (B), or FasL delta 1-80 (lacking the N-terminal aa 1-80 that constitutes the complete intracellular domain) (C) were prepared and then solubilized in Brij 98 detergent before they were subjected to sucrose gradient separation, as described in “Materials and methods.” Western blot analysis of the single gradient fractions was performed with anti-FasL, anti-Fyn (raft marker), and anti-Rab5 (non–raft marker) antibodies. Flow cytometry analysis of FasL expression was performed on each cell line to ensure that the deletion mutants FasL delta 1-40 and FasL delta 1-80 were properly expressed at the cell surface. The thin line indicates secondary antibody alone, thick line, Anti–FasL antibody (Nok-1) plus secondary antibody.
Deletion of the intracellular FasL domain abolishes raft localization of the ligand. PNSs from HEK293 cells stably expressing full-length human FasL (A), FasL delta 1-40 (lacking the N-terminal aa 1-40 (B), or FasL delta 1-80 (lacking the N-terminal aa 1-80 that constitutes the complete intracellular domain) (C) were prepared and then solubilized in Brij 98 detergent before they were subjected to sucrose gradient separation, as described in “Materials and methods.” Western blot analysis of the single gradient fractions was performed with anti-FasL, anti-Fyn (raft marker), and anti-Rab5 (non–raft marker) antibodies. Flow cytometry analysis of FasL expression was performed on each cell line to ensure that the deletion mutants FasL delta 1-40 and FasL delta 1-80 were properly expressed at the cell surface. The thin line indicates secondary antibody alone, thick line, Anti–FasL antibody (Nok-1) plus secondary antibody.
Inducible translocation of FasL into lipid membrane rafts. (A) HEK293 cells expressing hFasL were cocultured with L1210 cells (no Fas receptor; left panel) or with L1210 cells stably transfected with Fas (right panel) for 80 minutes at 37°C before solubilization in Brij 98 and were subjected to sucrose gradient separation. Single fractions were analyzed by Western blot with anti-FasL, anti-Fyn, and anti-Rab5 antibodies. (B) Samples were taken at different time points from the same coculture experiments described in panel A to quantify cell death in the target cell population (L1210 and L1210 Fas cells) by flow cytometry analysis (sub-G1 content; left panel). In addition, after 80 minutes of coculture, total protein lysates were prepared from an aliquot of the cells. Western blot analysis with the antiphosphotyrosine antibody 4G10 revealed increased protein phosphorylation in the sample derived from Fas receptor–expressing cells, indicating efficient Fas/FasL interaction (right panel).
Inducible translocation of FasL into lipid membrane rafts. (A) HEK293 cells expressing hFasL were cocultured with L1210 cells (no Fas receptor; left panel) or with L1210 cells stably transfected with Fas (right panel) for 80 minutes at 37°C before solubilization in Brij 98 and were subjected to sucrose gradient separation. Single fractions were analyzed by Western blot with anti-FasL, anti-Fyn, and anti-Rab5 antibodies. (B) Samples were taken at different time points from the same coculture experiments described in panel A to quantify cell death in the target cell population (L1210 and L1210 Fas cells) by flow cytometry analysis (sub-G1 content; left panel). In addition, after 80 minutes of coculture, total protein lysates were prepared from an aliquot of the cells. Western blot analysis with the antiphosphotyrosine antibody 4G10 revealed increased protein phosphorylation in the sample derived from Fas receptor–expressing cells, indicating efficient Fas/FasL interaction (right panel).
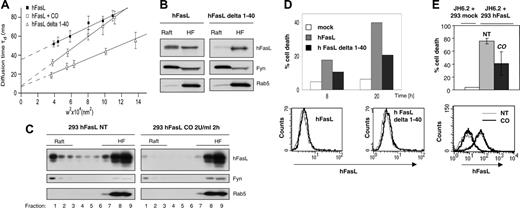
We first tested the influence of cholesterol oxidase treatment or removal of the N-terminal aa 1-40 on FasL raft localization by FCS analysis of GFP-tagged FasL in the presence of cholesterol oxidase and of the GFP-tagged FasL delta 1-40 (Figure 5A). Cholesterol oxidase treatment induced significant changes in the diffusion behavior of GFP-FasL. Molecular confinement strongly decreased, giving an intercept t0 value of 7.3 ± 2.9 milliseconds. Similarly, the GFP-tagged FasL delta 1-40 displays an intercept t0 value of 6.41 ± 3.02 milliseconds. These values imply a dislocation of FasL from rafts after cholesterol oxidase treatment or removal of the first 40 intracellular FasL aa.
We confirmed this raft dislocation by biochemical raft separation. As already described in Figure 3 for HEK293T cells, the delta 1-40 FasL mutant, in contrast to full-length FasL, is mainly expressed in non–raft fractions in Jurkat cells (Figure 5B).
As expected, the cholesterol oxidase treatment of FASLG–transfected HEK293T cells led to raft disruption and exclusive localization of FasL in the non–raft heavy fractions (Figure 5C), whereas in untreated control cells, FasL was still detected in the undisturbed raft fractions.
We then analyzed the consequences of this raft dislocation for the killing capability of FasL in coculture experiments. We first transfected Fas-deficient Jurkat cells (J16 Rapo ref) with full-length FasL or delta 1-40 FasL. Because deletion of the intracellular FasL domain increases FasL localization to the cell surface,51 we again selected these clones for comparable FasL cell surface expression (Figure 5D, bottom panel). When we compared their behavior in coculture experiments with the Fas-expressing JH6.2 Jurkat target cells, we found that the killing capacity of FasL delta 1-40 was significantly lower than that of full-length FasL (Figure 5D, upper panel).
In the next experiment, we cocultured for 5 hours Fasl-transfected 293 cells (either treated with cholesterol oxidase or untreated) with Fas-expressing JH6.2 Jurkat target cells. Although FasL expressed in cells with intact rafts efficiently triggered cell death, disruption of rafts led to a strong decrease in the killing capacity of FasL (Figure 5E, upper panel), though the ligand was still detectable and was expressed on the cell surface in amounts comparable to those of untreated cells with intact rafts, as determined by flow cytometry analysis (Figure 5E, bottom panel). Together our results, obtained on cholesterol oxidase treatment and usage of FasL delta 1-40, demonstrate that FasL must be localized within rafts for the maximum induction of cell death.
Discussion
Rafts are considered microdomains of ordered lipids that selectively partition functional groups of proteins within the membrane.29 They are distinct from the disordered fluid mosaic membrane that act as the relatively inert solvent for most membrane proteins. Our biochemical separation studies show that exogenously expressed and endogenous FasL are constitutively partitioned into raft membranes, a finding we have confirmed in live cells by demonstrating cholesterol-sensitive constrained diffusion in the plasma membrane for GFP-labeled FasL using a new FCS approach42,43 (Figures 2, 5A).
Biochemical data obtained with FasL mutants expressed in HEK293 cells and FCS diffusion behaviors of the GFP hFasL constructs show that deletion of the N-terminal 40 aa of the intracellular domain is sufficient to significantly decrease FasL localization in rafts, whereas removal of the complete cytoplasmic tail entirely abolishes raft partitioning. The physical requirement for the intracellular part of FasL for recruitment into rafts is of particular interest because this has not been found for the Fas receptor, a significant fraction of which (like FasL) is constitutively localized in rafts. Raft partitioning has been shown to be essential for the cell death function of Fas, at least in certain cell types.33-37 Importantly, Fas mutants with as few as 6 amino acid residues below the endoplasmic leaflet of the membrane behave identically to the wild-type receptor in terms of membrane raft distribution,36 arguing that the intracellular Fas domain is not important for raft localization (in contrast to FasL).
Our functional investigations demonstrate that FasL raft partitioning is not a static process; rather, it is a dynamic process. With the binding of the ligand to the Fas receptor (which induces cell death in the receptor-bearing target cell), the amount of raft-associated FasL increases, indicating the translocation of additional FasL molecules into rafts. The mechanism controlling this translocation is unknown, and it is not even clear whether interaction of the ligand with its receptor occurs before or after recruitment into rafts (in other words, we do not know whether FasL must be in the rafts for optimal contact with the Fas receptor or whether its interaction with the receptor results in raft localization; neither possibility is incompatible with the other).
Our data suggest an important role for the cytoplasmic portion of FasL in raft localization. This domain fulfills an important role in FasL sorting into intracellular secretory lysosomes in hematopoietic cells. In addition, this domain is most likely involved in reverse signaling into the ligand-bearing cell, a function that may also depend on raft localization. It is conceivable that FasL raft partitioning is regulated by other proteins binding to the intracellular FasL domain, possibly in an inducible manner.
Disruption of rafts and absence of the first 40 intracellular amino acids lead to dislocation of FasL from rafts into disordered membrane fractions and to diminished FasL-induced cell death. (A) Dynamic confinement of GFP-FasL into discrete domains at the plasma membrane is independently prevented by enzymatic modification of cholesterol and deletion of the first 40 intracellular FasL amino acids. COS-7 cells transiently expressing the recombinant GFP-FasL protein were left untreated (▪) or were treated with cholesterol oxidase (□). Alternatively, COS-7 cells were transfected with GFP-FasL delta 1-40 (▵). FCS measurements were performed at 37°C immediately after cellular treatment. (B) Jurkat cells stably transfected with human FasL (hFasL) or hFasL delta 1-40 were solubilized in Brij 98 detergent and subjected to sucrose gradient separation. Immunoblots were performed on pooled heavy (8-9; HF) and light (1-4; Raft) fractions with the indicated antibodies. (C) HEK293 cells stably transfected with human FasL were treated with cholesterol oxidase (2 U/mL) for 2 hours (CO) or were left untreated (NT). Cells were then solubilized in Brij 98 detergent and subjected to sucrose gradient separation before analysis of the raft and nonraft fractions by Western blot. (D) JH6.2 cells were cocultured for 8 or 20 hours with Jurkat cells stably transfected with hFasL or hFasL delta 1-40 or with mock-transfected Jurkat cells. Cell death was then quantified by flow cytometry analysis of propidium iodide–stained ethanol-fixed cells. The graph represents the average of 3 independent experiments, with error bars indicating the SD. Equal cell surface expression of FasL and FasL delta 1-40 in the Jurkat cell clones was demonstrated by flow cytometry analysis (bottom panel). The thin line indicates secondary antibody alone; thick line, Anti-FasL antibody (Nok-1) plus secondary antibody. (E) Aliquots of the FasL-transfected 293 cells (left untreated or treated with 2 U/mL cholesterol oxidase from the experiment described in panel B) were used for a 5-hour coculture with Fas-expressing JH6.2 Jurkat target cells to quantify the killing capacity of FasL. As a control, JH6.2 cells were cocultured with mock-transfected HEK293 cells. The graph represents the average of 3 independent experiments, with error bars indicating SD. Flow cytometry analysis was performed with anti-FasL antibody (Nok-1) to ensure that cell surface expression of FasL was not modified by the cholesterol oxidase treatment (bottom panel). Overlay of profiles obtained for cells incubated with fluorescence-labeled secondary antibody alone or with Nok-1 plus secondary antibody are shown. The thin line indicates not treated (NT) cells; thick line, cholesterol oxidase (CO)–treated cells.
Disruption of rafts and absence of the first 40 intracellular amino acids lead to dislocation of FasL from rafts into disordered membrane fractions and to diminished FasL-induced cell death. (A) Dynamic confinement of GFP-FasL into discrete domains at the plasma membrane is independently prevented by enzymatic modification of cholesterol and deletion of the first 40 intracellular FasL amino acids. COS-7 cells transiently expressing the recombinant GFP-FasL protein were left untreated (▪) or were treated with cholesterol oxidase (□). Alternatively, COS-7 cells were transfected with GFP-FasL delta 1-40 (▵). FCS measurements were performed at 37°C immediately after cellular treatment. (B) Jurkat cells stably transfected with human FasL (hFasL) or hFasL delta 1-40 were solubilized in Brij 98 detergent and subjected to sucrose gradient separation. Immunoblots were performed on pooled heavy (8-9; HF) and light (1-4; Raft) fractions with the indicated antibodies. (C) HEK293 cells stably transfected with human FasL were treated with cholesterol oxidase (2 U/mL) for 2 hours (CO) or were left untreated (NT). Cells were then solubilized in Brij 98 detergent and subjected to sucrose gradient separation before analysis of the raft and nonraft fractions by Western blot. (D) JH6.2 cells were cocultured for 8 or 20 hours with Jurkat cells stably transfected with hFasL or hFasL delta 1-40 or with mock-transfected Jurkat cells. Cell death was then quantified by flow cytometry analysis of propidium iodide–stained ethanol-fixed cells. The graph represents the average of 3 independent experiments, with error bars indicating the SD. Equal cell surface expression of FasL and FasL delta 1-40 in the Jurkat cell clones was demonstrated by flow cytometry analysis (bottom panel). The thin line indicates secondary antibody alone; thick line, Anti-FasL antibody (Nok-1) plus secondary antibody. (E) Aliquots of the FasL-transfected 293 cells (left untreated or treated with 2 U/mL cholesterol oxidase from the experiment described in panel B) were used for a 5-hour coculture with Fas-expressing JH6.2 Jurkat target cells to quantify the killing capacity of FasL. As a control, JH6.2 cells were cocultured with mock-transfected HEK293 cells. The graph represents the average of 3 independent experiments, with error bars indicating SD. Flow cytometry analysis was performed with anti-FasL antibody (Nok-1) to ensure that cell surface expression of FasL was not modified by the cholesterol oxidase treatment (bottom panel). Overlay of profiles obtained for cells incubated with fluorescence-labeled secondary antibody alone or with Nok-1 plus secondary antibody are shown. The thin line indicates not treated (NT) cells; thick line, cholesterol oxidase (CO)–treated cells.
FasL raft partitioning seems essential for its capability to induce Fas-mediated cell death, a finding which is, to our knowledge, reported here for the first time. Combined with the observation of an activation-dependent translocation of FasL to the immunologic synapse in primary T lymphocytes (O.J., unpublished data, April 2004) and the notion that the subsequent FasL/Fas ligation leads to a stabilization of long-lasting focal ligand/receptor interactions,54 these data suggest an amplifying “feedback” mechanism of FasL killing on first encounter of ligand molecules with the receptor.
The mere localization of FasL in rafts has been described previously but only in vitro. Gajate and Mollinedo55 investigated the molecular mechanism of cell death induction by the antitumor drug Aplidin (PharmaMar, Madrid, Spain). They found that Jurkat cell killing by Aplidin (PharmaMar) is Fas dependent and that cell treatment with this drug leads to its localization in rafts, along with the Fas receptor, FasL, and apoptotic downstream signaling molecules, including FADD and caspases. In light of our findings, such translocation of FasL into rafts would increase its potency to induce cell death in Fas-bearing neighboring cells. As discussed, the Fas receptor has to reside within intact rafts to launch an effective death signal.
In a study by Henkler et al, 54 YFP-tagged FasL was almost exclusively detected in the detergent-insoluble fraction of HeLa cells, indicating a constitutive association with lipid rafts. The authors describe the formation of stable and persistent Fas/FasL clusters, induced by membrane FasL, which still occurred in the presence of the cholesterol-depleting agent methyl-β-cyclodextrin (MβCD), though cluster assembly was somehow delayed. A possible decrease in the amount of cell death induced by FasL in Fas-sensitive cells after MβCD-treatment (despite formation of the Fas/FasL clusters) was not addressed in these experiments. Questions remain as to what extent the hydrophobic patches within GFP and its derivatives, which were used as tags for Fas and FasL, contributed to the observed clustering.
Lalor et al56 reported an almost exclusive localization of FasL in specialized membrane domains, though they detected the ligand in caveolae membrane domains of thymic epithelial cells using a detergent-free separation method for caveolae. Like rafts, caveolae are enriched in sphingolipids and cholesterol, but they form noncoated invaginations of the surface that are substantially bigger than rafts.29 Despite the copurification of caveolin by Lalor et al,56 it remains likely that rafts had in fact been isolated together with the caveolae. Our own conclusion that FasL is localized to rafts rather than to caveolae (at least in those cells analyzed by us) is based on the fact that Jurkat cells, in which we detected FasL in detergent-resistant membranes, do not contain caveolae.57
It is unknown which of the FasL properties that change to rafts on recruitment is important for its increased killing capacity. The often-quoted assumption that proteins are somehow concentrated on partitioning into rafts is at least disputable because the concentrating effect is probably the exact opposite: less than 95% of membrane proteins excluded from the rafts are concentrated within 30% to 50% of the membrane left for them to occupy.29 However, localization of receptors in rafts prevents their internalization, which may lead to sustained signaling (in this case, cell death induction). One could also envision that the raft environment favors the formation of FasL oligomers, a prerequisite for triggering Fas-mediated cell death.2 In any case, it is interesting that partitioning of the appropriate Fas receptor is equally important for its ability to transduce the death signal. Perhaps the necessary clustering of FasL and Fas—both expressed on neighboring cells—is facilitated if the molecules are localized in identical membrane structures with similar properties that do not repel on cell–cell contact.
As mentioned, FasL, after synthesis in cytotoxic T and NK cells, is directed to and stored in specialized secretory lysosomes/granules.22,58 It is expressed at the cell surface only on activation. Having used Brij 98 solubilization and sucrose gradient separation to isolate the rafts, we cannot exclude the possibility that the FasL-containing rafts in fact do not originate from the plasma membrane. Rather, they may originate from intracellular compartments such as secretory lysosomes. This specific localization might facilitate the quick expression of FasL at the surface on TCR stimulation, such as through a raft fusion phenomenon.
Modulating the extent of FasL partitioning appears to be another level at which the cell can regulate FasL activity. This concept may be adapted to clinical applications with applicable drugs that would influence raft stability and thereby FasL killing competence. This may represent a therapeutic option to inhibit excessive FasL killing under pathologic conditions (eg, type 1 diabetes mellitus20 ) or to kill tumor cells by using chemotherapeutic agents such as Aplidin (PharmaMar).55
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-07-2883.
Supported by institutional funds from the Centre National de la Recherche Scientifique (A.-O.H.) and the Institut National de la Santé et de la Recherche Médicale (D.M.) and by grants from the Association pour la Recherche contre le Cancer (A.-O.H.), the Emerald Foundation (A.-O.H.), Canceropole–Provence-Alpes Côte d'Azur (PACA) ACI 2004 (A.-O.H.), Deutsche Forschungsgemeinschaft (ZO 110/2-1 [W.B., M.Z.], SFB415/A9 [O.J.]), the German National Genome Research Network (NGFN project N1KR-S12T23) (V.K., M.Z.), and the French Research Ministry “Action concerteé incitative (ACI) Dynamique et réactivité des assemblages biologiques (DRAB) and ACI Nanosciences” (D.M.).
M.Z. and A.-O.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sebastien Huault and Mouna Sekoni for excellent technical assistance and Hai-Tao He for critical reading of the manuscript and helpful discussions.