Ligand binding to the thrombopoietin receptor (TpoR) is thought to impose a dimeric receptor conformation(s) leading to hematopoietic stem cell renewal, megakaryocyte differentiation, and platelet formation. Unlike other cytokine receptors, such as the erythropoietin receptor, TpoR contains an amphipathic KWQFP motif at the junction between the transmembrane (TM) and cytoplasmic domains. We show here that a mutant TpoR (Δ5TpoR), where this sequence was deleted, is constitutively active. In the absence of ligand, Δ5TpoR activates Jak2, Tyk2, STAT5, and mitogen-activated protein (MAP) kinase, but does not appear to induce STAT3 phosphorylation. Δ5TpoR induces hematopoietic myeloid differentiation in the absence of Tpo. In the presence of Tpo, the Δ5TpoR mutant appears to enhance erythroid differentiation when compared with the Tpo-activated wild-type TpoR. Strikingly, individual substitution of K507 or W508 to alanine also induces constitutive TpoR activation, indicating that the K and W residues within the amphipathic KWQFP motif are crucial for maintaining the unliganded receptor inactive. These residues may be targets for activating mutations in humans. Such a motif may exist in other receptors to prevent ligand-independent activation and to allow signaling via multiple flexible interfaces.
Introduction
The thrombopoietin receptor (c-mpl or TpoR) and its ligand thrombopoietin (Tpo) regulate the proliferation of megakaryocytic progenitors, their differentiation into mature megakaryocytes, and formation of platelets.1,2 Tpo or TpoR knockout mice exhibit a significant reduction of megakaryocytes and circulating platelets, and they also show reduced number of hematopoietic stem cells (HSCs) in the bone marrow, indicating a role for the TpoR and its ligand in HSC self-renewal.3 Like the erythropoietin receptor (EpoR), the TpoR is thought to function as a homodimer after binding Tpo. Downstream signaling pathways activated by the TpoR are triggered by 2 cytoplasmic tyrosine kinases, the Janus kinase (Jak) 2, and Tyk2.4-6 Upon receptor activation, phosphorylated tyrosine residues in the cytoplasmic domain of the TpoR and in Jaks provide docking sites for the SH2 domains of many signaling proteins, such as STAT3 and STAT5 (the signal transducers and activators of transcription 3 and 5), shc, SHIP, Grb2, and PI3K.4,5,7-9
Several activating mutations in the TpoR have been identified. In one, the envelope protein of the myeloproliferative virus replaces the extracellular domain of the receptor and activates it by oligomerization.10 Introduction of a cysteine residue in the extracellular domain of the TpoR at a position equivalent to that of the constitutively active R129C EpoR mutant11,12 leads to active disulfide-bonded TpoR dimers.13 Replacement of S498 (or S505) in the transmembrane (TM) domain by asparagine results in constitutively active receptors,9,14 most likely by ligand-independent dimerization.9,10,15 Deletion of the membrane distal extracellular cytokine receptor module of the TpoR results in a constitutive activation,16 but so far no data were reported about other negative regulatory motifs.
While a redundancy between cytokine receptors, such as between the erythropoietin (EpoR) and prolactin receptors, has been reported,17 EpoR and TpoR appear to exert exclusive effects, especially when expressed in embryonic stem cells.18 The cytoplasmic juxtamembrane (JM) domains of the TpoR, EpoR, and other cytokine receptors can be aligned with respect to the hydrophobic motif preceding the proline-rich Box 1.19 One exception is represented by a 5-amino acid amphipathic motif (KWQFP or RWQFP in the mouse and human receptors, respectively), which appears to be unique to the TpoR. We show here that deletion (Δ5TpoR) of these 5 residues, or mutation of K507 or W508 to alanine, results in constitutive activation of the TpoR. Furthermore, the mutant TpoR resembles the EpoR in its JM region. Δ5TpoR is hypersensitive to Tpo. Ligand activation of Δ5TpoR and of the AWQFP and KAQFP TpoR mutants leads to differentiation of myeloid colonies and an enhanced erythroid differentiation. We propose that the KWQFP motif evolved to prevent ligand-independent activation and to impart specific signaling, and suggest that such sequences may exist in other families of receptors.
Materials and methods
cDNA constructs
The Mpl (murine TpoR) and MPL (human TpoR) mutants containing cytosolic substitutions or deletions were subcloned into the pMX-IRES-GFP or MSCV-IRES-GFP bicistronic retroviral vector upstream of the internal ribosome entry site (IRES).20 All TpoR constructs (residue numbering for both mouse and human TpoR as in Onishi et al9 ) used for this work were generated by overlap extension using polymerase chain reaction (PCR) with synthetic oligonucleotides and verified by sequencing. All TpoR constructs contained an HA tag at their N-terminus.
Generation of cell lines
To obtain Ba/F3 cell lines stably expressing the wild-type TpoR (wtTpoR) or the TpoR mutants, retroviral supernatants were generated by transient transfection of BOSC packaging cell line with bicistronic vectors encoding the gene of interest and green fluorescent protein (GFP) as marker and used for infection.20 Populations of cells expressing GFP at levels above 25% were isolated by fluorescence-activated cell sorting (FACS). To obtain UT7 cell lines stably expressing the wtTpoR or the Δ5TpoR mutant, vesicular stomatitis virus G envelope protein pseudotype retroviral supernatants were generated by transfection of 293-EBNA packaging cells, as described.21
Assay for Tpo-independent growth
Ba/F3 cells expressing the wtTpoR, the Δ5TpoR, or mutants with alanine substitutions were grown in interleukin-3 (IL-3). For testing growth factor independence, cells were washed with RPMI medium, than placed in RPMI medium containing 10% fetal bovine serum (FBS) without cytokines. Cell numbers were counted using a Coulter counter (Beckman Coulter, Fuller-ton, CA) after 2 to 12 days. UT7 cells expressing the wtTpoR or Δ5TpoR were grown in granulocyte-macrophage colony-stimulating factor (GM-CSF). Cells were washed with α-modified Eagle medium (α-MEM; Invitrogen, Carlsbad, CA) and placed in α-MEM medium containing 10% FBS without cytokines. Cell numbers (average of triplicates ± standard deviation) were counted after 3 to 15 days.
Immunoprecipitation and immunoblots
For examination of P-Jak2 or P-Tyk2 levels, Ba/F3 cells expressing the wtTpoR or the Δ5TpoR were washed, starved for 3 hours in RPMI/1mg/mL bovine serum albumin (BSA) and stimulated for 7 minutes with 50 ng/mL Tpo or left untreated. Cells were lysed by addition of 2 × lysis buffer as described.22 After preclearing the lysates by incubation with protein A, immunoprecipitations were carried out using antiphosphotyrosine anti-P-Y 4G10 antibodies (Upstate Biotechnology, Lake Placid, NY). Analysis was performed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with anti-P-Jak2 (P-Y1007, 1008) or anti-P-Tyk2 (P-Y1054) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Lysates were analyzed by immunoblotting with anti-Jak2 or -Tyk2 antibodies to ensure equal protein level. For analyzing P-STAT5/3 levels Ba/F3 cells were washed, starved in RPMI/1 mg/mL BSA, and stimulated for 7 minutes with 50 ng/mL Tpo. Cells were lysed with 200 μL 1 × lysis buffer. Lysates were analyzed on 12% SDS-PAGE and by immunoblotting with anti-P-STAT5 and anti-P-STAT3 (P-Y705) antibodies (Cell Signaling Technology, Beverly, MA). For reprobing, blots were stripped and reprobed with either anti-STAT5 or anti-STAT3 antibodies (Santa Cruz Biotechnology).
Dual luciferase assays
STAT5 or STAT3 activation was assessed by measuring luciferase production of cells transfected with the pLHRE-luc23,24 or pGL3bPpr2-luc constructs.25 Mitogen-activated protein kinase (MAPK) activation was assessed with the pSRE-luc construct.26 As an internal control, we used the pRL-TK vector (Promega, Madison, WI) containing the renilla luciferase gene under control of the TK promoter. Ba/F3 cells expressing the wtTpoR or Δ5TpoR were washed and starved for 3 hours in RPMI/1mg/mL BSA. Luciferase assays were performed as described.27
Surface expression of HA-tagged wtTpoR and Δ5TpoR
Surface expression of HA-TpoR and HA-Δ5TpoR was measured in Ba/F3 cells by flow cytometry using monoclonal anti-HA antibodies (10 μg/mL; Covance, Princeton, NJ) and R-phycoerythrin (PE)-conjugated donkey F(ab′)2 anti-mouse immunoglobulin G (IgG) secondary antibody (12.5 g/mL) as described.28
In vitro colony assay
Bone marrow cells were isolated from 6- to 8-week-old C57/Black6 mice treated with 5-fluorouracil to enrich for highly proliferating progenitors. To obtain hematopoietic progenitors expressing wtTpoR, Δ5TpoR, TpoR-AWQFP, or TpoR-KAQFP vesicular stomatitis virus G envelope protein pseudotype retroviral supernatants were generated. GFP-positive cells were sorted and plated on methylcellulose in the absence of Tpo or in the presence of 10 ng/mL Tpo. After 7 and 14 days the plates were evaluated for formation of granulocyte-monocyte colony-forming units (CFU-GM), megakaryocyte CFUs (CFU-Meg), and colonies that contain Meg, GM, and erythroid (CFU-granulocyte erythroid macrophage mixed [GEMM]) as well as erythroid burst-forming unit (BFU-E) colonies.
Determination of the structure of the KWQFP motif
1-13C-labeled leucine was purchased from Cambridge Isotope Laboratories (Andover, MA). Octyl-β-glucoside was obtained from Sigma Chemical (St Louis, MO). DMPC and DMPG were obtained from Avanti Polar Lipids (Alabaster, AL) and used without further purification.
Peptide synthesis and purification
Peptides corresponding to the TM and JM domains of TpoR (amino acids 481-522) were synthesized by solid-phase chemistry. For the TM-JM peptides, 3 arginine residues were added to the N-terminus for solubility, and the C-terminus was amidated. The synthetic peptides were purified by reverse-phase high-performance liquid chromatography (HPLC) on a C4 column with an acetonitrile-water gradient and lyophilized. The purity was confirmed by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry and HPLC.
Reconstitution of peptides into membrane bilayers
Purified TpoR TM-JM peptides were reconstituted by detergent dialysis using the procedure described.29 The reconstituted membranes were pelleted to form multilamellar dispersions and loaded into nuclear magnetic resonance (NMR) rotors.
Solid-state NMR spectroscopy
NMR experiments were performed at a 13C frequency of 90.2 MHz using a 4.0-mm magic-angle spinning probe. The spinning frequency was 8 000 ± 5 Hz. Ramped amplitude cross-polarization contact times were 2 ms in all experiments and 2 pulse-phase-modulated decouplings were used during the evolution and acquisition periods. The decoupling field strength was typically 90 kHz. 13C chemical shifts were referenced to external glycine (13C=O) at 176.02 parts per million (ppm) relative to tetramethylsilane (TMS). The temperature was maintained at -75°C.
Results
Design of a new mutant form of the murine TpoR
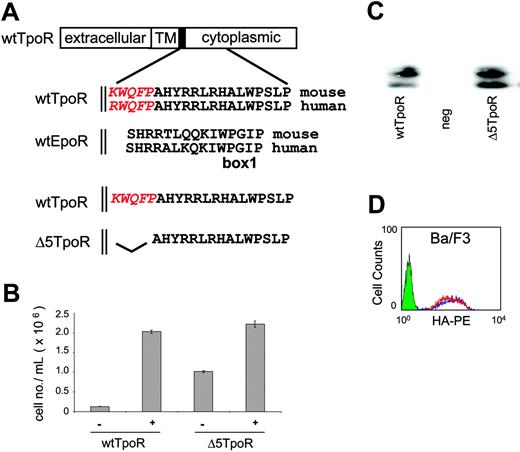
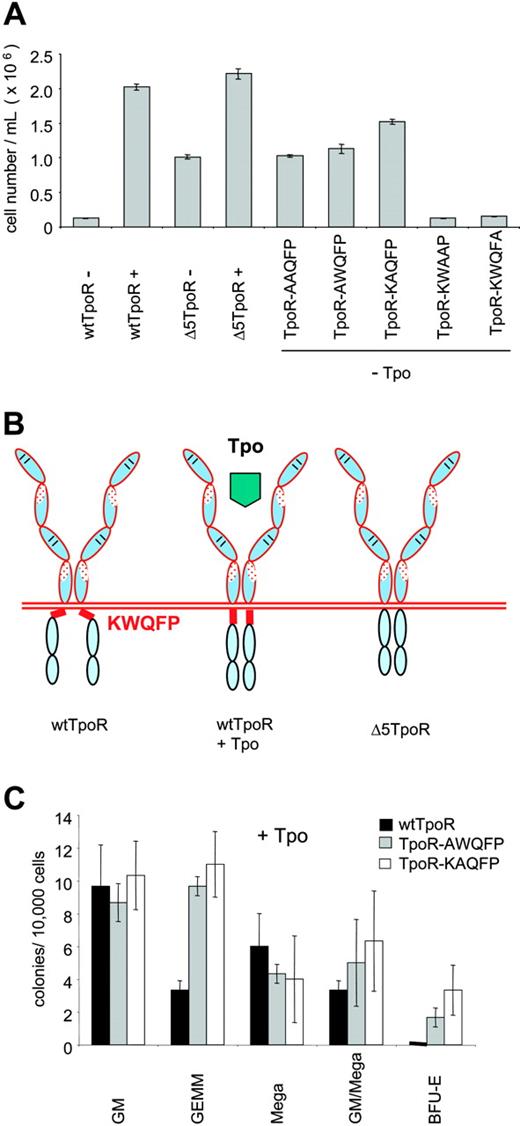
Cytokine receptors share 2 conserved motifs, Box1 and Box2, in the cytoplasmic domain that are crucial for signaling.30 The EpoR TM domain is connected to the Box1 motif by a stretch of 11-amino acid residues (SHRRTLQQKIW) that can be perfectly aligned between the EpoR and TpoR cytoplasmic domains and which appear to form a functional helical unit with the TM domain.19 Alignment of the EpoR and TpoR sequences reveals that TpoR contains a unique sequence, KWQFP in the murine and RWQFP in the human receptor, that forms the hinge between the TM and cytoplasmic domains (Figure 1A). To investigate whether this KWQFP motif is relevant to the function of the TpoR, we deleted it and tested the mutant Δ5TpoR for its biological activity (Figure 1B).
TpoR contains a cytoplasmic KWQFP motif that maintains the unliganded receptor inactive. (A) The TpoR JM domain contains 5 additional residues that show no homology to the EpoR (shown in red italics). To construct theΔ5TpoR, the KWQFP motif in the TpoR cytosolic JM domain was deleted by PCR mutagenesis. (B) Proliferation assays performed in Ba/F3 cells expressing the wtTpoR or Δ5TpoR at equal GFP levels in the absence of any cytokines or stimulated with 5 ng/mL Tpo as indicated. Cell numbers (averages of triplicates ± SD) were counted at day 12. (C) Total protein levels of the wtTpoR or Δ5TpoR in Ba/F3 cells infected to GFP levels at 40% to 50%, as revealed by Western blotting using anti-HA antibodies. (D) HA staining for cell-surface levels of the wtTpoR (red) or Δ5TpoR (blue) in Ba/F3 or hematopoietic progenitor cells infected to GFP levels at 40%. Green indicates Ba/F3 cells (negative control).
TpoR contains a cytoplasmic KWQFP motif that maintains the unliganded receptor inactive. (A) The TpoR JM domain contains 5 additional residues that show no homology to the EpoR (shown in red italics). To construct theΔ5TpoR, the KWQFP motif in the TpoR cytosolic JM domain was deleted by PCR mutagenesis. (B) Proliferation assays performed in Ba/F3 cells expressing the wtTpoR or Δ5TpoR at equal GFP levels in the absence of any cytokines or stimulated with 5 ng/mL Tpo as indicated. Cell numbers (averages of triplicates ± SD) were counted at day 12. (C) Total protein levels of the wtTpoR or Δ5TpoR in Ba/F3 cells infected to GFP levels at 40% to 50%, as revealed by Western blotting using anti-HA antibodies. (D) HA staining for cell-surface levels of the wtTpoR (red) or Δ5TpoR (blue) in Ba/F3 or hematopoietic progenitor cells infected to GFP levels at 40%. Green indicates Ba/F3 cells (negative control).
Proliferation of Ba/F3 cells that express the Δ5 TpoR
Ba/F3 cells are murine pro-B cells that are dependent on IL-3 for growth.31 Transfection of other cytokine receptors in these cells can render them dependent on the respective cytokines for growth. Culture of these cells in the absence of cytokines normally leads to rapid cell death.31 Ba/F3 cells were infected using the bicistronic retroviral vector pMX-IRES-GFP20 in order to compare the biologic activity of the murine Δ5TpoR and wtTpoR. The level of expression of the gene cloned upstream of the IRES correlates with the level of GFP downstream of the IRES, and expression levels can easily be monitored by FACS analysis.20 The cell lines obtained after infection (GFP levels of 40%-50%) were washed and grown in RPMI/10% FBS in the absence of any cytokines or stimulated with Tpo at a concentration of 5 ng/mL.
Figure 1B shows that expression of the Δ5TpoR enabled Ba/F3 cells to proliferate in the absence of IL-3 or Tpo, whereas cells expressing the wtTpoR died a few days after removal of cytokines. The levels of cell proliferation induced by the Δ5TpoR in the absence of cytokines were reduced compared to those induced by the wtTpoR stimulated with 5 ng/mL Tpo, but were similar to the levels of proliferation supported by lower Tpo concentrations (data not shown). The Δ5TpoR was also able to support cytokine-independent growth of UT7 cells. UT7 cells are cytokine-dependent human megakaryocytic leukemia cells.32 Both receptors (wtTpoR and Δ5TpoR) were expressed as doublets of 72 to 84 kDa, as shown by Western blot analysis of Ba/F3 cells expressing either the Δ5TpoR or wtTpoR (Figure 1C). The faster migrating band represents the immature (Endo H-sensitive) form, while the slower migrating band represents the mature (Endo H-resistant) form of the receptor, as described.33
We examined the cell-surface expression of the Δ5TpoR or wtTpoR in Ba/F3 cells and mouse bone marrow progenitors. It is well established that a higher cell-surface expression of other receptors, such as the Her-2/neu tyrosine kinase receptor, causes self-activation and transformation of NIH3T3 cells by constitutive signaling, and Her-2/neu receptor overexpression plays an important role in human breast cancer.34-36 In the case of wtTpoR and Δ5TpoR, both receptors were detected at equal levels at the surface of Ba/F3 cells (Figure 1D) and of mouse bone marrow progenitors (Figure 2A). Thus, we ruled out that growth factor-independent proliferation induced by the Δ5TpoR was simply due to much higher receptor levels at the cell surface. While the wtTpoR exhibited predominantly the mature form, examination of the Δ5TpoR showed a higher level of immature receptor form. This may suggest that the Δ5TpoR is less efficient in trafficking to the cell surface, or in degrading the immature form. However, the Δ5TpoR behaved indistinguishably from the wtTpoR in Jak2-dependent traffic assays33 (data not shown), and the steady-state cell-surface levels were similar between wtTpoR and Δ5TpoR.
To further ensure that the Δ5TpoR was not activated by the presence of low amounts of Tpo in the serum, we treated cells expressing the wtTpoR or the Δ5TpoR in the absence or presence of Tpo with antibodies that neutralize Tpo. Cells expressing the wtTpoR could not grow in Tpo after addition of neutralizing anti-Tpo antibodies. These cells show no ligand-independent proliferation regardless of the presence of anti-Tpo antibodies. In contrast, cells expressing the Δ5TpoR were permissive for growth in the presence of anti-Tpo antibodies (data not shown). As expected, deletion of the homologous motif in the human TpoR, RWQFP, resulted in a constitutively active human TpoR (data not shown). We have shown that a motif within the JM domain of the TpoR was crucial for maintaining the TpoR in a signaling inactive conformation in the absence of its ligand Tpo.
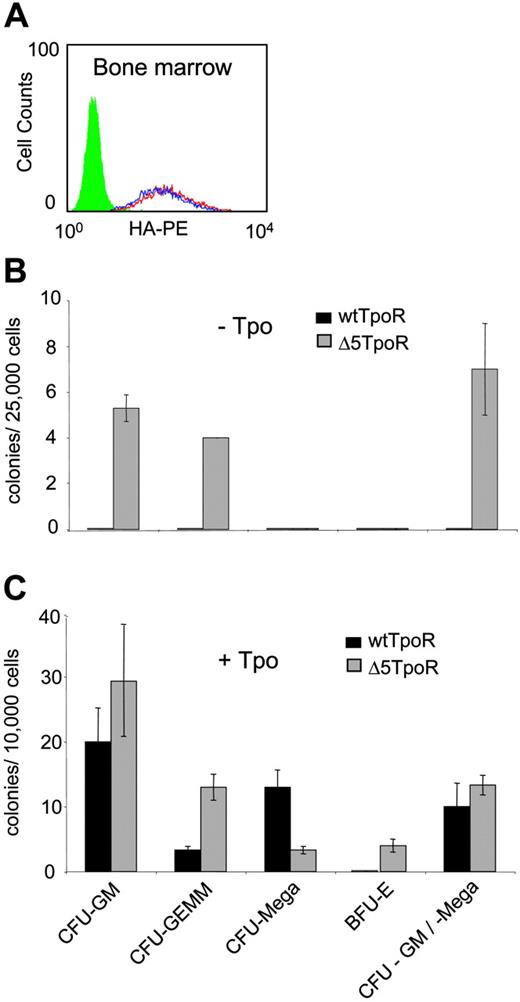
Constitutive and ligand-induced activation of Δ5TpoR in primary myeloid progenitors. (A) Bone marrow cells were infected with retroviruses coding for wtTpoR or Δ5TpoR and sorted for equivalent GFP before plating. Cell-surface levels of wtTpoR (red) or Δ5TpoR (blue) were determined by FACS with anti-HA antibodies. Green indicates Ba/F3 cells (negative control). (B) Colony formation of hematopoietic progenitor cells expressing the wtTpoR or Δ5TpoR in the absence of Tpo (- Tpo). (C) Colony formation of hematopoietic progenitor cells expressing the wtTpoR or Δ5TpoR in the presence of 10 ng/mL Tpo (+ Tpo). Shown in panels B and C are averages of colony numbers (averages of triplicates ± SD) from 1 representative experiment. Data were similar among 3 independent experiments.
Constitutive and ligand-induced activation of Δ5TpoR in primary myeloid progenitors. (A) Bone marrow cells were infected with retroviruses coding for wtTpoR or Δ5TpoR and sorted for equivalent GFP before plating. Cell-surface levels of wtTpoR (red) or Δ5TpoR (blue) were determined by FACS with anti-HA antibodies. Green indicates Ba/F3 cells (negative control). (B) Colony formation of hematopoietic progenitor cells expressing the wtTpoR or Δ5TpoR in the absence of Tpo (- Tpo). (C) Colony formation of hematopoietic progenitor cells expressing the wtTpoR or Δ5TpoR in the presence of 10 ng/mL Tpo (+ Tpo). Shown in panels B and C are averages of colony numbers (averages of triplicates ± SD) from 1 representative experiment. Data were similar among 3 independent experiments.
We next tested the ability of the Δ5TpoR to respond to lower doses of Tpo, such as a concentration of 0.1 ng/mL. The wtTpoR was not able to promote cell proliferation when stimulated with 0.1 ng/mL Tpo, whereas the Δ5TpoR induced significant proliferation under the same conditions (not shown). This hypersensitivity to Tpo was not due to higher total or cell-surface receptor levels (Figure 1C-D).
Colony formation of hematopoietic progenitors mediated by the Δ5TpoR
We examined the ability of the Δ5TpoR to mediate hematopoietic differentiation in the presence or absence of Tpo. Bone marrow cells were isolated from mice that were treated with 5-fluorouracil to enrich for highly proliferating progenitors. The hematopoietic progenitors were infected with retroviruses coding for the Δ5TpoR or wtTpoR and sorted for GFP-positive cells. Surface expression of wtTpoR and Δ5TpoR were similar (Figure 2A). These cells were plated on methylcellulose in the absence of Tpo or in the presence of 10 ng/mL Tpo. After 7 days the plates were evaluated for formation of CFU-GM, CFU-Meg, and CFU-GEMM, as well as BFU-E.
The Δ5TpoR promoted formation of CFU-GM, CFU-GM/Meg, and CFU-GEMM in the absence of any growth factors, whereas wtTpoR did not (Figure 2B). Thus, the Δ5 TpoR is constitutively active also in primary hematopoietic progenitors, not only in cell lines. Interestingly, when performing the assay in the presence of ligand (10 ng/mL Tpo), we found that the Δ5TpoR significantly increased the formation of erythroid colonies when compared with the stimulated wtTpoR (Figure 2C), although it did promote formation of CFU-Meg, which is the hallmark of the wtTpoR. Since both the wtTpoR and Δ5TpoR were transduced in bone marrow progenitors to similar levels, we suggest that ligand-activated Δ5TpoR may signal differently than the wtTpoR. The enhanced erythroid differentiation induced by ligand-activated Δ5TpoR may reflect not only a difference in signaling between the 2 receptors, but also the levels of transduction of our retroviral constructs in megakaryocyte progenitors. It is possible that higher expression of our retroviral constructs in megakaryocyte progenitors would result in higher megakaryocyte differentiation by the ligand-activated Δ5TpoR.
Downstream signaling mediated by the Δ5TpoR
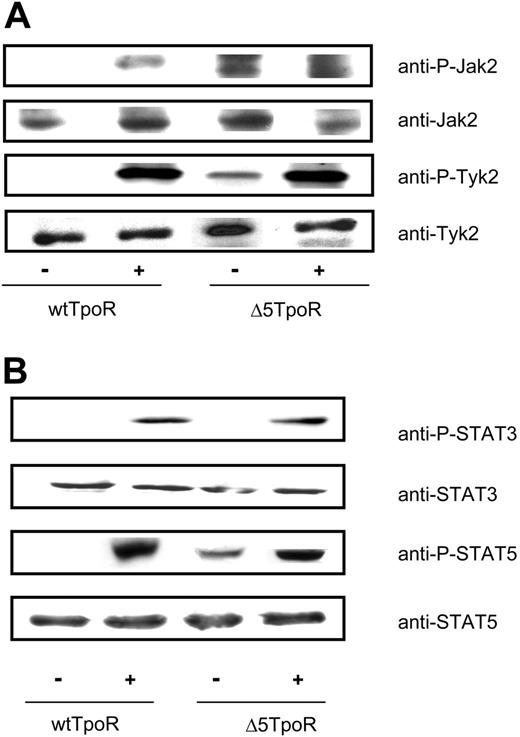
Activation of the TpoR by its ligand leads to activation of several signaling molecules including Jak2, Tyk2, STAT5, STAT3, and MAP kinases Erk1 and Erk2.7,37 We compared activation of these molecules in Ba/F3 cells that expressed either the Δ5TpoR or wtTpoR, grown in the absence of any cytokines in case of the Δ5TpoR or in the presence of 5 ng/mL Tpo when expressing the wtTpoR. The selected cells were washed, starved for 3 hours in RPMI/1 mg/mL BSA, and stimulated with Tpo (50 ng/mL) or not, as indicated. For Jak2 and Tyk2, cell lysates were immunoprecipitated with the 4G10 antiphosphotyrosine (anti-P-Y) antibody and immunoblotting was carried out using anti-P-Jak2 (Y1007, Y1008) or anti-P-Tyk2 (Y1054) antibodies. As shown in Figure 3A, there was constitutive tyrosine phosphorylation of Jak2 and Tyk2 in Ba/F3 cells expressing the Δ5TpoR, which was not detectable in cells expressing the wtTpoR. In the presence of ligand, both receptors supported Jak2 and Tyk2 activation, as revealed by antiphosphotyrosine-specific Western blotting (Figure 3A). TpoR is known to activate Tyk2 as well as Jak2, but Jak2 is much more active in downstream signaling.33 We used generic antiphosphotyrosine antibodies for immunoprecipitation, and phosphotyrosine-specific antibodies for detection of phosporylated Jak2 and Tyk2. The higher apparent signal for Tpo-induced Tyk2 phosphorylation may be due to more extensive phosphorylation of Tyk2 (many other sites than activation loop sites) compared with Jak2, or to differences in the affinities of phospho-specific antibodies for their respective phosphotyrosine residues in Jak2 and Tyk2.
For studying activation of STAT5 or STAT3, cells were washed, starved, and then stimulated or not with Tpo (50 ng/mL), as indicated. Cell lysates were immunoblotted with anti-P-STAT5 or anti-P-STAT3 antibodies. We detected phosphorylated STAT5 molecules in the absence of Tpo for the Δ5TpoR, but not for the wtTpoR. No STAT3 phosphorylation was observed in cells expressing Δ5TpoR (Figure 3B). This might be due to lower levels of phosphorylated STAT3 in nonstimulated Δ5TpoR cells that could not be detected by our immunoblot analysis. However, similar levels of STAT3 phosphorylation could be observed after ligand stimulation of cells expressing the wtTpoR or the Δ5TpoR, suggesting the latter may have a defect in sustaining STAT3 activation in the absence of ligand.
Constitutive and ligand-activated signaling by Δ5TpoR. Tyrosine phosphorylation of Jak2 and Tyk2 (A) and STAT5 and STAT3 (B) in cells expressing the wtTpoR or the Δ5TpoR was determined in Ba/F3 cells expressing the wtTpoR or Δ5TpoR at equal GFP and protein levels, and after selection with Tpo (for wtTpoR cells) or without any cytokines (for Δ5TpoR). Cells were stimulated or not with 50 ng/mL Tpo for 7 minutes. (A) Cells were lysed and immunoprecipitated with antiphosphotyrosine (P-Y 4G10) antibodies and analyzed by Western blotting with anti-P-Jak2 or anti-P-Tyk2 antibodies. Western blotting using anti-Jak2 or anti-Tyk2 antibodies, respectively, revealed total protein levels of cell lysates used for immunoprecipitations. (B) Western blotting using anti-P-STAT5 or anti-P-STAT3 antibodies revealed levels of STAT5 or STAT3 phosphorylation. Blots were stripped and analyzed for equal protein levels using anti-STAT5 or anti-STAT3 antibodies.
Constitutive and ligand-activated signaling by Δ5TpoR. Tyrosine phosphorylation of Jak2 and Tyk2 (A) and STAT5 and STAT3 (B) in cells expressing the wtTpoR or the Δ5TpoR was determined in Ba/F3 cells expressing the wtTpoR or Δ5TpoR at equal GFP and protein levels, and after selection with Tpo (for wtTpoR cells) or without any cytokines (for Δ5TpoR). Cells were stimulated or not with 50 ng/mL Tpo for 7 minutes. (A) Cells were lysed and immunoprecipitated with antiphosphotyrosine (P-Y 4G10) antibodies and analyzed by Western blotting with anti-P-Jak2 or anti-P-Tyk2 antibodies. Western blotting using anti-Jak2 or anti-Tyk2 antibodies, respectively, revealed total protein levels of cell lysates used for immunoprecipitations. (B) Western blotting using anti-P-STAT5 or anti-P-STAT3 antibodies revealed levels of STAT5 or STAT3 phosphorylation. Blots were stripped and analyzed for equal protein levels using anti-STAT5 or anti-STAT3 antibodies.
Transcriptional activity in Ba/F3 cells that express the Δ5TpoR
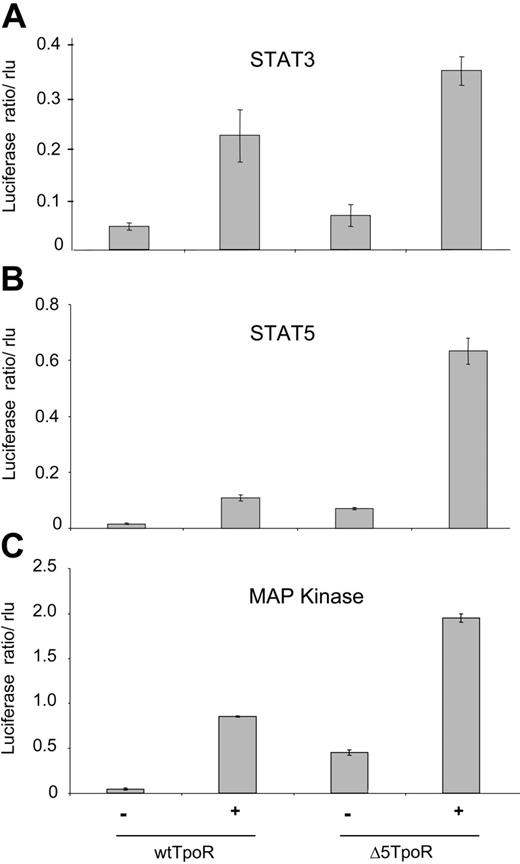
We next performed reporter gene assays using the luciferase gene under control of STAT5, STAT3, or MAP kinase-specific promoters. Cells were washed and starved for 3 hours in RPMI/1 mg/mL BSA, and luciferase assays were performed, as described in “Materials and methods.” STAT3-dependent transcription was assayed using the pancreatitis-associated protein 1 (rPAP) promoter (Figure 4A), which was shown to specifically reveal STAT3 activation.25 The induction of STAT3 activity in cells expressing the Δ5TpoR compared with nonstimulated wtTpoR cells was not statistically significant and confirmed that STAT3 was either not activated or activated with significantly lower efficiency by the unliganded Δ5TpoR. STAT5-dependent transcription was assessed using the pLHRE-luc promoter (Figure 4B) that specifically responds to STAT5.23 The TpoR is also known to induce the Mek-dependent activation of MAP kinase Erk1 and Erk2.4,9 MAP kinase-driven transcription can be measured using the luciferase gene under control of the serum response element.26 As shown in Figure 4C, the Δ5TpoR mutant activated MAP kinase in the absence of ligand. Transcriptional activation of STAT5, STAT3, and MAP kinase was increased in Ba/F3 cells after ligand stimulation of the Δ5TpoR compared with the wtTpoR (Figure 4A-C, rightmost histograms). This indicated that the TpoR mutant was hypersensitive to its ligand Tpo. Since both the wtTpoR and the Δ5TpoR respond to Tpo by activating STAT3 (Figures 3B, 4A), we suggest that the enhanced erythroid differentiation we detected with the Δ5TpoR is not simply related to the inability of unliganded Δ5TpoR to activate STAT3. Interestingly, there was a much higher activation of STAT5-dependent transcription by Tpo-activated Δ5TpoR than Tpo-activated wtTpoR (Figure 4B). This hyperactivation of STAT5 signaling may be due to additional posttranslational modification of STAT5 or to synergy with other Δ5TpoR-activated pathways downstream of STAT5 because the levels of STAT5 activation as detected by phosphorylation at the crucial STAT5 Tyr694 were similar (Figure 3B).
Alanine scanning of the TpoR KWQFP motif
In order to identify the residues that are necessary for the constitutive activation exhibited by the Δ5TpoR mutant, we constructed TpoR mutants where the KWQFP residues were substituted individually by alanine as shown in Table 1. Infected Ba/F3 cells were sorted for equal GFP levels and cells were washed and grown in the absence of any cytokines or in the presence of Tpo (5 ng/mL). All TpoR mutants were able to induce proliferation when stimulated with Tpo (data not shown), but only Ba/F3 cells expressing the TpoR mutants, where K507 and W508 were substituted by alanine, could grow in the absence of any cytokines (Figure 5A), and exhibited the same signaling profile as the Δ5TpoR, with a notable absence of activation of STAT3 (not shown). TpoR mutants, where the amino acids Q509 and F510 were substituted by alanine, did not promote proliferation in the absence of Tpo (Figure 5A); also, substitution of P511 by alanine did not result in growth factor-independent proliferation. The K and W residues within the KWQFP motif were therefore responsible for maintaining the TpoR in an inactive conformation in the absence of its ligand, Tpo (Figure 5B). We examined the ability of the TpoR-AWQFP and TpoR-KAQFP mutants to mediate hematopoietic differentiation in the absence or presence of Tpo. Not only were the point mutants able to drive colony formation in the absence of Tpo (not shown), as was the case for the TpoR (Figure 2B), but we again detected an apparent enhancement of erythroid differentiation in the presence of 10 ng/mL Tpo (Figure 5C), as also observed in the case of Δ5TpoR (Figure 2C).
Constitutive and ligand-induced transcriptional activation by Δ5TpoR. Ba/F3 cells selected in Tpo or without cytokines were starved for 3 hours in RPMI/1mg/mL BSA and electroporated with pGL3bPpr2-luc, pLHRE-luc, or pSRE-luc in order to measure transcriptional activity of STAT3 (A), STAT5 (B), or MAP kinase (C), respectively. pRL-TK encoding the renilla luciferase was coelectroporated for normalization of luciferase values. Cells were stimulated with 50 ng/mL Tpo or mock-treated as indicated. Cell lysates were prepared 2 hours after stimulation and analyzed for luciferase activity. Results shown here reflect averages of triplicate values ± SD. Similar results were obtained performing 3 independent experiments.
Constitutive and ligand-induced transcriptional activation by Δ5TpoR. Ba/F3 cells selected in Tpo or without cytokines were starved for 3 hours in RPMI/1mg/mL BSA and electroporated with pGL3bPpr2-luc, pLHRE-luc, or pSRE-luc in order to measure transcriptional activity of STAT3 (A), STAT5 (B), or MAP kinase (C), respectively. pRL-TK encoding the renilla luciferase was coelectroporated for normalization of luciferase values. Cells were stimulated with 50 ng/mL Tpo or mock-treated as indicated. Cell lysates were prepared 2 hours after stimulation and analyzed for luciferase activity. Results shown here reflect averages of triplicate values ± SD. Similar results were obtained performing 3 independent experiments.
Helical structure of the KWQFP motif
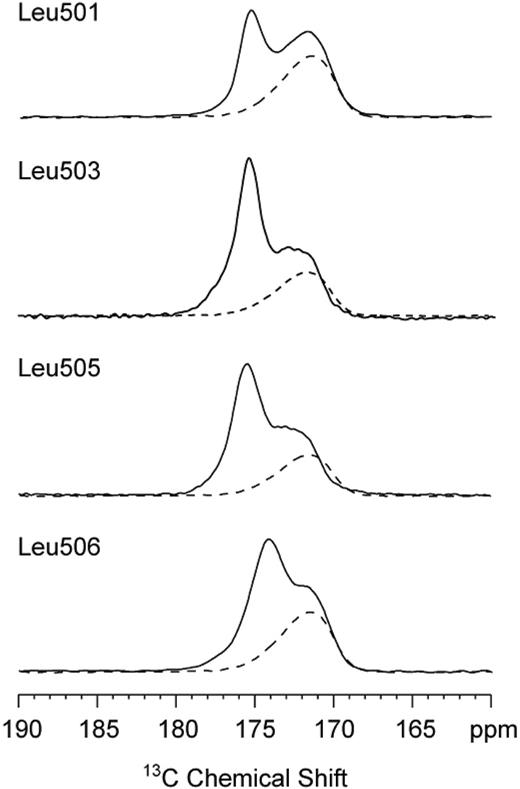
In order to interpret the results of the alanine scanning mutagenesis, we used magic-angle spinning NMR spectroscopy to probe the secondary structure at the boundary between the TM and JM domains of the TpoR. The 13C NMR chemical shifts of amide carbonyl carbons are sensitive to backbone torsion angles and hydrogen bonding of the C=O group. Helical conformations and i + 4 hydrogen bonding (eg, L506-13C=O · H-N-F510) result in 13C chemical shifts of greater than 174 ppm. Disruption of helical structure and hydrogen bonding to water lowers the 13C chemical shift to values between 168 and 172 ppm. We have specifically labeled the carbonyl carbons at the boundary between the TM and JM region in peptides corresponding to the human TpoR. The sequences of the human and mouse Tpo receptors are identical between L501 and W522 except for the Arg to Lys substitution at position 507. Figure 6 shows the region of the NMR spectrum of 13C-labeled TpoR peptides. Four peptides were specifically 13C = O labeled at the backbone carbonyl carbon of L501, L503, L505, and L506 (mouse TpoR sequence for peptides as in Onishi et al9 ). The spectra were obtained of DMPC and DMPG multilayers with (solid line) and without (dashed line) the reconstituted TpoR. The lipid membranes without peptide (dashed line) exhibit 13C NMR resonances due to the natural abundance 13C carbons of the lipid acyl chain carbonyls at approximately 172 ppm. The detected 13C=O chemical shifts of 175 ppm are consistent with 13C=O · H-N hydrogen bonding and thus α-helicity extending from L501 toF510. The small shift of the 13C=O resonance of L506 to 174 ppm indicates a weakening of the L506-F510 hydrogen bond, before the α-helix is broken at P511.
Identification of K507 and W508 as the residues responsible for the inhibitory function of the KWQFP motif on the unliganded TpoR. (A) Proliferation assays of the TpoR mutants containing alanine substitutions were performed with Ba/F3 cells expressing equal GFP levels. Cells were treated with 5 ng/mL Tpo where indicated or grown in the absence of any cytokines. Cell numbers were counted at day 9. Results shown here reflect averages of triplicates ± SD from 1 representative experiment. (B) Model of inhibition by the cytoplasmic KWQFP motif of the activity of TpoR. In the presence of Tpo, the receptor adopts a conformation where the KWQFP motif cannot bind to membranes or membrane proteins and interdict signaling. (C) Colony formation induced by Tpo-activated AWQFP and KAQFP point mutants of the TpoR. Colonies were assayed as in Figure 2C.
Identification of K507 and W508 as the residues responsible for the inhibitory function of the KWQFP motif on the unliganded TpoR. (A) Proliferation assays of the TpoR mutants containing alanine substitutions were performed with Ba/F3 cells expressing equal GFP levels. Cells were treated with 5 ng/mL Tpo where indicated or grown in the absence of any cytokines. Cell numbers were counted at day 9. Results shown here reflect averages of triplicates ± SD from 1 representative experiment. (B) Model of inhibition by the cytoplasmic KWQFP motif of the activity of TpoR. In the presence of Tpo, the receptor adopts a conformation where the KWQFP motif cannot bind to membranes or membrane proteins and interdict signaling. (C) Colony formation induced by Tpo-activated AWQFP and KAQFP point mutants of the TpoR. Colonies were assayed as in Figure 2C.
Solid-state NMR spectra of TpoR TM-JM peptides. Only the carbonyl region of the 13C spectrum is shown. The spectra of lipid alone (dashed line) exhibit only the resonance of the lipid acyl chain carbonyl due to the natural abundance of 13C. The specific 13C labels at the backbone carbonyl of L501, L503, L505, and L506 are observed at approximately 175 ppm (solid line), characteristic of helical secondary structure extending from L501 to F510.
Solid-state NMR spectra of TpoR TM-JM peptides. Only the carbonyl region of the 13C spectrum is shown. The spectra of lipid alone (dashed line) exhibit only the resonance of the lipid acyl chain carbonyl due to the natural abundance of 13C. The specific 13C labels at the backbone carbonyl of L501, L503, L505, and L506 are observed at approximately 175 ppm (solid line), characteristic of helical secondary structure extending from L501 to F510.
Discussion
Our main observation is that the TpoR contains an amphipathic α-helical KWQFP motif (RWQFP in the human) at the junction between the TM and cytoplasmic domain that is required for maintaining the receptor in a signaling-inactive conformation and, importantly, for imparting specific signaling to ligand-activated receptors.
Among cytokine receptors, the KWQFP motif is unique to the TpoR. We propose a model in which, for the unliganded wtTpoR, the KW residues of the motif interact with the plasma membrane and thus block receptor dimerization/activation (Figure 5B). Deletion of the KWQFP motif or substitution of K507 or W508 by alanine result in constitutive activation of TpoR (Figures 1B, 5A). We suggest that Tpo binding changes the conformation of the α-helical JM region, interrupting the interaction of the critical K507 and W508 residues with the membrane or with membrane-associated proteins and allowing Jak2 activation. In this regard, McLaughlin et al have shown that that basic and hydrophobic residues in the JM domain of the epidermal growth factor (EGF) receptor are responsible for mediating binding of the JM sequence to negatively charged membranes and thereby inhibiting EGF receptor activity.38 It is not known whether the unliganded TpoR exists on the cell surface as a preformed dimer, like the EpoR,39 as a monomer or in a monomer-dimer equilibrium. A spontaneous activating mutation was recently described for the TpoR, where W508 is replaced by serine.40 Since serine and alanine are quite equivalent, we suggest that all these mutants function by removing the inhibitory structural constraint induced by the KWQFP motif.
Signaling by Δ5TpoR is different from that of the activated wtTpoR both in the absence and in the presence of ligand. On one hand, Δ5TpoR constitutively activates many of the pathways activated by ligand-activated TpoR (ie, Jak2, Tyk2, STAT5, and MAP kinase), with the notable exception of STAT3. This in itself may promote cell proliferation, due to a shift toward predominant STAT5 activation, as has been suggested for mutants of the granulocyte colony-stimulating factor receptor.41 On the other hand, Δ5TpoR was hypersensitive to Tpo, and responded to ligand by activation of all tested pathways including STAT3, and supported myeloid differentiation. Compared with Tpo-activated TpoR at similar levels of expression, the Tpo-activated Δ5TpoR or Tpo-activated AWQFP or KAQFP mutants were more efficient in inducing the formation of erythroid cell-containing colonies (BFU-E, CFU-GEMM; Figures 2B, 5B). This suggests that ligand activation induces different signals via the wtTpoR and the Δ5TpoR. Our readout is of course dependent on the levels of expression of the retroviral constructs in the different myeloid progenitors. It is possible that higher levels of expression in megakaryocyte progenitors would enhance the effects of Tpo-activated Δ5TpoR. While ligand-activated wtTpoR and Δ5TpoR activate similar levels of STAT3, the activated Δ5TpoR induces much higher STAT5-dependent transcriptional activation. Thus, the enhanced erythroid differentiation may be due to a higher ratio of STAT5 versus STAT3 activation. After deletion of the KWQFP motif, the TpoR JM sequence greatly resembles the homologous region of the EpoR, which induces erythroid differentiation and induces much higher levels of STAT5 than STAT3 activation.
There is a substantial amount of evidence that several conformations of the TpoR may be competent for signaling. First, Richard et al showed that fusion of an inducible FK506-binding protein (FKBP) to the intracellular domain of the TpoR resulted in a receptor fusion protein that promoted expansion of primary hematopoietic cells as shown for the wtTpoR.42,43 However, in this study the orientation of the cytoplasmic domain was not controlled. In contrast, for the EpoR we showed that essentially 1 dimeric orientation is fully competent for signaling.27 Second, Onishi et al9 showed that substitution of serine at position 498 in the TM by asparagine results in a constitutively active TpoR. Third, in a case of familial essential thrombocythemia, all family members express a constitutively active TpoR mutant where the S505 (or S498) was replaced by an asparagine.14 Fourth, we substituted other sites in the TpoR TM domain to asparagine and found several positions that are not on the same helical face as S498/S505 that induce constitutive activation of TpoR (J.S. and S.N.C., unpublished results, July 2004). Fifth, it is quite easy to obtain agonistic anti-TpoR monoclonal antibodies. Furthermore, simply adding anti-FLAG antibodies to cells expressing FLAG-tagged TpoR at the amino-terminus leads to activation of cell proliferation.44 This again is in contrast with the EpoR, where a very low fraction of anti-EpoR monoclonal antibodies are agonistic.45 While antibodies and other small molecules may activate the TpoR in cell lines, our data would suggest that careful studies will be necessary to establish exactly which biologic function of the TpoR would be induced by these agonistic molecules. The biologic activity would be dependent on the particular dimeric conformation that would be activated by the agonistic molecule among the many permissive ones.
In conclusion, we show that the TpoR contains a unique amphipathic motif that maintains the unliganded receptor inactive and which is required for Tpo-induced specific signaling. Amphipathic motifs, such as the TpoR KWQFP sequence, may exist in other receptors to allow signaling via multiple flexible interfaces and prevent ligand-independent activation. We predict that point mutations in the KWQFP, especially replacing K507 and W508, may induce constitutive TpoR activation and myeloproliferation in humans.
Prepublished online asBlood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-06-2600.
Supported by grants from La Ligue Nationale contre le Cancer (équipe labellisée 2003, W.V.), la Fédération Belge contre le Cancer, the “de Hovre” Foundation, the Fonds National de la Recherche Scientifique (FNRS), Belgium (Mandat d'impulsion) to S.N.C., and a grant from the National Institutes of Health (GM 46732) to S.O.S. J.S. was supported by a Daimler-Benz PhD fellowship, an exchange Marie Curie fellowship, and a Télévie fellowship. W.V. is supported by an interface contract between INSERM and Institut Gustave Roussy (IGR). S.N.C. is a Research Associate of the FNRS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank André Tonon for expert flow cytometry expertise, Yan Yin and Julie Klein for technical and graphics assistance, and Stuart McLaughlin for communicating results before publication.