Langerhans cells (LCs) are highly abundant dendritic cells (DCs) in epidermal and mucosal tissues. The transcription factors PU.1 and Id2 have been implicated as positive regulators of LC development from hematopoietic progenitor cells. LC differentiation from progenitors is absolutely dependent on transforming growth factor beta 1 (TGF-β1) in vitro as well as in vivo; however, downstream mechanisms are poorly defined. We found that both PU.1 and Id2 are induced by TGF-β1 in human CD34+ monocyte/LC (M/LC) progenitor cells, and that neither ectopic PU.1 or Id2 alone, nor both together, could replace TGF-β1 in its instructive function on LC commitment. However, both factors critically contributed to LC differentiation by acting at 2 distinct intersection points. Ectopic PU.1 strongly enhanced TGF-β1-dependent LC development. Additionally, Notch-induced generation of interstitial-type DCs was associated with PU.1 up-regulation. Thus, PU.1 is generally increased during myeloid DC development. Ectopic Id2 inhibits the acquisition of early monocytic characteristics by cells generated in the absence of TGF-β1 and also inhibits monocyte induction by alternative stimuli. Since TGF-β1 represses a default monocyte pathway of common progenitor cells, PU.1 and Id2 seem to modulate lineage options of M/LC precursors, downstream of TGF-β1.
Introduction
CD1a+ human dendritic cells (DCs) comprise 2 functionally distinct subsets including Langerhans-type DCs (LCs) and dermal/interstitial DCs (DDC-IDCs).1-4 Both DC subsets arise from monopoietic intermediates in cultures of human CD34+ hematopoietic progenitor cells. LCs develop from early monocytic cells identified as lysozyme+, CD14+/-, CD11b- in serum-free cultures of CD34+ cells in response to TGF-β1.5-10 Conversely, DDC-IDCs arise from more differentiated CD14+/CD11b+ monocytes in progenitor cultures.8,9 In both instances, CD1a+ DC generation is associated with repression of monocyte features by cultured cells.
In vivo, LC differentiation requires epithelial TGF-β1.11,12 TGF-β1 addition to single-cell cultures of CD34+ cells induces LC colony formation at the expense of monocyte/macrophage-containing colonies.7 In line with an early lineage instructive effect of TGF-β1 on monocyte/LC (M/LC) progenitors, CD34+ cells rapidly lose TGF-β1-responsive LC differentiation potential upon in vitro expansion.13
Transcriptional mechanisms underlying TGF-β1-dependent LC commitment remain unknown. Recently, positive transcriptional regulators of LC induction have been identified. The ETS-domain transcription factor PU.1 is essential for myelopoiesis and myeloid DC development.14-19 Recent studies demonstrated that ectopic PU.1 expression induces CD1a+ LC-like cells from CD34+ progenitor cells20 and induces DC fate in transformed chicken myeloid progenitors as well as in the absence of cytokines in human HL60 myeloblast/promyelocytic cells and monocyte clones.21 Similarly the helix-loop-helix (HLH) regulatory protein inhibitor of DNA binding/differentiation 2 (Id2) is required for LC differentiation in vivo.22 Id2 is induced in day-10-generated DC progenitors in response to TGF-β1 stimulation,22 suggesting that Id2 is functionally involved in TGF-β1-dependent LC generation.
Given previous observations that TGF-β1 instructs progenitor cells to undergo LC differentiation,6,7 together with recent reports that PU.1 and Id2 are candidates as positive regulators of LC commitment,20,22 we considered the study of a possible functional interrelationship between TGF-β1 and these factors to be of substantial importance. For our analysis, we used a model in which TGF-β1 stimulation instructs freshly isolated or short-term expanded (< 96 hours) CD34+ hematopoietic progenitor cells to undergo LC commitment. We demonstrate that TGF-β1 induces PU.1 and Id2 in CD34+ progenitor cells undergoing LC commitment. Since omission of TGF-β1 abrogates LC differentiation and progenitors, in turn, developed into monocytes, we studied whether ectopic PU.1 or Id2 functionally replaces exogenous TGF-β1 for these effects. Id2 repressed monocyte differentiation and PU.1 strongly promoted LC differentiation. However, PU.1 strictly required exogenous TGF-β1. Our data support a model suggesting that PU.1 is generally increased by stimuli that induce CD1a+ myeloid DC generation from monocytic cells irrespective of the DC subset induced.
Materials and methods
Cytokines and ligands
Macrophage colony-stimulating factor (M-CSF), stem-cell factor (SCF), thrombopoietin (TPO), and tumor necrosis factor alpha (TNFα) were purchased from PeproTech (London, United Kingdom); transforming growth factor beta 1 (TGF-β1) was purchased from R&D Systems (Wiesbaden, Germany); fms-related tyrosine kinase 3 ligand (Flt3L) was obtained from Amgen (Seattle, WA); granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6 were kindly provided by Novartis Research Institute (Vienna, Austria); 1,25-dihydroxyvitamin D3 (vitamin D3) was obtained from Sigma-Aldrich (Vienna, Austria). The recombinant extracellular domain of Notch ligand Delta-1 fused to the Fc portion of human IgG1 (Delta-1ext-IgG)23 was kindly provided by I. Bernstein (Seattle, WA).
Retroviral constructs and gene transduction
The Moloney murine leukemia virus-based retroviral vector pBMN-I-GFP was obtained from G. P. Nolan (Stanford, CA); and pMX-Id2-GFP, from Y. Yokota (Fukui, Japan).24 pBMN-PU.1-I-GFP was generated by subcloning of the mouse PU.1 cDNA from pGCsam-PU.1-I-NGFR (a kind gift from A. Iwama, Ibaraki, Japan)20 into the multiple cloning site of pBMN-I-GFP. pBMN-PU.1S142A-I-GFP and pBMN-PU.1S148A-I-GFP were generated by subcloning of cDNAs encoding PU.1 mutations Ser142Ala and Ser148Ala from pcDNA3/HA-PU.1S142A and pcDNA3/HA-PU.1S148A (kindly provided by J. M. Wang, Taipei, Taiwan)25 into the multiple cloning site of pBMN-I-GFP. For double-transduction experiments, PU.1 cDNA was inserted into the retroviral vector pBMN-I-lyt2 encoding lyt2 (mouse CD8; obtained from G. P. Nolan, Stanford, CA). Methods used for production of recombinant amphotropic retrovirus and for gene transduction of progenitor cells were recently described.10
Isolation of cord-blood CD34+ cells
Cord-blood samples from healthy donors were collected during healthy full-term deliveries. Approval was obtained from the Medical University of Vienna institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. CD34+ cells were isolated as described previously.10 The purity of the isolated CD34+ cells analyzed by flow cytometry was more than 95%.
In vitro culture of CD34+ cord-blood stem cells
CD34+ cells were plated at a density of 1 to 2 × 104 cells/mL in 24-well cell-culture plates (NUNC, Rochester, NY; or Costar, Cambridge, MA) in serum-free X-VIVO 15 medium and were supplemented with SCF (20 ng/mL), Flt3L (50 ng/mL), and GM-CSF (200 ng/mL) ± TNFα (2.5 ng/mL) ± TGF-1 (0.5 ng/mL), according to previously described cytokine titration and optimization experiments.6,7 For evaluation of the effects of Id2 on monopoiesis, 2 additional monocyte stimuli were used. These comprised SCF (20 ng/mL), Flt3L (50 ng/mL), M-CSF (100 ng/mL) plus IL-6 (20 ng/mL) or SCF (20 ng/mL), and Flt3L (50 ng/mL) plus vitamin D3 (25 ng/mL), as previously described.10 Cells were stimulated with Delta-1ext-IgG as described.23 Cytokine combinations applied in Notch stimulation experiments comprised GM-CSF, SCF, TNFα, and FLT3 with or without TGF-β1 as indicated. Alternatively, cultures containing Flt3L, SCF, TNFα, and GM-CSF were further supplemented with 10% FCS to preferentially induce monocytic cells and interstitial-type DCs. For TGF-β1 neutralization experiments, gene-transduced CD34+ cells were stimulated in the presence of SCF (20 ng/mL), Flt3L (50 ng/mL), GM-CSF (200 ng/mL), and 10% fetal calf serum (FCS). TGF-β1-neutralizing antibody (mouse IgG1, at a saturating final concentration of 10 μg/mL, clone 9016; R&D Systems, Minneapolis, MN) was added at day 1 to the cultures as described previously by us6 and others.8
Flow cytometry and microscopy
For flow cytometry, immunofluorescence stainings and analyses were performed as previously described.10 Murine monoclonal antibodies (mAbs) of the following specificities were used: phycoerythrin (PE)-conjugated mAbs specific for CD86 (purchased from Pharmingen, Heidelberg, Germany); CD11b (BD Biosciences, Palo Alto, CA); CD80, CD83, and lyt2 (Caltag Laboratories, Hamburg, Germany); and Langerin (Immunotech, Marseille, France); biotinylated mAbs specific for CD1a (clone VIT 6), CD40 (clone G28-5), CD14 (clone MEM 18), and CD11b (LM-2) were kindly provided by O. Majdic (Vienna, Austria). As second-step reagent, we used streptavidin (SA)-PerCP (BD Biosciences). Allophycocyanin (APC)-conjugated mAbs specific for CD1a were purchased from BD Biosciences. APC conjugates specific for CD14 and CD83 were from Caltag Laboratories. Isotype control mAbs were kindly provided by O. Majdic.
Western blotting
An equal amount of 1 to 3 × 105 CD34+ cells cultured in the presence of different cytokines (see “Results”) were directly lysed with protein sample buffer (Santa Cruz Biotechnology, Heidelberg, Germany) and loaded on a 12% SDS-PAA gel. Alternatively, cells were lysed with lysis buffer (20 mM Tris/HCl [pH 7.5], 150 mM NaCl, 2.5 mM EDTA, 1% Triton X-100, and 1× protease inhibitor cocktail, Set III; Calbiochem, San Diego, CA) and 30 μg/lane was loaded on a 12% SDS-PAA gel. The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylene difluoride membrane. The membrane was probed with anti-PU.1 (sc-352), anti-Id2 (sc-489), anti-alpha-tubulin (sc-5546; Santa Cruz Biotechnology, Santa Cruz, CA), or anti-actin (A-2066; Sigma-Aldrich, Vienna, Austria) antibodies, followed by peroxidase-conjugated ImmunoPure goat anti-rabbit IgG (H+L) antibodies (Pierce Biotechnology, Rockford, IL).
Real-time PCR
Differentially stimulated CD34+ cells (1 × 105; with or without TGF-β1) were harvested and total RNA was isolated using TRI Reagent (Sigma, Vienna, Austria) according to the manufacturer's instructions. Contaminating DNA was removed by incubation with RNase-free DNaseI (Roche Diagnostics, Vienna, Austria) at 22°C for 30 minutes and subsequent phenol extraction was performed. DNA-free total RNA (1 μg) was subjected to cDNA synthesis using oligo-dT-primers and reverse transcriptase (M-MLV RT H-; Promega, Mannheim, Germany) according to the manufacturer's instructions. Real-time polymerase chain reaction (PCR) was performed in a LightCycler instrument (Roche Diagnostics) in a total volume of 15 μL using the LightCycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics) and 1.5 μL of the cDNA as template. Samples were heated to 95°C for 10 minutes followed by 50 cycles of denaturation at 95°C for 5 seconds, annealing at 65°C for 5 seconds, and extension at 72°C for 25 seconds. After the last cycle, a melting curve analysis was performed to verify the specificity of the amplified PCR products. The following primers for human Id2 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used: Id2/F, 5′-CGGTCTCGCCTTCCTCGCGGTC-3′; Id2/R, 5′-CTTATTCAGCCACACAGTGCT-3′; GAPDH/F, 5′-GAAATCCCATCACCATCTTCCAGG-3′; and GAPDH/R, 5′-CGCGGCCATCACGCCACAGTTTCC-3′. Relative Id2 mRNA expression ratio was calculated as described.26
Statistical analysis
Statistical analysis was performed using the paired, 2-tailed Student t test; P values of less than .05 were considered significant.
Results
Up-regulation and sustained expression of PU.1 during TGF-β1-induced LC differentiation from CD34+ cells
DC growth medium without exogenous TGF-β1 promotes the generation of CD11b+/CD14+ monocytes but not of CD1abright Langerin+ LCs from CD34+ cells (Figure 1A). Addition of TGF-β1 to these cultures significantly represses the generation of monocytes (P = .022) and induces LCs (P = .020), accompanied by formation of typical round homotypic LC clusters (Figure 1A, microphotographs).27 The basic growth medium used in these experiments contains a combination of cytokines including SCF, Flt3L, GM-CSF, and TNFα, previously shown to support DDC-IDC and LC development in serum-supplemented cultures.2,3 Our experiments showed that these cytokines in serum-free medium in the absence of TGF-β1 are not sufficient to induce LCs (Figure 1A).7 Furthermore, clonal analyses revealed that TGF-β1 addition to these cytokines induces LC colonies at the expense of myelomonocytic colonies.7 Therefore, our culture model is well suited to study TGF-β1 cosignaling processes during LC commitment. Iwama et al recently demonstrated that ectopic PU.1 expression in CD34+ cells instructs progenitors to acquire LC characteristics in serum-containing cultures.20 A possible role of PU.1 activation downstream of TGF-β1 signaling during LC commitment has not been investigated. TGF-β1 addition to CD34+ progenitors resulted in the induction of high endogenous PU.1 expression levels within 72 hours (Figure 1B, lanes 3 vs 5). TGF-β1 addition to SCF/Flt3L/GM-CSF-supplemented cultures (ie, basic DC cytokines in the absence of TNFα) similarly enhanced PU.1 expression (Figure 1B, lanes 2 vs 4), albeit lower amounts. In contrast, addition of TNFα to SCF/Flt3L/GM-CSF-supplemented cultures clearly failed to enhance PU.1 expression. TGF-β1 versus TNFα addition to SCF, Flt3L, and GM-CSF alternatively promoted LC versus monocyte differentiation (data not shown). Time kinetics analyses further revealed that TGF-β1 addition to the basic cytokine combination SCF/Flt3L/GM-CSF/TNFα is essential for the maintenance of high PU.1 levels later during cultures (day 7; Figure 2C, compare lanes 3 vs 7).
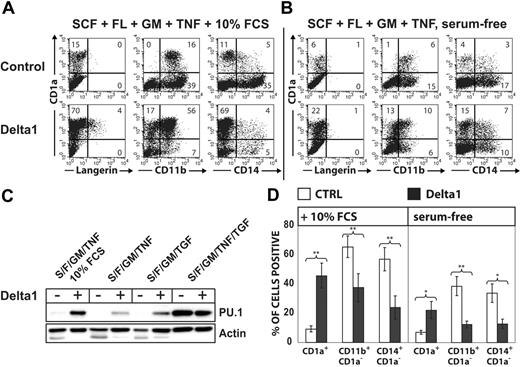
Regulation of endogenous PU.1 protein levels during TGF-β1-induced LC differentiation in serum-free medium. (A) Fluorescence-activated cell sorter (FACS) diagrams show representative CD34+ cells stimulated with “LC mix” (right, TGF-β1 plus SCF, Flt3L, GM-CSF, and TNFα) or LC mix without TGF-β1 (left) for 9 days analyzed for CD11b versus CD14 or Langerin versus CD1a. Bar diagrams represent the means and SEM of 4 independent experiments. The differences between -TGF-β1 and +TGF-β1 were significant (P = .022 for CD14+CD11b+, P = .020 for Langerin+CD1abright, paired 2-tailed t test). Microphotographs show typical LC cluster morphology by cells generated in LC mix (right). TGF-β1-nonsupplemented parallel cultures are compared (left). (B) The Western blot shows endogenous PU.1 or α-tubulin protein levels by CD34+ cells after 72-hour stimulation with the indicated cytokines. Lane 3 (center) and lane 5 (right) represent growth conditions giving rise to cells shown in the left and right FACS diagrams in panel A, respectively. Numbers in the quadrants of each diagram represent the percentage of cells in that quadrant. Data in panel A are representative of 5 independent experiments. Data in panel B are representative of 3 independent experiments. Cytokines: S indicates SCF (20 ng/mL); F, Flt3L (50 ng/mL); GM, GM-CSF (200 ng/mL); TNF, TNFα (2.5 ng/mL); and TGF, TGF-β1 (0.5 ng/mL).
Regulation of endogenous PU.1 protein levels during TGF-β1-induced LC differentiation in serum-free medium. (A) Fluorescence-activated cell sorter (FACS) diagrams show representative CD34+ cells stimulated with “LC mix” (right, TGF-β1 plus SCF, Flt3L, GM-CSF, and TNFα) or LC mix without TGF-β1 (left) for 9 days analyzed for CD11b versus CD14 or Langerin versus CD1a. Bar diagrams represent the means and SEM of 4 independent experiments. The differences between -TGF-β1 and +TGF-β1 were significant (P = .022 for CD14+CD11b+, P = .020 for Langerin+CD1abright, paired 2-tailed t test). Microphotographs show typical LC cluster morphology by cells generated in LC mix (right). TGF-β1-nonsupplemented parallel cultures are compared (left). (B) The Western blot shows endogenous PU.1 or α-tubulin protein levels by CD34+ cells after 72-hour stimulation with the indicated cytokines. Lane 3 (center) and lane 5 (right) represent growth conditions giving rise to cells shown in the left and right FACS diagrams in panel A, respectively. Numbers in the quadrants of each diagram represent the percentage of cells in that quadrant. Data in panel A are representative of 5 independent experiments. Data in panel B are representative of 3 independent experiments. Cytokines: S indicates SCF (20 ng/mL); F, Flt3L (50 ng/mL); GM, GM-CSF (200 ng/mL); TNF, TNFα (2.5 ng/mL); and TGF, TGF-β1 (0.5 ng/mL).
Ectopic PU.1 requires TGF-β1 signaling for promoting LC differentiation from CD34+ progenitor cells
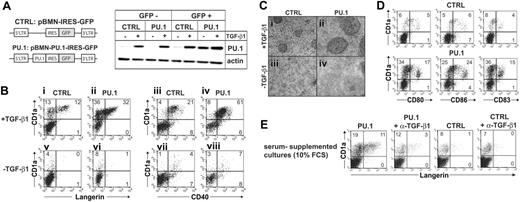
We next transduced CD34+ cells with a retroviral vector encoding PU.1-IRES-GFP, or an empty control vector (Figure 3A), and studied whether enforced PU.1 is sufficient to complement exogenous TGF-β1 during LC commitment. Progenitors were gene transduced in the presence of SCF, Flt3L, and TPO (see “Materials and methods”). Within 96 hours, cells were replated in above-described LC differentiation cultures (see “Materials and methods”) with or without TGF-β1. After a period of 7 days in these secondary cultures, cells were examined for LC characteristics, and GFP+ cells were analyzed using flow cytometry. First, we analyzed PU.1 under TNFα-nonsupplemented conditions, since TGF-β1 in the absence of TNFα is sufficient to induce LCs, and ectopic PU.1 was previously shown to replace TNFα costimulation in LC induction experiments from CD34+ cells.20 Ectopic retroviral PU.1 alone, in the absence of TGF-β1, failed to induce typical LC cluster morphology (Figure 3Civ). In line with this, most cells remained Langerin-/CD1a- (Figure 3Bvi). TGF-β1 stimulation of control-transduced cells (empty vector) induced small to intermediate LC clusters (Figure 3Ci) accompanied by low percentages of Langerin+CD1abright LCs (Figure 3Bi; mean ± SEM: 11.2% ± 0.4%, n = 9). Conversely, PU.1-transduced cultures stimulated with TGF-β1 contained typical large LC clusters (Figure 3Cii; compare with Figure 1A). In line with this, significantly higher percentages of differentiated CD1abright LCs were observed in these latter cultures (Figure 3Bii; mean ± SEM of Langerin+CD1abright cells: 25.2% ± 0.4%, n = 9) compared with control-transduced cultures (P < .01, n = 9) or with PU.1-transduced cultures without TGF-β1 (P < .01, mean ± SEM of Langerin+CD1abright cells without TGF-β1: 2.2% ± 0.6%, n = 9). Control Western blot analyses showed similar PU.1 expression levels by sorted GFP+ cells from PU.1-transduced cultures (PU.1) in the absence of TGF-β1 compared with GFP+ cells from control-transduced cultures (CTRL) in the presence of TGF-β1 (Figure 3A, lanes 7 vs 6). Furthermore, GFP+ cells, but not GFP- cells, from PU.1-transduced cultures showed high PU.1 expression levels (Figure 3A, lanes 7 vs 3). Therefore, enforced PU.1 requires exogenous TGF-β1 for promoting LC induction. Ectopic PU.1 enhanced TGF-β1-dependent LC generation without promoting DC maturation, as revealed by a similar surface marker profile of CD1a+ cells with or without ectopic PU.1 expression (ie, most cells CD40+; neg/dim for CD80, CD86, CD83 molecule expression; Figure 3B,D).
Delta-1ext-IgG induces PU.1 and redirects monocyte to CD1a+ DC development. CD34+ cord-blood cells were stimulated in 10% FCS (A) or in serum-free medium (B) supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF. Basic cytokines were further supplemented with TGF-β1 or TNFα as indicated. FACS diagrams show the phenotype of cells stimulated with plate-bound Delta-1ext-IgG or control. Cells were harvested at day 7, and bivariate analyses for CD1a versus Langerin, CD11b, or CD14 are displayed. Numbers in quadrants indicate the percentage of cells in that quadrant. (C) The Western blot shows endogenous PU.1 versus actin control of cells stimulated with the indicated cytokines with or without Delta-1ext-IgG. Data are representative of 3 experiments. S indicates SCF; F, Flt3L; GM, GM-CSF; TNF, TNFα; and TGF, TGF-β1. (D) Bars represent mean percentages ± SEM of the indicated cell populations without or with Delta-1ext-IgG. The differences between values were significant at *P < .05 and **P < .01 (paired 2-tailed t test; n = 5 independent experiments for CD1a+ and CD11b+CD1a- and n = 4 independent experiments for CD14+CD1a-).
Delta-1ext-IgG induces PU.1 and redirects monocyte to CD1a+ DC development. CD34+ cord-blood cells were stimulated in 10% FCS (A) or in serum-free medium (B) supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF. Basic cytokines were further supplemented with TGF-β1 or TNFα as indicated. FACS diagrams show the phenotype of cells stimulated with plate-bound Delta-1ext-IgG or control. Cells were harvested at day 7, and bivariate analyses for CD1a versus Langerin, CD11b, or CD14 are displayed. Numbers in quadrants indicate the percentage of cells in that quadrant. (C) The Western blot shows endogenous PU.1 versus actin control of cells stimulated with the indicated cytokines with or without Delta-1ext-IgG. Data are representative of 3 experiments. S indicates SCF; F, Flt3L; GM, GM-CSF; TNF, TNFα; and TGF, TGF-β1. (D) Bars represent mean percentages ± SEM of the indicated cell populations without or with Delta-1ext-IgG. The differences between values were significant at *P < .05 and **P < .01 (paired 2-tailed t test; n = 5 independent experiments for CD1a+ and CD11b+CD1a- and n = 4 independent experiments for CD14+CD1a-).
The results described in Figure 3B differ from a previous study that showed that retroviral expressed PU.1 can direct LC-type DC differentiation.20 Those experiments, however, were done in the presence of serum. Addition of anti-TGF-β1 antibody at culture onset to serum-containing DC generation cultures of CD34+ cells inhibits the generation of LC-like DCs.8 We therefore added a TGF-β1-neutralizing antibody to PU.1-transduced cells initiated in the presence of 10% FCS (containing the basic cytokines GM-CSF, SCF, and Flt3L) to show that PU.1 is cooperating with TGF-β1 to direct LC differentiation. In line with Iwama et al,20 we observed that ectopic PU.1 in the presence of 10% FCS is sufficient to induce Langerin+CD1a+ LCs (Figure 3E). Adding a TGF-β1-neutralizing antibody to the cultures strongly reduces percentages of generated LCs (Figure 3E).
We further studied the effects of TNFα supplementation, since TNFα and TGF-β1 costimulate LC induction in our model. In the absence of TGF-β1, TNFα promotes CD11b+ monocytes but not LC generation, and this effect is associated with low endogenous PU.1 protein levels (Figure 1). TGF-β1 addition to TNFα-supplemented cultures leads to an up-regulation of endogenous PU.1 protein levels, represses monocytes, and induces LC characteristics (Figure 1). Given this cooperation, we first asked whether ectopic PU.1 is sufficient to induce LCs in these TNFα-supplemented cultures. PU.1 plus TNFα fails to induce CD1abright LCs (n = 9; Figure 4Aii). In the absence of TGF-β1, CD1a+ cells showed low CD1a expression levels (Figure 4Ai,ii), and PU.1 significantly (P < .01) promoted these CD1adim cells (Figure 4Aii). In contrast, most CD1a+ cells from TGF-β1-supplemented cultures were CD1abright, and only these cells showed typical LC features (ie, Langerin expression, Figure 4Aiii,iv; and LC clusters, data not shown). Therefore, PU.1 promotes the generation of both CD1adim cells that lack LC features as well as LC-type DCs. Next, we investigated whether ectopic PU.1 shows TNFα-like costimulatory activity on the generation of monocytes in the absence of TGF-β1 or on LC differentiation in its presence. As also observed for TNFα, ectopic PU.1 significantly (P < .01) increased percentages of CD11b+ monocytes (Figure 4B, -TGF-β1, ± TNFα). Conversely, in the presence of TGF-β1, PU.1 strongly enhanced the generation of CD1abrightCD40+ LCs, and this effect equaled or even exceeded TNFα costimulation (Figure 4C-D, +TGF-β1, ±TNFα). Therefore, ectopic PU.1 shows a “TNFα-like” costimulatory effect on LC generation. Furthermore, ectopic PU.1 lacks LC instructive activity in the absence of TGF-β1 even when all the costimulatory cytokine signals (SCF, Flt3L, GM-CSF, and TNFα) are present in culture.
Cooperation of PU.1 and TGF-β1 in LC induction from progenitor cells. (A-D) CD34+ cord-blood cells were transduced with PU.1-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the cytokine combinations SCF/Flt3L/GM-CSF or SCF/Flt3L/GM-CSF plus TGF-β1 as indicated. (A) Schematic representation of the retroviral vectors used in the experiments (left). Representative day-5-generated gene-transduced cultures were FACS sorted into GFP+ and GFP- cell fractions and were subsequently analyzed for PU.1 or actin using Western blot analysis (right). (B-D) Day 7-generated PU.1- or control-transduced cultures are compared. GFP+ cells were gated on a separate FACS diagram (not shown) and were further analyzed for the expression of informative marker molecules (B: Langerin or CD40 vs CD1a; C: CD80, CD86, or CD83 vs CD1a). (C) Culture morphology of PU.1- or control-transduced cultures with or without TGF-β1 as indicated. Data in panels B, C, and D are representative of 9, 6, and 3 independent experiments, respectively. Data in panel D are representative of 3 independent experiments. (E) CD34+ cord-blood cells were transduced with PU.1-IRES-GFP (PU.1) or empty control vector (CTRL) and were then stimulated in culture medium supplemented with the cytokine combinations SCF/Flt3L/GM-CSF and 10% FCS in the absence or presence of 10 μg/mL neutralizing antibody specific for TGF-β1. Gated GFP+ cells were analyzed for Langerin versus CD1a expression. The data shown represent 1 of 3 experiments with comparable results. Numbers in quadrants of diagrams indicate the percentage of cells in that quadrant.
Cooperation of PU.1 and TGF-β1 in LC induction from progenitor cells. (A-D) CD34+ cord-blood cells were transduced with PU.1-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the cytokine combinations SCF/Flt3L/GM-CSF or SCF/Flt3L/GM-CSF plus TGF-β1 as indicated. (A) Schematic representation of the retroviral vectors used in the experiments (left). Representative day-5-generated gene-transduced cultures were FACS sorted into GFP+ and GFP- cell fractions and were subsequently analyzed for PU.1 or actin using Western blot analysis (right). (B-D) Day 7-generated PU.1- or control-transduced cultures are compared. GFP+ cells were gated on a separate FACS diagram (not shown) and were further analyzed for the expression of informative marker molecules (B: Langerin or CD40 vs CD1a; C: CD80, CD86, or CD83 vs CD1a). (C) Culture morphology of PU.1- or control-transduced cultures with or without TGF-β1 as indicated. Data in panels B, C, and D are representative of 9, 6, and 3 independent experiments, respectively. Data in panel D are representative of 3 independent experiments. (E) CD34+ cord-blood cells were transduced with PU.1-IRES-GFP (PU.1) or empty control vector (CTRL) and were then stimulated in culture medium supplemented with the cytokine combinations SCF/Flt3L/GM-CSF and 10% FCS in the absence or presence of 10 μg/mL neutralizing antibody specific for TGF-β1. Gated GFP+ cells were analyzed for Langerin versus CD1a expression. The data shown represent 1 of 3 experiments with comparable results. Numbers in quadrants of diagrams indicate the percentage of cells in that quadrant.
Ectopic PU.1 strictly requires TGF-β1 stimulation to promote LCs in serum-free medium. CD34+ cord-blood cells were transduced with PU.1-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF with or without TNFα and/or TGF-β1 as indicated. (A) FACS diagrams show representative GFP+ cells analyzed for Langerin versus CD1a. Numbers in each quadrant indicate the percentage of cells in that quadrant. Bar diagrams show the percentages (± SEM) of generated cells expressing the indicated marker molecules: (B) CD11b; (C) CD1a; and (D) CD40. Cells were harvested and analyzed at day 7 after culture initiation. FACS diagrams are representative of 9 experiments. Bars represent mean values (± SEM) of 9 independent experiments. The differences between values indicated were significant at *P < .05 and **P < .01 (paired, 2-tailed t test).
Ectopic PU.1 strictly requires TGF-β1 stimulation to promote LCs in serum-free medium. CD34+ cord-blood cells were transduced with PU.1-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF with or without TNFα and/or TGF-β1 as indicated. (A) FACS diagrams show representative GFP+ cells analyzed for Langerin versus CD1a. Numbers in each quadrant indicate the percentage of cells in that quadrant. Bar diagrams show the percentages (± SEM) of generated cells expressing the indicated marker molecules: (B) CD11b; (C) CD1a; and (D) CD40. Cells were harvested and analyzed at day 7 after culture initiation. FACS diagrams are representative of 9 experiments. Bars represent mean values (± SEM) of 9 independent experiments. The differences between values indicated were significant at *P < .05 and **P < .01 (paired, 2-tailed t test).
Notch ligand Delta-1ext-IgG induces PU.1 associated with monocyte to non-LC/DDC-IDC conversion
The recombinant Notch ligand Delta-1ext-IgG shows an effect reminiscent of TGF-β1 in that Delta-1ext-IgG similarly represses monocyte and induces CD1a+ DC features in CD14+/CD1a- cells generated from CD34+ cells.23,28 Furthermore, Notch activation induces PU.1 expression in FDCPmix progenitors and regulates DC generation in the murine system.29-31 To investigate whether Delta-1ext-IgG resembles TGF-β1 in LC induction, we performed side-by-side stimulation experiments of CD34+ cells with Delta-1ext-IgG and TGF-β1. In cultures supplemented with the basic cytokines (SCF/Flt3L/GM-CSF and TNFα) plus 10% FCS, CD11b+/CD14+ monocytes predominated, and only low percentages of CD1a+ cells were induced (Figure 2A, control). Plate-bound Delta-1ext-IgG23 significantly (P < .01) repressed CD14+/CD11b+ monocyte generation and in turn significantly (P < .01) induced CD1a+ DC development (Figure 2A, Delta-1ext-IgG). Similarly, as observed for TGF-β1, Delta-1ext-IgG-dependent CD1a+ cell generation was associated with induction of high endogenous PU.1 protein levels (Figure 2C, lanes 1 vs 2). However, CD1a+ cells did not resemble LCs, since in all 5 experiments analyzed, the majority of CD1a+ cells showed a phenotype characteristic of DDC-IDCs (CD1a+/Langerin-/CD11b+) (Figure 2A).32 Omission of 10% FCS significantly reduced percentages of CD14+ and CD11b+ cells in the absence of Delta-1ext-IgG (P < .01) and significantly reduced percentages of CD1a+ cells (P = .03, n = 5) in its presence (Figure 2A-B,D). Under serum-free conditions, Delta-1ext-IgG stimulation resulted in intermediate endogenous PU.1 levels (Figure 2C, lanes 3 vs 4). In comparison, stimulating cells in parallel with TGF-β1 instead of Delta-1ext-IgG induces very high PU.1 levels (Figure 2C, lane 7). Overall, PU.1 protein levels at day 7 of culture positively correlated with percentages of CD1a+ cells under the various conditions tested (Figure 2). Furthermore, neither did Delta-1ext-IgG induce Langerin+ LCs in serum-free medium in the absence of TGF-β1 (Figure 2), nor did Delta-1ext-IgG significantly modulate TGF-β1-dependent LC generation (n = 3, data not shown). Therefore, phenotypic repression of monocyte characteristics and induction of CD1a+ DC characteristics are associated with high endogenous PU.1 protein levels irrespective of the fact that distinct DC subsets (LC or DDC-IDC) are induced by TGF-β1 or Delta-1ext-IgG.
Id2 is induced by TGF-β1 during LC commitment
Id2 is up-regulated in day-10-generated DC progenitors in cultures of CD34+ cells in response to TGF-β1.22 We analyzed Id2 mRNA expression levels in early progenitors undergoing LC commitment using real-time PCR analysis (Figure 5A). Thus, CD34+ progenitor cells were stimulated for 48 hours or less under expansion conditions (Flt3L, SCF, and TPO) and were subsequently replated into optimized LC generation conditions with or without exogenous TGF-β1. Cells stimulated without TGF-β1 showed a slight relative increase in Id2 mRNA expression within 12 hours (compared with unstimulated cells; 0 hours). Conversely, addition of TGF-β1 resulted in a marked increase in Id2 mRNA levels within 12 hours. In 3 independent stimulation experiments, we observed 2.2-fold versus 5.4-fold (experiment 1), 2.1-fold versus 4.1-fold (experiment 2), and 2.0-fold versus 4.9-fold (experiment 3) increases in Id2 mRNA for conditions without versus with TGF-β1, respectively. At later time points (24 to 48 hours), Id2 mRNA levels dropped in all 3 experiments under both conditions. Between 24 and 48 hours, TGF-β1-stimulated cells showed higher relative Id2 mRNA levels compared with TGF-β1-nonstimulated cells in all 3 experiments. This pattern of initial rise in Id2 mRNA is reminiscent of Id2 mRNA kinetics as revealed from Northern blot analyses of day-10 DC progenitors.22
Id2 is induced by TGF-β1 costimulation in LC progenitors, but ectopic Id2 is not sufficient to substitute for TGF-β1. (A) Relative Id2 mRNA levels in CD34+ progenitors stimulated with SCF, Flt3L, GM-CSF, and TNFα ± TGF-β1 for the indicated time points determined by real-time PCR. Values were normalized to GAPDH expression and compared with Id2 mRNA levels in unstimulated cells (0 h). One representative of 3 independent experiments is shown. (B) CD34+ cord-blood cells were transduced with Id2-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the cytokine combination SCF/Flt3L/GM-CSF/TNFα with or without TGF-β1 as indicated. Day-7-generated Id2-transduced (center diagram, without TGF-β1) or control-transduced (left: without TGF-β1, right: with TGF-β1) cultures are compared. GFP+ cells were gated in a separate FACS diagram (not shown) and were further analyzed for the expression of Langerin versus CD1a. One representative of 5 independent experiments is shown. Numbers in each quadrant indicate the percentage of cells in that quadrant.
Id2 is induced by TGF-β1 costimulation in LC progenitors, but ectopic Id2 is not sufficient to substitute for TGF-β1. (A) Relative Id2 mRNA levels in CD34+ progenitors stimulated with SCF, Flt3L, GM-CSF, and TNFα ± TGF-β1 for the indicated time points determined by real-time PCR. Values were normalized to GAPDH expression and compared with Id2 mRNA levels in unstimulated cells (0 h). One representative of 3 independent experiments is shown. (B) CD34+ cord-blood cells were transduced with Id2-IRES-GFP or empty control vector and then stimulated in serum-free medium supplemented with the cytokine combination SCF/Flt3L/GM-CSF/TNFα with or without TGF-β1 as indicated. Day-7-generated Id2-transduced (center diagram, without TGF-β1) or control-transduced (left: without TGF-β1, right: with TGF-β1) cultures are compared. GFP+ cells were gated in a separate FACS diagram (not shown) and were further analyzed for the expression of Langerin versus CD1a. One representative of 5 independent experiments is shown. Numbers in each quadrant indicate the percentage of cells in that quadrant.
Id2 represses monocyte generation
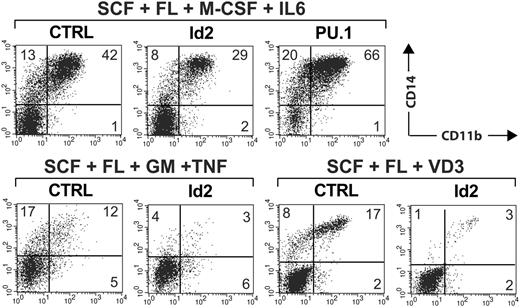
We next analyzed whether enforced Id2 expression might functionally substitute for exogenous TGF-β1 during LC commitment. Thus, CD34+ cells were transduced with a retroviral vector encoding Id2-IRES-GFP or empty control vector. Cells were then induced under optimized LC conditions but without TGF-β1 (SCF, Flt3L, GM-CSF, and TNFα), and GFP+ cells were analyzed at day 7 for LC characteristics. These experiments revealed that enforced Id2 fails to re-establish Langerin+CD1abright LCs in the absence of TGF-β1 (n = 5, Figure 5B). In TGF-β-nonsupplemented cultures containing SCF, Flt3L, and GM-CSF, ectopic Id2 consistently reduced percentages of CD14+ monocytic cells (Table 1). The fold changes of percent CD14+ cells were significant in the absence of TNFα (P < .01, Table 1). Parallel cultures set up in the presence of TNFα revealed reduced percentages of CD14+ cells in 4 of 5 experiments (ie, except of experiment 2, Table 1). M-CSF plus IL-6 represents an alternative stimulus for the generation of monocytes from CD34+ cells in serum-free medium.33 As can be seen in Figure 6, Id2 substantially reduced percentages of CD14+/CD11b+ monocytic cells generated in response to M-CSF plus IL-6. Id2 also consistently inhibited the generation of vitamin D3-induced CD14+/CD11b+ monocytes from CD34+ cells in serum-free medium (Figure 6). Therefore, Id2 inhibits monocyte generation by different stimuli. Side-by-side experiments revealed that ectopic Id2 and PU.1 antagonistically modulate monocyte generation by M-CSF plus IL-6 (Figure 6).
Ectopic expression of Id2 inhibits monocyte generation. CD34+ cord-blood cells were transduced with vectors encoding Id2-IRES-GFP or PU.1-IRES-GFP and were then stimulated in serum-free medium supplemented with cytokines as indicated. Vitamin D3 (VD3) was added as indicated. Cells were harvested at day 7 and stained for CD11b versus CD14. GFP+ cells were gated in a separate FACS diagram (not shown). Representative CD11b versus CD14 analyses of gated GFP+ cells (Id2-IRES-GFP, empty control vector, or PU.1-IRES-GFP) are shown (top: SCF/Flt3L/M-CSF/IL-6; bottom left: SCF/Flt3L/GM-CSF/TNFα; bottom right: SCF/Flt3L/vitamin D3). The data are representative of 3 independent experiments. The number in each quadrant indicates the percentage of cells in that quadrant.
Ectopic expression of Id2 inhibits monocyte generation. CD34+ cord-blood cells were transduced with vectors encoding Id2-IRES-GFP or PU.1-IRES-GFP and were then stimulated in serum-free medium supplemented with cytokines as indicated. Vitamin D3 (VD3) was added as indicated. Cells were harvested at day 7 and stained for CD11b versus CD14. GFP+ cells were gated in a separate FACS diagram (not shown). Representative CD11b versus CD14 analyses of gated GFP+ cells (Id2-IRES-GFP, empty control vector, or PU.1-IRES-GFP) are shown (top: SCF/Flt3L/M-CSF/IL-6; bottom left: SCF/Flt3L/GM-CSF/TNFα; bottom right: SCF/Flt3L/vitamin D3). The data are representative of 3 independent experiments. The number in each quadrant indicates the percentage of cells in that quadrant.
PU.1 and Id2 cotransduction
When expressed alone, neither PU.1 nor Id2 can replace the effects of TGF-β1 on LC generation (Figures 3, 4, 5). We therefore analyzed whether in combination these 2 could substitute for TGF-β1. Thus, cells were coinfected with Id-2-IRES-GFP and PU.1-IRES-lyt2 (murine CD8), or with respective empty control vectors. Cells were then stimulated in LC generation conditions with or without TGF-β1, and those cells expressing both markers (ie, GFP plus lyt2) were gated and analyzed for LC phenotype (Langerin+CD1abright). Only in the presence of TGF-β1 did a portion of GFP+lyt2+ control-transduced cells develop into LCs (Langerin+CD1abright; Figure 7A, upper-left vs lower-left diagram). GFP+lyt2+ cells transduced with both PU.1 plus Id2 failed to show an LC phenotype in the absence of TGF-β1 (Figure 7Aviii). Therefore, combined ectopic expression of Id2 plus PU.1 in human progenitor cells is insufficient for inducing LCs in the absence of TGF-β1. In the presence of TGF-β1 (Figure 7Ai-iv), ectopic PU.1 increased percentages of Langerin+CD1abright LCs. Of interest, ectopic Id2 consistently reduced PU.1-mediated enhanced LC generation when both factors were coexpressed. (Figure 7Ai-iv; n = 3). This is reminiscent of recent observations that ectopic Id2 counteracts PU.1-dependent gene expression.34 Overexpression of both molecules was confirmed by Western blot analyses of FACS-sorted cell populations (Figure 7B-C). Representative sort region settings for isolating GFP+ and GFP- fractions (Figure 7B), or for isolating double-transduced lyt2+GFP+ cells (Figure 7C), are shown. Endogenous Id2 was below the detection limit of Western blot analysis in FACS-sorted primary CD34+ cell-derived progeny (Figure 7B-C).
Discussion
LC development requires TGF-β1 signaling; however, downstream transcriptional mechanisms remain poorly defined. Here we applied a model for LC differentiation in which TGF-β1 addition induces LC commitment of putative M/LC progenitors.7 The 2 transcriptional regulators PU.1 and Id2 are essential for the development of LCs, but both are similarly required for a number of other leukocyte lineages.14,15,35 Given the unique instructive role of exogenous TGF-β1 on LC commitment and the proposed function of PU.1 and Id2 as positive regulators of LC differentiation,20,22 we studied here a possible involvement of these candidate molecules downstream of TGF-β1 during LC commitment of monopoietic cells.
Combined ectopic expression of PU.1 plus Id2 is insufficient to induce LCs. CD34+ cord-blood cells were combined transduced with vectors encoding PU.1-IRES-lyt2 and Id2-IRES-GFP, or with respective empty control vectors (CTRL). Cells were then stimulated in serum-free medium supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF/TNFα with or without TGF-β1 as indicated. (A) Gated lyt2+GFP+ cells are analyzed for Langerin vs CD1a expression. Data are representative of 3 independent experiments. Numbers in quadrants indicate the percentage of cells in that quadrant. (B) Cells generated without TGF-β1 were sorted into GFP+ and GFP- fractions (see representative FACS sorting region settings), or into lyt2+ and lyt2- fractions. Sorted cells were analyzed for Id2, PU.1, and actin protein expression using Western blotting. (C) Double-transduced lyt2+/GFP+ cells were FACS-purified according to the sort region shown. Western blot analyses for Id2, PU.1, and actin are shown (n.s. indicates nonspecific).
Combined ectopic expression of PU.1 plus Id2 is insufficient to induce LCs. CD34+ cord-blood cells were combined transduced with vectors encoding PU.1-IRES-lyt2 and Id2-IRES-GFP, or with respective empty control vectors (CTRL). Cells were then stimulated in serum-free medium supplemented with the basic cytokine combination SCF/Flt3L/GM-CSF/TNFα with or without TGF-β1 as indicated. (A) Gated lyt2+GFP+ cells are analyzed for Langerin vs CD1a expression. Data are representative of 3 independent experiments. Numbers in quadrants indicate the percentage of cells in that quadrant. (B) Cells generated without TGF-β1 were sorted into GFP+ and GFP- fractions (see representative FACS sorting region settings), or into lyt2+ and lyt2- fractions. Sorted cells were analyzed for Id2, PU.1, and actin protein expression using Western blotting. (C) Double-transduced lyt2+/GFP+ cells were FACS-purified according to the sort region shown. Western blot analyses for Id2, PU.1, and actin are shown (n.s. indicates nonspecific).
First, we observed that both PU.1 and Id2 are induced in CD34+ progenitor cells in response to TGF-β1 during LC commitment. Since omission of TGF-β1 from LC generation cultures abrogated LC differentiation, we subsequently tested whether ectopic PU.1 or Id2 is sufficient to functionally replace TGF-β1 in our model. We observed that either factor alone or both together could not substitute for TGF-β1. Given the essential role of TGF-β1 in LC differentiation in vivo11,12 and in vitro,6 which cannot be substituted by any other known stimuli, these findings formally rule out that ectopic PU.1 or Id2 possesses LC lineage instructive function. However, both factors showed profound activities in our system consistent with an important role downstream of TGF-β1 during LC commitment of M/LC progenitors. First, ectopic PU.1 cooperated with TGF-β1 in increasing percentages of LCs. Conversely, PU.1 levels remained low during monocyte differentiation. Second, ectopic Id2 inhibited monocyte differentiation in cultures in which TGF-β1 had been omitted. Given that TGF-β1 represses M/LC progenitor-cell differentiation along a default monocyte pathway,7 our data suggest that lineage options of common M/LC progenitors are modulated by these molecules downstream of TGF-β1 signaling (Figure 8). However, our data revealed that additional transcriptional mechanisms downstream of TGF-β1 (apart from PU.1 and Id2 induction) are required for instructing these progenitors to undergo LC commitment.
Ectopic retroviral PU.1 was previously shown to instruct CD34+ progenitor cells to undergo LC differentiation.20 Conversely, we demonstrate that even when all 4 TGF-β1 costimulatory cytokine signals are present in culture, ectopic PU.1 fails to re-establish LCs in the absence of TGF-β1. Therefore, one important finding of our study is that ectopic PU.1 lacks LC lineage instructive activity in progenitor cells downstream of TGF-β1. Previous studies were performed in serum-supplemented cultures.20 TGF-β1 neutralization experiments performed by us demonstrated that TGF-β1 critically contributes to LC induction by PU.1 in serum-supplemented cultures. Therefore, serum-free models are mandatory for studying LC commitment in vitro.
Our data support a general important function of PU.1 in myeloid DC differentiation. First, we observed that ectopic PU.1 effectively cooperated with TGF-β1 in directing LC differentiation from progenitors. This effect was already visible morphologically when examining LC cluster formation of cultured cells. In the absence of TGF-β1, PU.1-transduced cultures failed to form LC clusters. Conversely, TGF-β1 addition to these cultures induced very large LC clusters. Second, we observed that PU.1 is strongly up-regulated by 2 distinct DC-inducing stimuli (ie, by TGF-β1 or by Delta-1ext-IgG). Both stimuli when applied side-by-side to cultures of CD34+ cells similarly redirected monocyte to CD1a+ DC development, and both strongly induced PU.1. In contrast to TGF-β1, however, Notch activation induced CD1a+ DCs that lacked LC characteristics. Therefore PU.1 up-regulation seems to generally mark myeloid DC generation irrespective of the specific DC subset induced. These observations fit to our findings that PU.1 lacks LC lineage instructive activity downstream of TGF-β1. Furthermore, they are in line with a recent study showing that PU.1 is up-regulated during DC differentiation from CD14+ peripheral-blood monocytes in response to GM-CSF plus IL-4.21 In further support for a general role of PU.1 in myeloid DC differentiation are our observations that PU.1 in the absence of TGF-β1 promoted CD1adim cells that lacked LC characteristics. In addition, ectopic expression of PU.1 in cells stimulated under conditions that induce high endogenous PU.1 levels (with TNFα and TGF-β1) only weakly enhanced CD1a+ DC generation.
Model of TGF-β1-dependent LC lineage commitment. Our serum-free differentiation model revealed that the basic cytokine combination SCF, Flt3L, GM-CSF, plus TNFα favors monopoiesis. If TGF-β1 is added, monocyte differentiation is repressed and LCs are induced.5,7 This lineage-modulating effect of TGF-β1 is associated with marked PU.1 and Id2 up-regulation by progenitor cells. We show here that neither ectopic PU.1 nor ectopic Id2 is sufficient to re-establish LCs in cultures in the absence of TGF-β1. Nevertheless, our data suggest that both factors critically contribute to LC lineage commitment by acting at 2 distinct intersection points. PU.1 strongly enhances LC induction by TGF-β1. Conversely, ectopic Id2 inhibits acquisition of monocytic characteristics by cells generated in the absence of TGF-β1. PU.1 is generally up-regulated during myeloid DC generation (eg, also by Delta-1 [our study] or by IL-4 costimulation21 ). Furthermore, ectopic PU.1 induces DC differentiation in several cell systems.20,21 However, for LCs (DCs of the epithelial/mucosal type), PU.1 lacks lineage instructive activity. These cells still required exogenous TGF-β1 even when PU.1-transduced progenitor cells were stimulated with all the other DC cytokines. Therefore, our data suggest that TGF-β1 downstream signaling processes (X) have to cooperate with PU.1 for LC lineage commitment.
Model of TGF-β1-dependent LC lineage commitment. Our serum-free differentiation model revealed that the basic cytokine combination SCF, Flt3L, GM-CSF, plus TNFα favors monopoiesis. If TGF-β1 is added, monocyte differentiation is repressed and LCs are induced.5,7 This lineage-modulating effect of TGF-β1 is associated with marked PU.1 and Id2 up-regulation by progenitor cells. We show here that neither ectopic PU.1 nor ectopic Id2 is sufficient to re-establish LCs in cultures in the absence of TGF-β1. Nevertheless, our data suggest that both factors critically contribute to LC lineage commitment by acting at 2 distinct intersection points. PU.1 strongly enhances LC induction by TGF-β1. Conversely, ectopic Id2 inhibits acquisition of monocytic characteristics by cells generated in the absence of TGF-β1. PU.1 is generally up-regulated during myeloid DC generation (eg, also by Delta-1 [our study] or by IL-4 costimulation21 ). Furthermore, ectopic PU.1 induces DC differentiation in several cell systems.20,21 However, for LCs (DCs of the epithelial/mucosal type), PU.1 lacks lineage instructive activity. These cells still required exogenous TGF-β1 even when PU.1-transduced progenitor cells were stimulated with all the other DC cytokines. Therefore, our data suggest that TGF-β1 downstream signaling processes (X) have to cooperate with PU.1 for LC lineage commitment.
The identity of the molecular events that have to cooperate with PU.1 downstream of TGF-β1 for LC induction remains to be identified. Cotransduction of PU.1 and Id2 revealed that both factors together fail to re-establish LC in the absence of TGF-β1. Therefore, additional TGF-β1 downstream effectors seem to be required for LC induction. In initial experiments using retroviral delivery of serine to alanine mutants of PU.1 (Ser142Ala or Ser148Ala), we already found that phosphorylation-induced activation of PU.1 at these sites is dispensable for LC enhancement (n = 3, data not shown). Since phosphorylation of Ser148 was shown to be required for dimerization with IRF4 or IRF8,36,37 the latter required for optimal LC development in vivo,38 our data seem to rule out functional participation of these interaction partners in the here-described PU.1 effects.
One key finding of this study is that Id2 inhibits the generation of monocytic cells in response to several growth stimuli, suggesting that Id2 generally represses initiation of monopoiesis from CD34+ cells. Since TGF-β1-dependent LC commitment in our model involves repression of monocyte generation, and TGF-β1 induces Id2 in CD34+ cells during LC commitment, we speculate that Id2 mediates monocyte repression downstream of TGF-β1 signaling. How Id2 represses monocytes remains to be analyzed. Among several possibilities, Id2 might inhibit bHLH proteins involved in monopoiesis or exert effects on molecules involved in cell-cycle regulation and/or in monopoiesis such as the retinoblastoma protein.34,39 We did not notice differences in cell proliferation of Id2 compared with control-transduced cells in our short-term cultures (data not shown). Id2 coexpression together with PU.1 results in a dose-dependent inhibition of the binding of PU.1 to DNA, and, moreover, Id2 inhibits PU.1-dependent transcriptional activation of reporter genes.34 Furthermore, Id2 physically interacts with PU.1.34 In line with this, we observed that ectopic Id2 antagonizes PU.1-mediated enhancement of LC generation. Inhibition of monocyte generation by Id2 furthermore corresponds to previous observations that adenoviral vector-mediated expression of Id2 in U937 cells inhibits their induced differentiation into M-CSF receptor-positive monocyte/macrophages.34 It is interesting to speculate that high levels of PU.1 as observed in myeloid DCs might override a possible inhibitory effect of Id2, whereas monocyte precursors, which express lower levels of PU.1, might be more sensitive to Id2 inhibition. Such a model would be compatible with previously described inhibitory effects of ectopic Id2 on pDC development.40 These cells show low endogenous PU.1 protein levels41,42 and develop from progenitors via an M-CSF receptor-positive precursor pathway.43 Several cell types might be capable of reconstituting LCs in vivo.44-49 TGF-β1-responsive M/LC precursors can be substantially enriched by purification of CD45RA+CD34+ progenitor-cell subsets (data not shown), known to include granulomonocytic progenitors.50-52 These cells express PU.1.52,53
LCs seem to possess specialized function in tolerance and immunity induction. Therefore, targeting TGF-β1 downstream regulators of LC generation might allow for modulation of the DC system in a subset-specific manner and thus open new avenues in immunomodulation.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-04-1721.
Supported by grants START-Y156 and SFB-F2304 from the Austrian Science Fund (FWF), and grant 10294 from the Austrian National Bank.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the collaborating nurses and doctors from the obstetric departments at Lainz Hospital and Kaiser Franz Josef Hospital, Vienna. Furthermore, we thank W. Ellmeier and J. Stockl for critically reading the paper and helpful discussions.





![Figure 8. Model of TGF-β1-dependent LC lineage commitment. Our serum-free differentiation model revealed that the basic cytokine combination SCF, Flt3L, GM-CSF, plus TNFα favors monopoiesis. If TGF-β1 is added, monocyte differentiation is repressed and LCs are induced.5,7 This lineage-modulating effect of TGF-β1 is associated with marked PU.1 and Id2 up-regulation by progenitor cells. We show here that neither ectopic PU.1 nor ectopic Id2 is sufficient to re-establish LCs in cultures in the absence of TGF-β1. Nevertheless, our data suggest that both factors critically contribute to LC lineage commitment by acting at 2 distinct intersection points. PU.1 strongly enhances LC induction by TGF-β1. Conversely, ectopic Id2 inhibits acquisition of monocytic characteristics by cells generated in the absence of TGF-β1. PU.1 is generally up-regulated during myeloid DC generation (eg, also by Delta-1 [our study] or by IL-4 costimulation21). Furthermore, ectopic PU.1 induces DC differentiation in several cell systems.20,21 However, for LCs (DCs of the epithelial/mucosal type), PU.1 lacks lineage instructive activity. These cells still required exogenous TGF-β1 even when PU.1-transduced progenitor cells were stimulated with all the other DC cytokines. Therefore, our data suggest that TGF-β1 downstream signaling processes (X) have to cooperate with PU.1 for LC lineage commitment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/4/10.1182_blood-2005-04-1721/2/m_zh80040691050008.jpeg?Expires=1769284478&Signature=E1rM~nve16gw-uPZrPW9cfZPvuC7xMR0NpG-2Aw5ckQN1JsSWKu~yIMMXejTC2Lkd2QwmtQbhf1Rbr3pze-J186Lamzm4Tmk~lisiWy70os6rvEb9yK9eunNEM0lIVfmGEv6XJpRHh07guNP7unCTd4JJJy4EKxUCsquVVzIMzfd-Le5TPy66zn0zR9M9DJ8twN6OKsdxQhzrzLvcL1HvqT4ExrGKrXVwo-BBui85RHOthp-Yxo7N8m9tz~6PjGt~m9k4LLqoPSivvESLBFSfEkQD7mWnJYntoRKIedkhMx8JPag4YMpoy6z0yWa2fgkfh8Ax74yeDm1GY5Qpdd8Uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
