MHC class II (MHCII) deficiency or bare lymphocyte syndrome (BLS) is a severe immunodeficiency characterized by deficient T helper (Th)-cell-dependent immunity. The disease is caused by defects of the MHCII promoter complex resulting in low or absent MHCII expression. We demonstrate in a murine model of MHCII deficiency (RFX5- or CIITA-deficient mice) that residual MHCII expression by professional antigen-presenting cells (APCs) is sufficient to support activation of adoptively transferred Th cells. Furthermore, upon transplantation of WT thymic epithelium, we observed development of endogenous Th cells with restoration of Th-cell-dependent antibody responses and immunity to cytomegalovirus infection, thus opening the possibility of an alternative treatment regimen for BLS. Residual MHCII expression was further induced by the presence of Th cells and also other stimuli. Analysis of CIITA/RFX5 double-deficient animals revealed that this inducible MHCII expression is genetically independent of the known promoter complex and thus constitutes an alternative MHCII expression pathway. In these experiments, we also detected a novel repressive function of the RFX complex in the absence of CIITA.
Introduction
MHC class II (MHCII) molecules play a pivotal role in the development of protective T-cell immunity by displaying antigenic peptides from the endocytic pathway to CD4+ T helper (Th) cells.1 MHCII gene expression is tightly regulated and mostly restricted to thymic epithelium2,3 and professional antigen-presenting cells (APCs)4-6 but inducible also in other cell types. Coordinated expression of all MHCII genes is controlled by a conserved promoter region.7,8 This promoter contains the so-called X, X2, and Y boxes, which bind the heterotrimeric RFX complex9 consisting of RFX5, RFXAP, and RFXANK,10-13 CREB (cAMP-responsive element-binding protein),14-16 and NF-Y.17 Transcriptional activity of MHCII genes is, however, not only dependent on these DNA-binding molecules but requires the cell type-specific18,19 or inducible presence20,21 of the transcriptional coactivator CIITA.22
Defects in one of the genes of the RFX proteins or CIITA led to almost complete absence of MHCII expression. This phenomenon was first observed in a human hereditary immunodeficiency, called MHCII deficiency or bare lymphocyte syndrome (BLS).5,23,24 BLS patients have reduced Th-cell numbers and are extremely susceptible to infections with bacterial, viral, and fungal pathogens.25 The development of Th-cell-dependent class-switched immunoglobulin responses is impaired in these patients, despite reports of residual MHCII expression on B-cell lines from some patients and the presence of CD4+ T cells.26,27 It seems unlikely that the Th cells of BLS patients develop in the thymus since the thymic cortex, which is normally responsible for positive selection, showed no MHCII expression in BLS patients.28-30 To date, the only available treatment for this fatal disease is allogeneic BM transplantation (BMT), which in the case of BLS has a low success rate.27,31
To further investigate the mechanisms underlying this immunodeficiency and to test novel treatment strategies, we and others have generated mouse models of BLS by inactivating the genes coding for CIITA and for RFX5.32-35 Similar to the human disease, CIITA-/- and RFX5-/- mice lack MHCII expression on the majority of peripheral APCs. Unexpectedly, residual MHCII expression was found in the thymic medulla, on a subset of dendritic cells (DCs) in peripheral lymphoid organs, and on in vitro-activated RFX5-/- B cells. Due to the absence of MHCII in thymic cortex, both mutants are unable to generate Th cells and thus fail to mount Th-cell-dependent immune responses.32-34
To test whether the residual MHCII expression in RFX5-/- and CIITA-/- mice (“BLS mice”), and potentially also in patients, could support Th-dependent immune responses we reconstituted BLS mice with peripheral Th cells by implantation of WT embryonic thymi. The reconstituted mice were found to mount Th-cell-dependent humoral responses and were able to show Th-cell-mediated control of murine cytomegalovirus (MCMV) infection. In addition, reconstitution with Th cells led to an increase in MHCII expression on professional APCs. This “alternative” MHCII expression was found to be inducible and independent of RFX5 and CIITA. Furthermore, CIITA/RFX5 double-deficient (CR-/-) mice revealed a novel repressive function of RFX5.
Patients, materials, and methods
Mouse maintenance and typing
RFX5-/- mice33 and CIITA-/- mice32 were on a mixed 129/Ola/C57BL/6 or 129/Sv/C57BL/6 background, respectively, and were intercrossed to generate CR-/- mice. The double knock out was confirmed by polymerase chain reaction (PCR) and Southern blot. For the RFX5 deficiency, the primers RFX5-N8B 5′ACATAATGACCGTTCTCGAGG3′, RFX5-N4B 5′AGCAGACTTGGCTCTGAGCTG3′, and RFX5-N3 5′TCTACCTTCAGCTCCCATCGG3′ (WT, 500 bp; KO, 860 bp) and for the CIITA deficiency the primers JaxCIITA-1 5′GATCGGAGACAAGGGTGTGT3′, JaxCIITA-2 5′GTCAGGGAGCAGGATCTTTG3′, and Neo-reverse 5′GACTAGTGAGACGTGCTACT3′ (WT, 550 bp; KO, 400 bp) were used. These PCR primers were also used to detect the presence of contaminating WT cells after transplantation. The Southern blot analysis for the detection of the CIITA alleles was described previously.32 Aα-/- mice36 were on a pure C57BL/6 background and were provided by H. Bluethmann (Basel, Switzerland). C57BL/6nu/nu mice were purchased from Bomholtgard (Ry, Denmark). The animals were housed in specific pathogen-free conditions.
Thymus transplantation
Thymic lobes were prepared from C57BL/6 embryos at gestational day 14.5. In some experiments, these lobes were irradiated with 30 Gy and cultured for 5 days, or cultured in medium containing 1.35 mM 2-deoxyguanosine (Sigma, St Louis, MO) for 5 days before transplantation. After anesthetizing 4- to 6-week-old mice of the respective strains with Ketanest/Rompun, 6 thymic lobes were transplanted under the kidney capsule.37
Cell preparation and culture
To enrich for DCs from splenic-cell or thymic-cell preparations, the organ was digested with collagenase D (Roche Diagnostics, Mannheim, Germany) prior to preparation of a single-cell suspension.38 Anti-CD11c (N418) magnetic microbeads were used to positively enrich for DCs from total splenocytes (Miltenyi Biotec, Bergisch Gladbach, Germany). For analysis of MHCII expression on B cells, splenocytes were cultured O/N in the presence of 10 μg/mL anti-CD40 (Pharmingen, San Diego, CA), CpG-oligodeoxynucleotide (ODN) 1668 (TCC-ATG-ACG-TTC-CTG-ATG-CT; TIB MOLBIOL, Berlin, Germany), or LPS (Sigma) and analyzed by flow cytometry the next day.
To enrich Vα3+ T cells from LN and spleen of 2D2 mice, biotinylated anti-Vα3 (Pharmingen) and streptavidin-coupled magnetic microbeads (Miltenyi Biotec) were used. To enrich B cells for adoptive transfer from CD45.1 congenic C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany), anti-CD19 magnetic microbeads (Miltenyi Biotec) were used.
Human peripheral-blood lymphocytes (PBLs) were enriched on a Pancoll gradient (PanBiotech, Aidenbach, Germany) and cultured in RPMI 1860 (Gibco, Carlsbad, CA). For activation, LPS (10 μg/mL final concentration), CpG 2006 (TCGTCGTTTTGTCGTTTTGTCGTT; 0.5 μM final concentration; TIB MOLBIOL), polyI/C (50 μg/mL final concentration; Sigma), or CD40L (Alexis, Lausen, Switzerland) was added to the cultures.
SJO and RO cells10 were gifts from B. Grospierre (Paris, France) and M. Müschen (Düsseldorf, Germany) and cultured in DMEM (Gibco) supplemented with 15% FCS, glutamine, nonessential amino acids, sodium pyruvate, β-mercaptoethanol, and penicillin/streptomycin.
Cytofluorometric analysis
Fluorescence staining was performed as previously described.39 The following antibodies were purchased from Pharmingen: RM4-5 for CD4, 53-6.7 for CD8, 25-9-17 for I-Aβb, HL3 for CD11c (N418), H57 for TCRβ, M1/70 for CD11b. M5/114 for MHCII staining, anti-mouse B220 (RA3-6B2), and antinitrophenyl (N1G9, isotype control for HLA-DQ) were purified from hybridoma supernatants.33 FITC-coupled anti-TCRβ, CD11b, and CD11c antibodies were combined as non-B-cell marker. Anti-HLA-DQ and anti-HLA-DP (Pharmingen) were revealed with anti-mouse IgG1 (Pharmingen). Anti-human CD19 and anti-human CD3 were a gift of ImmunoTools (Friesoythe, Germany). Dead cells were excluded by Topro-3, Propidium Iodide, or Cytox Green staining (Molecular Probes, Eugene, OR). Analysis was performed on a FACScalibur (Becton Dickinson, Mountain View, CA).
Immunohistochemistry
Frozen tissue sections (3 μm) were air dried, fixed in ice-cold acetone, and blocked in phosphate-buffered saline containing 0.6% H2O2, 5% goat serum, and 0.1% NaN3 followed by 4% fetal calf serum in Tris-buffered saline; the sections were stained with digoxigenin-conjugated M5/114 and biotinylated RA3-6B2. The MHCII antibody was detected with anti-digoxigenin peroxidase, followed by 3-amino-9-ethyl carbazole (Sigma), and the B220 antibody was detected with streptavidin-alkaline phosphatase and alkaline phosphatase substrate kit III/blue (Vector, Burlingame, CA). Slides were photographed on a Leica DMRBE (Leica, Heidelberg, Germany) microscope using a PL Fluotar 10 ×/0.30 objective lens; pictures were scanned and processed using Adobe Photoshop 5.02 software (Adobe Systems, San Jose, CA). For analysis by confocal microscopy, cryosections of thymi were stained with UEA-1-FITC (Vector) biotin-antimouse I-Ab (Becton Dickinson) and anti-mouse-CD11c (Becton Dickinson). The antibodies were revealed with biotin-PE (Becton Dickinson) and anti-Armenian Hamster-Cy5 antibody (Dianova, West Grove, PA). Slides were mounted in Fluoromount G (Southern Biotechnology, Birmingham, AL) and analyzed with a Zeiss LSM 510 microscope (Carl Zeiss, Jena, Germany) equipped with LSM510 software version 2.02 and Ar/Kr (458/488 nm) and He/Ne (543 nm) lasers. A Plan-NEOFLUAR 20 ×/0.5 objective lens was used. Images recorded with the microscope were exported as single-image files in JPEG format with LSM5 Image Browser 2.8 software, and then processed with Adobe Photoshop 5.02.
Adoptive transfers
For transfer of Th cells, Vα3+ T cells were purified by magnetic sorting from 2D2 TCR transgenic mice and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE). Of these cells, 5 × 106 were injected into RFX5-/-, CIITA-/-, Aα-/-, and C57BL/6 mice. Two days later, the recipients were immunized at the tail base with 50 μg MOG p35-55 in CFA. Four days after the immunization, LN cells were stained with antibodies for CD4 and Vα3 and analyzed on a FACScalibur.
For transfer of B cells, 2 × 107 CD19+ B cells from CD45.1 congenic C57BL/6 mice were injected into Aα-/- and RFX5-/- animals. Three weeks later, splenocytes from these mice were analyzed by fluorescence-activated cell sorter (FACS) for expression of CD45.1, I-Ab, B220, and non-B-cell marker.
NP-CG immunizations
Mice of the different strains were injected intraperitoneally with 100 μg alumn-precipitated and γ-irradiated NP15-chicken gamma globulin (CG) and boosted with 50 μg NP-CG after 3 weeks. Serum was collected as described and NP-specific IgG1 or IgM titers were measured by enzyme-linked immunosorbent assay (ELISA).40
MCMV infection and virus plaque assay
PFUs (2 × 105) of the tissue culture-grown Smith strain of MCMV (VR-194; ATCC, Manassas, VA) were injected into the footpads of the different mouse strains. Three weeks after infection, salivary glands and lungs were collected under sterile conditions and stored at -70°C. The virus titers in organ homogenates were determined by in vitro plaque assay after centrifugal enhancement of infectivity.41 To prevent secondary plaque formation, we used methylcellulose (Merck, West Point, PA), and the plaques were counted after 3 to 5 days of incubation at 37°C. Detection limit of the assay was 100 PFU MCMV per organ. MCMV-specific antibodies in sera were determined by ELISA.42
Patients
The patients with MHCII deficiency were treated at the University Children's Hospital, Ulm, and were 1 and 4 years of age. The molecular defect of both patients was located in the RFX-ANK gene and results in a amino acid exchange at position 121 (D→V).43 PBC samples from patients were obtained and analyzed after informed consent was provided in accordance with the Declaration of Helsinki. The University of Ulm Institutional Review Board approved the protocols of this study.
Reverse-transcriptase (RT)-PCR
SJO cells, RO cells, or blood lymphocytes (all 105) were lysed in TRIzol (Gibco) reagent. RNA was prepared according to instructions of the manufacturer and DNaseI (Promega, Madison, WI) digested. After reverse transcription (Gibco), 1 μL cDNA was used for PCRs with the following primers: 5′CGTCTTCCCCTCCATCGTGG3′ and 5′GTCATCTTCTCGCGGTTGGCC3′ for β-actin (273 bp), 5′ATGGCCATAAGTGGAGTCCCTGTGC3′ and 5′CCCTGCGTTCTGCTGCATTGC3′ for HLA-DRα (754 bp), and 5′GTCATTTCTTCAATGGGACGGAGC3′ and 5′CCGTAGTTGTGTCTGCAGTAGGTGTCC3′ for HLA-DRβ (208 bp). Magnetically purified mouse B cells and DCs were lysed in Trizol (Gibco), RNA prepared, and reverse transcribed by Superscript II (Invitrogen, Frederick, MD). The following probes from a probe library (Exiqon/Roche, Vedbaek, Denmark) and primers were used for the amplification: I-Aβ: probe mouse 16, 5′CACAGGAGTCAGAAAGGACCTC3′ and 5′GTCAAAACACTCTGAGTCACTGC3′; I-Aα: probe mouse 90, 5′TCTGATTCTGGGGGTCCTC3′ and 5′ACCATAGGTGCCTACGTGGT3′; β-actin: probe mouse 82, 5′TGACAGGATGCAGAAGGAGA3′ and 5′CGCTCAGGAGGAGCAATG3′. The data were acquired on a ABI7500 PCR machine (Applied Biosystems, Foster City, CA).
Results
Residual MHCII expression in RFX5- and CIITA-deficient mice supports Th-dependent immune responses
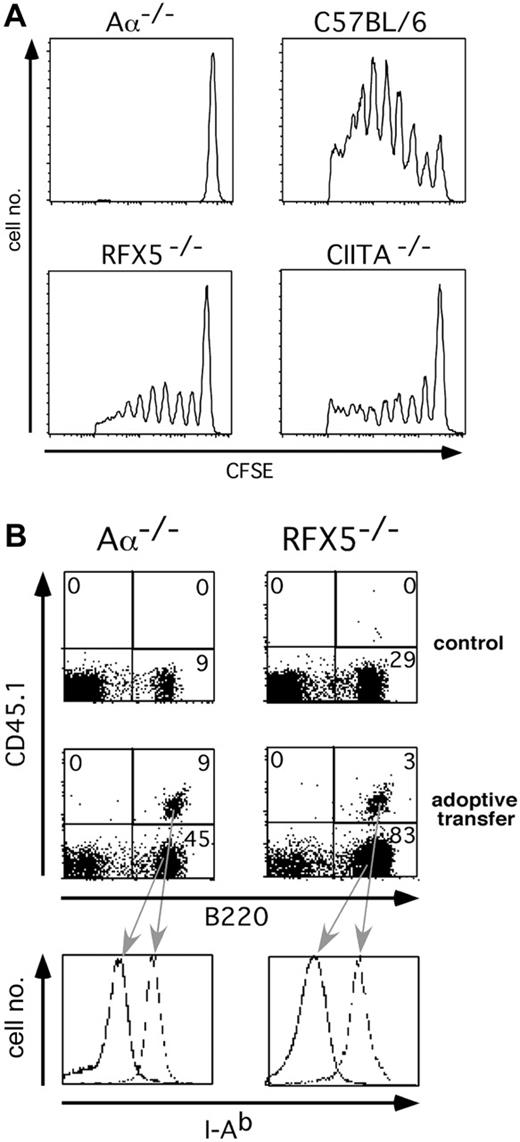
Since RFX5-/- mice and, to a lesser extent, CIITA-/- mice express MHCII on a proportion of peripheral APCs, we wanted to explore the possibility if these APCs can support MHCII-dependent immune responses when appropriately selected Th cells are present. CFSE-labeled Vα3+ Th cells from 2D2 TCR transgenic mice, whose TCR is specific for the MOG-peptide p35-55 in the context of I-Ab,44 were adoptively transferred into RFX5-/-, CIITA-/-,Aα-/-, and C57BL/6 mice. The hosts were immunized with p35-55 and 4 days later the proliferation of the Vα3+ CFSE-labeled LN and spleen cells of graft origin was determined by FACS analysis. We used the Aα-/- strain,36 which lacks the MHCII structural genes, as a negative control to rule out that WT APCs potentially introduced with the grafted cells restored immune function independent of host APCs. C57BL/6 mice served as controls, with WT I-Ab expression allowing optimal activation of the transferred Th cells. We found that the transferred T cells proliferated only in C57BL/6, RFX5-/-, and CIITA-/- but not Aα-/- hosts (Figure 1A), indicating that residual MHCII expression on endogenous APCs in the mouse models of BLS is sufficient to support Th-cell activation. The lower T-cell proliferation in RFX5-/- and CIITA-/- mice is likely explained by the fact that not all APCs express MHCII in the mice. To validate the Aα-/- control, we ascertained that MHCII+ APCs were not rejected in Aα-/- mice in which MHCII expression is completely absent. For this purpose, WT B cells carrying the CD45.1 surface marker were adoptively transferred into Aα-/- mice and RFX5-/- mice, which are likely to be tolerant to MHCII. In both recipients, MHCII+ B cells survived equally for at least 3 weeks (Figure 1B), demonstrating that no allorejection of MHCII+ cells takes place in Aα-/- mice.
Adoptive transfer of T and B cells into mouse models of BLS. (A) Proliferation of CFSE-labeled Vα3+ T cells from 2D2 TCR transgenic mice after adoptive transfer into RFX5-/-, CIITA-/-, Aα-/-, and C57BL/6 mice and immunization with MOG p35-55. The histogram analysis of CFSE fluorescence of CD4+Vα3+PI- lymphocytes is shown. (B) Survival of CD45.1+ B cells after transfer into Aα-/- or RFX5-/- hosts. The dot plots show B220 and CD45.1 expression of non-B-cell marker-negative splenocytes 3 weeks after transfer. The histogram analysis depicts the I-Ab expression of the indicated populations. The number in each quadrant represents the percentage of cells in that quadrant.
Adoptive transfer of T and B cells into mouse models of BLS. (A) Proliferation of CFSE-labeled Vα3+ T cells from 2D2 TCR transgenic mice after adoptive transfer into RFX5-/-, CIITA-/-, Aα-/-, and C57BL/6 mice and immunization with MOG p35-55. The histogram analysis of CFSE fluorescence of CD4+Vα3+PI- lymphocytes is shown. (B) Survival of CD45.1+ B cells after transfer into Aα-/- or RFX5-/- hosts. The dot plots show B220 and CD45.1 expression of non-B-cell marker-negative splenocytes 3 weeks after transfer. The histogram analysis depicts the I-Ab expression of the indicated populations. The number in each quadrant represents the percentage of cells in that quadrant.
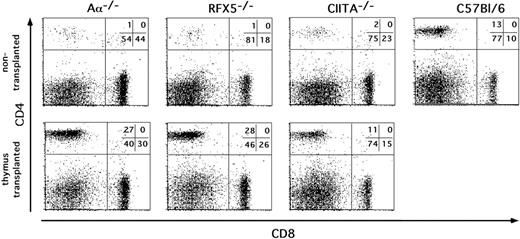
Th-cell reconstitution in MHCII-deficient mice after thymic transplantation. Thymic lobes from embryonic day 14 (e14) C57BL/6 embryos were transplanted under the kidney capsule of Aα-/-, RFX5-/-, and CIITA-/- mice. Nine weeks after transplantation, inguinal LN cells of mice that underwent transplantation and control mice were analyzed for CD4 and CD8 expression by flow cytometry. The percentage of cells in the indicated quadrants is given in the top right corner of each dot plot. The CD4/CD8 ratio was calculated for each mouse and is shown in Table 1. The results are representative of several experiments performed.
Th-cell reconstitution in MHCII-deficient mice after thymic transplantation. Thymic lobes from embryonic day 14 (e14) C57BL/6 embryos were transplanted under the kidney capsule of Aα-/-, RFX5-/-, and CIITA-/- mice. Nine weeks after transplantation, inguinal LN cells of mice that underwent transplantation and control mice were analyzed for CD4 and CD8 expression by flow cytometry. The percentage of cells in the indicated quadrants is given in the top right corner of each dot plot. The CD4/CD8 ratio was calculated for each mouse and is shown in Table 1. The results are representative of several experiments performed.
Reconstitution with Th cells and normal humoral immune response in CIITA-/- and RFX5-/- mice after implantation of WT thymi
To analyze the ability of RFX5-/- and CIITA-/- mice to support MHCII-dependent immune responses in the presence of Th cells in a more physiologic setting, we allowed development of Th cells by transplanting embryonic thymi from C57BL/6 mice under the kidney capsule of BLS mice. We controlled for successful restoration of Th-cell-dependent immune responses by transplantation of WT thymi into athymic MHCII-expressing nude mice. We also included the Aα-/- mice mentioned in the previous section as controls for unintentionally cotransferred donor APCs. Nine weeks after implantation of the thymi, we found the compartment of CD4 T cells in inguinal LNs to be restored to almost WT levels in all mutants (Figure 2; Table 1).
Ratio of CD4 to CD8.
Thymus . | No transplantation . | Transplantation . |
|---|---|---|
| RFX5-/- | 0.06 | 1.1 |
| CIITA-/- | 0.07 | 0.7 |
| Aα-/- | 0.02 | 0.8 |
| C57BL/6 | 1.3 | — |
Thymus . | No transplantation . | Transplantation . |
|---|---|---|
| RFX5-/- | 0.06 | 1.1 |
| CIITA-/- | 0.07 | 0.7 |
| Aα-/- | 0.02 | 0.8 |
| C57BL/6 | 1.3 | — |
— indicates not applicable.
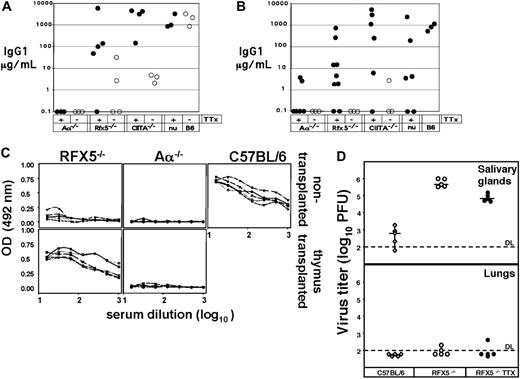
Subsequently, we tested whether after restoration of a peripheral Th-cell compartment RFX5-/- or CIITA-/- mice generated a Th-cell-dependent IgG1 response to the hapten nitrophenyl coupled to chicken globulin (NP-CG). At 10 and 13 weeks after transplantation, the mice were immunized with NP-CG and bled 3 weeks after the second immunization. Four of 5 RFX5-/-, 4 of 4 CIITA-/-, and 3 of 3 nude mice that underwent transplantation responded with high serum IgG1 titers (Figure 3A), showing the ability of functional T-B interaction in reconstituted CIITA-/- and RFX5-/- mice. Since the Aα-/- mice that underwent transplantation developed no NP-specific IgG1 responses despite the presence of Th cells (Figures 2,3A; Table 1), it is unlikely that donor APCs, which may have been transferred along with the thymi, were responsible for the recovery of Th-cell-dependent antibody responses in RFX5-/- or CIITA-/- mice. Nevertheless, we performed an additional experiment in which irradiated or 2-deoxyguanosine-treated thymi consisting only of radiation-resistant thymic stroma were used for transplantation. Although the efficiency of Th-cell reconstitution was transient and relatively low due to the treatment (data not shown), 4 of 9 RFX5-/- mice and 4 of 6 CIITA-/- mice showed increased serum levels of NP-specific IgG1 (> 10 μg/mL) in contrast to RFX5-/- (0/3) and CIITA-/- (0/3) mice that did not undergo transplantation (Figure 3B). Nude mice that underwent transplantation showed a similar efficiency of reconstitution as RFX5-/- and CIITA-/- mice after transplantation of treated thymi (2/6 mice; Figure 3B). Since NP-CG represents a nonpathogenic antigen, we challenged reconstituted RFX5-/- animals also with the viral pathogen MCMV 6 months after transplantation. We determined serum levels of MCMV-specific antibodies of the IgM and IgG isotypes at day 0 and day 21 after MCMV infection. Th-independent MCMV-specific IgM titers were present in all mice analyzed, including Aα-/- mice that did not undergo transplantation, and did not differ between the groups (data not shown). All of the 5 RFX5-/- mice that underwent transplantation produced MCMV-specific antibodies of the IgG type on day 21 at levels comparable with WT controls (Figure 3C). In contrast, RFX5-/- controls that did not undergo transplantation as well as Aα-/- mice that did and did not undergo transplantation did not generate MCMV-specific IgG antibodies (Figure 3C).
Increased CD4 T-cell-dependent clearance of MCMV after transplantation with WT thymi
Th cells are not only able to provide help to other cells of the immune system, but also exhibit effector functions themselves. Clearance of MCMV infection in salivary glands but not other organs is Th-cell dependent45-48 and mediated by IFNγ49 and TNFα.50 To test whether the Th cells in RFX5-/- mice that underwent transplantation were also able to control MCMV infection, virus titers were determined in salivary glands and lungs 3 weeks after infection with MCMV. RFX5-/- mice that did not undergo transplantation could not clear the virus from the salivary gland and the virus titers there were approximately 1000-fold higher than in C57BL/6 controls. The viral titers in the salivary glands of RFX5-/- mice that underwent transplantation, however, were 10-fold lower than in RFX5-/- controls that did not undergo transplantation (P < .01) (Figure 3D) but yet higher than in WT controls. In contrast, we detected identical virus titers in the salivary glands of Aα-/- mice that did and did not undergo transplantation (data not shown), indicating that the effect observed in RFX5-/- mice that underwent transplantation is specific and requires residual MHCII expression. In agreement with previous reports that Th cells are not required to resolve MCMV infection of the lung,47 we found that the infection was cleared from this organ by all animals without significant differences (Figure 3D and data not shown).
Th-cell-dependent immune responses in mice that underwent thymus transplantation. Aα-/-, RFX5-/-, CIITA-/-, or C57BL/6nu/nu mice received e14 thymi (TTX) either without further treatment (A,C-D) or after irradiation/2′-deoxyguanosine treatment (B). (A-B) Ten weeks after transplantation, the mice were immunized with NP-CG and boosted 3 weeks later. Blood was taken 3 weeks after the boost and analyzed for NP-specific IgG1. ○ indicates control mice; •, mice that underwent thymus transplantation. (C-D) MCMV-specific immune responses. Groups of 5 C57BL/6, RFX5-/-, and RFX5-/- mice that underwent thymus transplantation were infected with MCMV 6 months after transplantation, and viral titers were determined in salivary glands 3 weeks after infection. (C) IgG serum titers of MCMV-specific antibodies in RFX5-/-,Aα-/-, and C57BL/6 mice that did not undergo transplantation and RFX5-/- or Aα-/- mice that underwent thymus transplantation (TTX). OD indicates optical density. (D) Clearance of MCMV in salivary glands and lungs. PFU indicates plaque forming units. ⋄ indicates C57BL/6 mice; ○, RFX5-/- control mice; and •, RFX5-/- mice that underwent thymus transplantation.
Th-cell-dependent immune responses in mice that underwent thymus transplantation. Aα-/-, RFX5-/-, CIITA-/-, or C57BL/6nu/nu mice received e14 thymi (TTX) either without further treatment (A,C-D) or after irradiation/2′-deoxyguanosine treatment (B). (A-B) Ten weeks after transplantation, the mice were immunized with NP-CG and boosted 3 weeks later. Blood was taken 3 weeks after the boost and analyzed for NP-specific IgG1. ○ indicates control mice; •, mice that underwent thymus transplantation. (C-D) MCMV-specific immune responses. Groups of 5 C57BL/6, RFX5-/-, and RFX5-/- mice that underwent thymus transplantation were infected with MCMV 6 months after transplantation, and viral titers were determined in salivary glands 3 weeks after infection. (C) IgG serum titers of MCMV-specific antibodies in RFX5-/-,Aα-/-, and C57BL/6 mice that did not undergo transplantation and RFX5-/- or Aα-/- mice that underwent thymus transplantation (TTX). OD indicates optical density. (D) Clearance of MCMV in salivary glands and lungs. PFU indicates plaque forming units. ⋄ indicates C57BL/6 mice; ○, RFX5-/- control mice; and •, RFX5-/- mice that underwent thymus transplantation.
Residual MHCII expression is independent of both RFX5 and CIITA
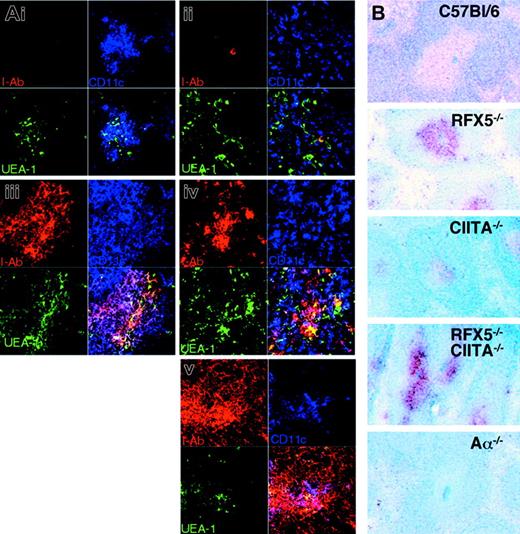
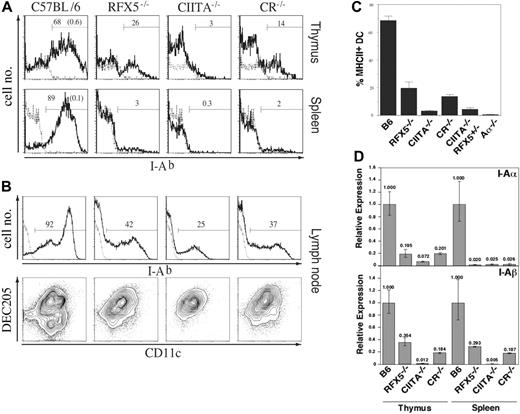
As shown in the two preceding sections, reconstitution of the Th-cell compartment by transplantation of WT thymi into BLS mice allows the generation of Th-dependent immune responses because of residual MHCII expression in the peripheral immune system. Although it was reported that (a) the transcription of MHCII is solely regulated by association of CIITA with the constitutively assembled promoter complex22 and (b) absence of RFX5 results in disassembly of this promoter complex,15,51 the data available from knock-out mice implied that RFX5 deficiency permits higher “residual” MHCII expression than CIITA deficiency.32-34 To directly compare the RFX5-/- and CIITA-/- strains and to investigate whether “residual” MHCII expression in these mice is completely independent of CIITA and RFX5, we assessed MHCII expression in various lymphoid organs of CR-/- mice in comparison with RFX5-/- and CIITA-/- mice. If CIITA was the sole regulator for the induction of MHCII transcription, we expected these mice to exhibit a phenotype similar to single CIITA-deficient mice with residual MHCII expression present on a few cells only. When we analyzed MHCII expression on thymic and splenic sections by immunohistochemistry, we made the surprising observation that CR-/- mice showed stronger residual MHCII expression than CIITA-/- mice, phenotypically resembling RFX5-/- mice (Figure 4). We further investigated the localization and phenotype of MHCII+ cells in the thymus by confocal microscopy. Sections were stained for MHCII, CD11c, and UEA-152 to reveal MHCII expression on DCs and medullary epithelial cells. We observed expression of MHCII on a fraction of both cell types in RFX5-/- and CR-/- mice and, in particular, on thymic DCs that were located in the vicinity of UEA-1+ epithelial cells (Figure 4A). In agreement with a previous report, CIITA-/- mice expressed high levels of MHCII on only a few medullary cells34 (Figure 4A). FACS analysis was then performed to quantify and further characterize the MHCII-expressing cell types. We found a sizable fraction of thymic MHCII+CD11c+ DCs in RFX5-/- and CR-/- mice (mean values of 20% and 14% of total CD11c+ cells, respectively) and only a few of these cells in CIITA-/- mice (3%) as well as CIITA-/-/RFX5+/- mice (4%) (Figure 5A-B). In the spleen, MHCII expression was found on 2% to 3% of DCs in RFX5-/- and CR-/- mice but not in CIITA-/- mice (Figure 5A). These findings were also confirmed by quantitative RT-PCR analysis (Figure 5D).
In contrast to spleen and thymus, a high proportion of MHCII+ DCs was detectable in inguinal and brachial LNs of all 3 mutant strains (Figure 5C). All LN MHCII+ DCs expressed the DEC205 marker53 (Figure 5C), in agreement with findings reported previously for CIITA-/- mice.34,54 B cells did not express MHCII in spleen or Peyer patches of all 3 BLS mouse strains analyzed (data not shown).
Taken together, it appears that CIITA is not essential for residual expression of MHCII in RFX5-/- mice and that, in the absence of CIITA, RFX5 represses rather than induces MHCII expression in some cell types.
MHCII expression in thymus and spleen of MHCII-deficient mouse strains. (A) Thymic sections from Aα-/- (i), CIITA-/- (ii), RFX5-/- (iii), C/R-/- (iv), and C57BL/6 (v) mice were analyzed by 3-color immunofluorescence staining and confocal microscopy. Medullary regions of the sections are shown. The sections were stained for I-Ab (red; top left quadrants), CD11c (blue; top right quadrants), and UEA-1 (green; bottom left quadrants); bottom right quadrants show overlay of all 3 stains (UEA+I-Ab+ cells = yellow; CD11c+I-Ab+ cells = pink; triple-positive cells = white). (B) Immunohistochemical analysis of I-Ab (red) and B220 (blue) expression on splenic sections of the different mouse strains. Staining for I-Ab (red) in splenic T-cell zones was consistently stronger in RFX5-/- and CR-/- mice than in the C57BL/6 control (see also Clausen et al33 ).
MHCII expression in thymus and spleen of MHCII-deficient mouse strains. (A) Thymic sections from Aα-/- (i), CIITA-/- (ii), RFX5-/- (iii), C/R-/- (iv), and C57BL/6 (v) mice were analyzed by 3-color immunofluorescence staining and confocal microscopy. Medullary regions of the sections are shown. The sections were stained for I-Ab (red; top left quadrants), CD11c (blue; top right quadrants), and UEA-1 (green; bottom left quadrants); bottom right quadrants show overlay of all 3 stains (UEA+I-Ab+ cells = yellow; CD11c+I-Ab+ cells = pink; triple-positive cells = white). (B) Immunohistochemical analysis of I-Ab (red) and B220 (blue) expression on splenic sections of the different mouse strains. Staining for I-Ab (red) in splenic T-cell zones was consistently stronger in RFX5-/- and CR-/- mice than in the C57BL/6 control (see also Clausen et al33 ).
Alternative MHCII expression is inducible in vitro and in vivo
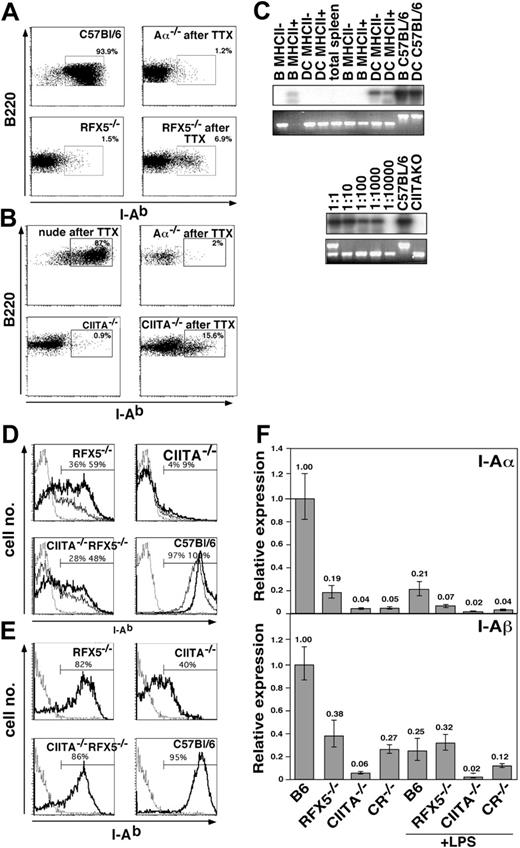
When we analyzed MHCII expression in RFX5-/- or CIITA-/- mice that underwent transplantation, we found that the fraction of MHCII-expressing B cells increased from background values of less than 2% in mice that did not undergo transplantation to values in the range of 5% to 15% (Figure 6A-B and data not shown), suggesting that alternative MHCII expression was further inducible. To exclude that these MHCII+ cells were derived from donor cells emigrating from the transplanted WT thymus, we amplified their RFX5 and CIITA alleles by PCR. Only in some samples a weak band for the WT allele could be detected following sensitive Southern blot hybridization (Figure 6C, top) but not in the ethidium bromide-stained gel (Figure 6C, bottom), indicating that only very few contaminating donor cells were present within the sorted MHCII+ B cells and DCs (Figure 6C and data not shown). In addition, we failed to detect MHCII+ B cells in Aα-/- animals that underwent transplantation (Figure 6A).
To analyze whether the residual MHCII expression in CIITA-/- or RFX5-/- mice could be up-regulated, we tested splenic B cells from mice that did not undergo transplantation for MHCII expression after overnight culture in the presence of various activating agents. We observed that stimulation with anti-CD40 antibody, CpG-oligodeoxynucleotide, and LPS but not pI/pC resulted in strong induction of MHCII expression in RFX5-/- and CR-/- B cells (Figure 6D and data not shown). In contrast, only a few B cells lacking expression of CIITA were induced to moderately increase MHCII expression after activation (Figure 6D). For both WT and mutant mice, the increased surface expression of MHCII on activated B cells was not accompanied by increased mRNA expression levels, indicating that posttranslational modifications of MHCII expression were responsible for this effect (Figure 6F). DCs isolated from the spleen of BLS mutant mice also responded to in vitro maturation on plastic cell-culture dishes by up-regulation of MHCII and B7.2 expression (Figure 6E and data not shown). Remarkably, more than 80% of splenic DCs from RFX5-/- and CR-/- mice but also 40% of DCs from CIITA-/- mice expressed MHCII after the culture period (Figure 6E).
Residual MHCII expression in human MHCII deficiency
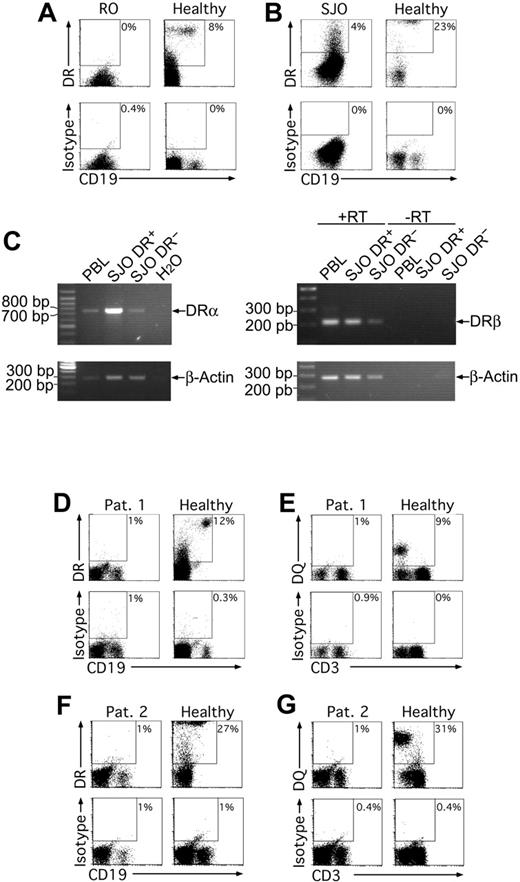
To assess whether residual MHCII expression was also detectable in human MHCII deficiency, in particular following activation through TLR ligands, we first analyzed the RFX5-deficient B-cell lines RO and SJO10,55 for MHCII expression in the absence or presence of CpG or LPS. Solely on a fraction of SJO cells, we found HLA-DR but not HLA-DP and HLA-DQ expression irrespective of the stimulation (Figure 7A-B), indicating that also in humans MHCII expression was indeed possible in the absence of RFX5. We confirmed this observation by RT-PCR for HLA-DR α- and β-chain transcripts and found that the HLA-DR+ cell population also contained more HLA-DR transcripts, especially for the α-chain.
We also had the possibility to analyze PBLs derived from 2 patients with MHCII deficiency, both with mutations in the RFX-ANK gene. We stimulated PBLs from these patients and a healthy control with LPS, CpG, polyI/C, or CD40L O/N. The next day, the cells were analyzed for HLA-DR, -DP, and -DQ expression. We could not detect MHCII expression in any of the cultures (Figure 7D-G and data not shown). Thus, in one RFX5-deficient cell line but not in RFX-ANK-deficient patients residual MHCII expression could observed.
Flow cytometric analysis of MHCII expression on thymic, splenic, and LN DCs in MHCII-deficient mouse strains. (A) Cells from collagenase-digested thymi (top row) and spleens (bottom row) were stained for CD11c and I-Ab. Shown are histograms of I-Ab expression on CD11c+ cells of the indicated mouse strains (bold line) or the Aα-/- control (dotted line). The percentage of MHCII-expressing cells in the indicated gates is given as bold numbers (those for the Aα-/- controls are given in parenthesis in the diagram on the left). (B) Mean frequencies ± SD of MHCII+ cells among thymic CD11c+ DCs of 3 mice analyzed in each group. To control for background differences in the analysis of the RFX5/CIITA double-deficient (CR-/-) mice, we also examined CIITA-/- RFX5+/- mice, which were derived from the same breeding stock as CR-/- mice. (C) Cells from collagenase-digested brachial and inguinal LNs were magnetically enriched for CD11c+ cells and subsequently stained for CD11c, DEC205, and I-Ab. Top: The histograms show I-Ab expression of CD11c+ cells (bold line, bold number). The dotted line indicates I-Ab staining on control cells from Aα-/- animals. Bottom: CD11c and DEC205 expression on I-Ab-expressing LN cells. (D) The expression of I-Ab α- and β-chain in magnetically purified DCs from collagenase-digested spleens and thymi of the indicated mouse strains was analyzed by qRT-PCR. Analysis of β-actin expression was used for standardization. Shown are expression levels relative to the ones in DCs from C57BL/6 mice. The error bars indicate the standard deviation.
Flow cytometric analysis of MHCII expression on thymic, splenic, and LN DCs in MHCII-deficient mouse strains. (A) Cells from collagenase-digested thymi (top row) and spleens (bottom row) were stained for CD11c and I-Ab. Shown are histograms of I-Ab expression on CD11c+ cells of the indicated mouse strains (bold line) or the Aα-/- control (dotted line). The percentage of MHCII-expressing cells in the indicated gates is given as bold numbers (those for the Aα-/- controls are given in parenthesis in the diagram on the left). (B) Mean frequencies ± SD of MHCII+ cells among thymic CD11c+ DCs of 3 mice analyzed in each group. To control for background differences in the analysis of the RFX5/CIITA double-deficient (CR-/-) mice, we also examined CIITA-/- RFX5+/- mice, which were derived from the same breeding stock as CR-/- mice. (C) Cells from collagenase-digested brachial and inguinal LNs were magnetically enriched for CD11c+ cells and subsequently stained for CD11c, DEC205, and I-Ab. Top: The histograms show I-Ab expression of CD11c+ cells (bold line, bold number). The dotted line indicates I-Ab staining on control cells from Aα-/- animals. Bottom: CD11c and DEC205 expression on I-Ab-expressing LN cells. (D) The expression of I-Ab α- and β-chain in magnetically purified DCs from collagenase-digested spleens and thymi of the indicated mouse strains was analyzed by qRT-PCR. Analysis of β-actin expression was used for standardization. Shown are expression levels relative to the ones in DCs from C57BL/6 mice. The error bars indicate the standard deviation.
Discussion
MHCII deficiency (BLS) represents a severe immunodeficiency syndrome in humans caused by mutations of parts of the MHCII promoter complex.24 The underlying defects in immune function are 2-fold: (1) inability in MHCII-restricted positive selection of Th cells due to lack of MHCII on thymic cortical epithelium and (2) absence of MHCII-dependent antigen presentation due to impaired MHCII expression on peripheral APCs. We report here that the presence of properly selected Th cells in mouse models of MHCII deficiency results in further up-regulation of residual MHCII expression on hematopoietic cells, restores Th-cell-dependent humoral immunity, and significantly improves CD4 T-cell-dependent clearance of MCMV. Beyond these clinically relevant results, our analysis of the genetic requirements for residual MHCII expression revealed a so-far-unknown mechanism of MHCII expression, which is independent of the known promoter complex and appears to be repressed by RFX5.
RFX5- and CIITA-independent expression of MHCII
Although CIITA-/- and RFX5-/- mice both possess some MHCII+ cells,32-34 MHCII expression is more prominent in RFX5-/- mice. Surprisingly, CR-/- mice were phenotypically indistinguishable from RFX5-/- mice, with increased expression levels of MHCII on DEC205+ DCs, activated B cells, and thymic UEA-1+ medullary epithelial cells. Although this result appears paradoxic at first, it could be explained if the RFX complex serves as a transcriptional suppressor in the absence of CIITA. Thus, the role of the RFX complex may be a dual one: (a) suppression of MHCII transcription in the absence of CIITA and (b) activation of MHCII transcription by binding of CIITA.22 This might apply to professional APCs in particular, since somatic cells were not found to be MHCII positive in the CR-/- mice. A suppressive activity of RFX5 has been reported only for a collagen promoter construct in rat fibroblasts,57 but the mechanism of suppression is different in this case because the RFX binding side in the collagen promoter overlaps with the transcriptional start site.
It is of particular interest for the understanding of the immune defect in BLS that in the absence of the RFX complex a substantial proportion of splenic and LN DCs express MHCII. Such cells are also present in LN but not spleen of CIITA-/- mice as previously reported.33,34,54 Remarkably, the majority of splenic DCs of all 3 BLS mutant strains analyzed in this study drastically up-regulated MHCII expression upon maturation in vitro (Figure 6E). This finding indicates that RFX5- and CIITA-independent expression of MHCII in DCs is not restricted to a certain subset of DCs but is rather dependent on the maturation status of the cells. A similar, but activation-dependent up-regulation of MHCII was observed for B cells of RFX5-/- and CR-/- mice and for a minor fraction of CIITA-/- B cells (Figure 6D). Of interest, a CIITA-independent mechanism of MHCII mRNA stabilization has been described for B cells stimulated with CpG DNA.58 However, using quantitative RT-PCR analysis we were not able to detect increased levels of MHCII mRNA in activated B cells, implying that posttranslational modifications of MHCII expression also occur in activated B cells similar to DCs.59 It seems possible that such mechanisms have evolved to allow MHCII expression to be continued in activated professional APCs, even after inhibition of the classic MHCII transcription pathway through a pathogen.60 Also, thymus transplantation led to an increase of MHCII+ B cells (Figure 6A-B), indicating that the mere presence of CD4 T cells in BLS mice increases the proportion of MHCII+ B cells. This effect may result from preferential expansion of B cells expressing MHCII or from up-regulation of MHCII expression in response to signals provided by CD4 T cells.
Induced MHCII expression in vivo and in vitro. (A-B) LN cells of Aα-/-, RFX5-/-, and CIITA-/- mice that did (TTX) and did not undergo transplantation and control C57BL/6 mice were analyzed for I-Ab, B220, and CD11c expression. The dot plots show I-Ab expression on gated B220+CD11c- cells. The percentage of MHCII+ cells is indicated in the respective windows. Parts A and B are from independent experiments. (C) I-Ab-expressing and I-Ab-negative B cells and DCs of CIITA-/- animals that underwent transplantation were sorted by FACS, and the genotype of the cells was assessed by a 3-point PCR for the WT allele (bottom row, top band) and KO allele (bottom row, bottom band). The products of the PCR reaction using primers for only the WT fragment were hybridized with a CIITA-specific probe (top row). Shown are results from 2 independent sorting experiments (left). The PCR reactions were standardized based on cell number, and the sensitivity of amplification was determined by dilution of WT cells in cells from CIITA-/- animals at ratios of 1:1 to 1:10 000 as indicated (right). (D) Splenocytes of Aα-/-, RFX5-/-, CIITA-/-, CR-/-, and C57BL/6 mice that did not undergo transplantation were cultured O/N in the presence (bold line) or absence (thin line) of anti-CD40. The histograms show I-Ab expression on CD19+ cells of the indicated mouse strains. The Aα-/- control is shown as a dotted line in each histogram. (E) Collagenase-digested spleen cells of the same mouse strains as shown in panel D were incubated O/N in medium without additional stimuli. Cells were stained with anti-CD11c and anti-I-Ab and gated on CD11c+ DCs. Percent of MHCII+ cells in the indicated gates is given in the figure. The Aα-/- control is shown as dotted line. (F) The expression of I-Ab α- and β-chain in magnetically purified splenic B cells of the indicated mouse strains was analyzed by qRT-PCR after overnight culture in the presence or absence of 10 μg/mL LPS. Analysis of β-actin expression was used for standardization. Shown are expression levels relative to the ones of untreated B cells from C57BL/6 mice. The error bars indicate the standard deviation. LPS indicates lipopolysaccharide.
Induced MHCII expression in vivo and in vitro. (A-B) LN cells of Aα-/-, RFX5-/-, and CIITA-/- mice that did (TTX) and did not undergo transplantation and control C57BL/6 mice were analyzed for I-Ab, B220, and CD11c expression. The dot plots show I-Ab expression on gated B220+CD11c- cells. The percentage of MHCII+ cells is indicated in the respective windows. Parts A and B are from independent experiments. (C) I-Ab-expressing and I-Ab-negative B cells and DCs of CIITA-/- animals that underwent transplantation were sorted by FACS, and the genotype of the cells was assessed by a 3-point PCR for the WT allele (bottom row, top band) and KO allele (bottom row, bottom band). The products of the PCR reaction using primers for only the WT fragment were hybridized with a CIITA-specific probe (top row). Shown are results from 2 independent sorting experiments (left). The PCR reactions were standardized based on cell number, and the sensitivity of amplification was determined by dilution of WT cells in cells from CIITA-/- animals at ratios of 1:1 to 1:10 000 as indicated (right). (D) Splenocytes of Aα-/-, RFX5-/-, CIITA-/-, CR-/-, and C57BL/6 mice that did not undergo transplantation were cultured O/N in the presence (bold line) or absence (thin line) of anti-CD40. The histograms show I-Ab expression on CD19+ cells of the indicated mouse strains. The Aα-/- control is shown as a dotted line in each histogram. (E) Collagenase-digested spleen cells of the same mouse strains as shown in panel D were incubated O/N in medium without additional stimuli. Cells were stained with anti-CD11c and anti-I-Ab and gated on CD11c+ DCs. Percent of MHCII+ cells in the indicated gates is given in the figure. The Aα-/- control is shown as dotted line. (F) The expression of I-Ab α- and β-chain in magnetically purified splenic B cells of the indicated mouse strains was analyzed by qRT-PCR after overnight culture in the presence or absence of 10 μg/mL LPS. Analysis of β-actin expression was used for standardization. Shown are expression levels relative to the ones of untreated B cells from C57BL/6 mice. The error bars indicate the standard deviation. LPS indicates lipopolysaccharide.
Residual MHCII expression in human MHCII deficiency. The RFX5-deficient human B-cell lines RO (A) and SJO (B) were cultured overnight with 10 μg/mL LPS, stained for CD19 and HLA-DR, and analyzed by flow cytometry. PBLs were used as healthy control cells. Only live cells are shown in all cases. RT-PCR analysis of HLA-DR expression by SJO cells (C). The cells were FACS sorted into HLA-DR+ and HLA-DR- fractions. Cells (105) were used for RT-PCR with primers for HLA-DRα and β. Primers were placed into highly homologous regions according to aligned HLA-DR alleles at the IMGT HLA database.56 The α-chain PCR was intron spanning, the β-chain PCR was not. For the latter control, reactions lacking reverse transcriptase (RT) were included. PBLs (105) from a healthy donor were used as positive controls. A PCR for β-actin served as control for the RT reaction. (D-G) MHCII expression on PBLs derived from 2 patients previously diagnosed to be MHCII deficient was analyzed after overnight culture in the presence of 10 μg/mL LPS and 0.5 μM CpG 2006 (patient 1) or 10 μg/mL LPS (patient 2). The patients were 1 year (F-G) and 4 years (D-E) of age, and the molecular defects were located in the RFX-ANK gene. After culture, the cells were stained for CD19 and HLA-DR (D,F) or CD3 and HLA-DQ (E,G) or CD3 and HLA-DP (data not shown) and analyzed by flow cytometry. Antibodies of irrelevant specificities were used as isotype controls for the HLA-DQ, HLA-DR, and HLA-DP stainings. Dot plots show cells in lymphocyte- and live-cell gates. In all experiments, similar results were obtained without treatment or treatment with 0.5 μM CpG, 50 μg/mL pI/pC, or CD40L.
Residual MHCII expression in human MHCII deficiency. The RFX5-deficient human B-cell lines RO (A) and SJO (B) were cultured overnight with 10 μg/mL LPS, stained for CD19 and HLA-DR, and analyzed by flow cytometry. PBLs were used as healthy control cells. Only live cells are shown in all cases. RT-PCR analysis of HLA-DR expression by SJO cells (C). The cells were FACS sorted into HLA-DR+ and HLA-DR- fractions. Cells (105) were used for RT-PCR with primers for HLA-DRα and β. Primers were placed into highly homologous regions according to aligned HLA-DR alleles at the IMGT HLA database.56 The α-chain PCR was intron spanning, the β-chain PCR was not. For the latter control, reactions lacking reverse transcriptase (RT) were included. PBLs (105) from a healthy donor were used as positive controls. A PCR for β-actin served as control for the RT reaction. (D-G) MHCII expression on PBLs derived from 2 patients previously diagnosed to be MHCII deficient was analyzed after overnight culture in the presence of 10 μg/mL LPS and 0.5 μM CpG 2006 (patient 1) or 10 μg/mL LPS (patient 2). The patients were 1 year (F-G) and 4 years (D-E) of age, and the molecular defects were located in the RFX-ANK gene. After culture, the cells were stained for CD19 and HLA-DR (D,F) or CD3 and HLA-DQ (E,G) or CD3 and HLA-DP (data not shown) and analyzed by flow cytometry. Antibodies of irrelevant specificities were used as isotype controls for the HLA-DQ, HLA-DR, and HLA-DP stainings. Dot plots show cells in lymphocyte- and live-cell gates. In all experiments, similar results were obtained without treatment or treatment with 0.5 μM CpG, 50 μg/mL pI/pC, or CD40L.
Residual MHCII expression in BLS mice supports Th-cell-mediated immune responses
In an initial adoptive transfer experiment, we observed that residual MHCII expression in RFX5-/- and CIITA-/- mice supports activation and expansion of specific Th cells in vivo (Figure 1A). This result seems to contradict previously published data that showed that Th cells from immunized CIITA-/- animals do not show an in vitro proliferative response against the same antigen.32 A possible explanation is that the endogenous CD4+ T cells present in CIITA-/- animals are mainly restricted to non-classic MHC class I molecules as has been described for Aβ-/- mice.61 To better study whether MHC+ APCs in BLS mice could support Th-dependent immune responses, WT thymi were transplanted into RFX5-/- or CIITA-/- or Aα-/- mice and led to reconstitution of the peripheral CD4 T-cell compartment, in accordance with most37,62-64 but not all65 previous experiments.
Following reconstitution with a functional CD4 T-cell compartment derived from endogenous T-cell precursors, BLS mice were able to mount normal Th-cell-dependent antibody responses. This applied not only to immunization with the model antigen NP-CG but also to infection with MCMV. The latter is of major importance since viral infections are the main cause of death in BLS patients before and after BMT.27,66 Furthermore, we also determined the CD4 T-cell-dependent clearance of MCMV in the salivary glands of infected mice.48 Although full clearance of the virus could not be achieved in RFX5-/- mice that underwent transplantation, the viral load was reduced by a factor of 10 compared with RFX5-/- mice that did not undergo transplantation. The lower viral clearance of RFX5-/- mice that underwent transplantation compared with WT controls may be explained by residual MHCII expression in RFX5-/- mice being restricted to professional APCs. Thus, induced MHCII expression on infected epithelial cells, which is IFNγ and thus CIITA dependent, may be required for more efficient clearance of the virus. In addition, the RFX complex regulates not only the expression of the classic MHCII genes but also of accessory molecules such as H2-M.67 Expression of the latter may still be impaired in the mice that underwent transplantation. Nevertheless, our findings that Th-cell-dependent immune responses were substantially improved in BLS mice that received versus did not receive transplants demonstrate that the presence of a WT thymus in BLS mice is therapeutically efficient, without the need for BMT.
Implications for treatment of bare lymphocyte syndrome
In the light of the present results that thymic transplantation represents a potent therapy in mouse models of BLS, it appears important to re-examine residual MHCII expression in patients with MHCII deficiency and to consider reconstitution of thymic selection as a treatment strategy. Due to the low number of patients and their young age at diagnosis, a comprehensive analysis of residual MHCII expression is difficult. We could detect MHCII expression only by one RFX5-deficient B-cell line, but not by unstimulated or stimulated PBLs of 2 patients with RFX-ANK deficiency (Figure 7). Since RFX-ANK-deficient mice have not been generated so far, it is unclear whether this deficiency would allow residual MHCII expression in the mouse. Residual MHCII expression was, however, reported on subsets of Langerhans cells, monocytes, and B lymphocytes from BLS patients after long-term culture.26,68 In particular, significant levels of HLA-DR expression could be detected on monocyte-derived DCs and epidermal Langerhans cells but not B cells or monocytes of twin brothers with RFX5 deficiency.69,70 However, compared with other patients with RFX5 deficiency, these twin brothers presented with a rather mild disease phenotype, indicating that differences in the type of RFX5 mutation may influence residual MHCII expression. Patient-derived B-cell lines failed to up-regulate MHCII upon IFNγ stimulation,71,72 but since IFNγ-induced MHCII expression depends on the action of CIITA and indirectly the RFX complex21 other stimuli should be tested in addition. An obvious difference between the mouse models and findings in BLS patients is the relatively high frequency of peripheral CD4 T cells in patients. These T cells were shown to express a polyclonal TCR repertoire,73 which could mean that thymic CD4 T-cell selection is at least partially functional in BLS patients. However, structural abnormalities were described for TCRs from BLS patients within the complementarity determining region 3,74,75 and it still remains to be clarified whether the CD4 T cells present in the patients are MHCII restricted. At least in one case, restriction of CD4 T cells to MHC class I rather than MHCII was reported.76 From the analysis of CD4 T cells of MHCII-deficient (Aβ-/-) mice, it is known that these cells are restricted to non-classic MHC class I molecules.61 In addition, the persisting immunodeficiency in many BLS patients after BMT, despite the presence of sufficient numbers of MHC-matched or haploidentical APCs, supports the notion that the available CD4 T cells are either not selected on MHCII, are functionally incompetent, or have a skewed repertoire.27,66 Since in the published cases of successfully cured BLS patients the grafted bone marrow was only partially or not depleted of T cells,27,75,77 expanded donor T cells but not de novo thymic T-cell generation may play a major role in the reconstitution of the immune system after BMT in BLS patients. It is striking in this context that the CD4 T-cell population of 3 BMT patients that exhibited a high chimerism in the B-cell and monocyte population was almost entirely donor derived.27,76 This is most likely explained by peripheral expansion of mature T cells transplanted with the BM graft and a failure of thymic CD4 T-cell development from stem-cell precursors. Alternatively, successful T-cell reconstitution in cases of MHC-matched BMT may be explained by positive selection of recipient T cells on donor-derived DCs, as has been described in the mouse.78
The application of thymic transplantation in human therapy has recently been shown to be technically feasible in patients with complete DiGeorge syndrome and did not require MHC matching.78-80 Difficulties for the treatment of human MHCII deficiency by thymus transplantation would arise, however, from the presence of T cells in BLS patients, probably making MHC matching necessary. As an alternative, local gene therapy, which reconstitutes expression of the mutated transcription factor in the thymic epithelium, could be combined with BMT for the generation of MHCII-expressing APCs. In principle, such an approach has been shown feasible for MHCII structural genes in Aα-/- mice using adenoviral vectors.63
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2004-09-3445.
Supported by the Deutsche Forschungsgemeinschaft through SFB456 and SFB576 (I.F.), AW1600/1-1 (A.W.), and BU1410/1-1; the Volkswagen Foundation (I.F.); the Croatian Ministry of Science and Technology through grants 0062004 and 0062005; and Fonds der Chemischen Industrie and the Land Nordrhein-Westfalen through fellowships to T.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to J. Kirberg for advice on thymic transplantation procedures; H. Bluethmann for providing Aα-/- mice; V. Kuchroo and F. Frommer for providing 2D2 mice; S. Bauer and ImmunoTools for providing antibodies; C. Uthoff-Hachenberg for technical help; and F. Rieux-Laucat, M. Alimzhanow, and C. Tertilt for comments on the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal