Abstract
The best treatment approach for children with B-precursor acute lymphoblastic leukemia (ALL) in second clinical remission (CR) after a marrow relapse is controversial. To address this question, we compared outcomes in 188 patients enrolled in chemotherapy trials and 186 HLA-matched sibling transplants, treated between 1991 and 1997. Groups were similar except that chemotherapy recipients were younger (median age, 5 versus 8 years) and less likely to have combined marrow and extramedullary relapse (19% versus 30%). To adjust for time-to-transplant bias, treatment outcomes were compared using left-truncated Cox regression models. The relative efficacy of chemotherapy and transplantation depended on time from diagnosis to first relapse and the transplant conditioning regimen used. For children with early first relapse (< 36 months), risk of a second relapse was significantly lower after total body irradiation (TBI)–containing transplant regimens (relative risk [RR], 0.49; 95% confidence interval [CI] 0.33-0.71, P < .001) than chemotherapy regimens. In contrast, for children with a late first relapse (≥ 36 months), risks of second relapse were similar after TBI-containing regimens and chemotherapy (RR, 0.92; 95% CI, 0.49-1.70, P = .78). These data support HLA-matched sibling donor transplantation using a TBI-containing regimen in second CR for children with ALL and early relapse.
Introduction
Despite dramatic improvements in survival for children with acute lymphoblastic leukemia (ALL) that have occurred over the last 3 to 4 decades, approximately 20% to 25% of children suffer a relapse.1-5 Over 70% of relapses occur within 3 years from diagnosis and most involve the bone marrow alone or together with extramedullary involvement.4 The likelihood of achieving and sustaining a second clinical remission (CR) is influenced by duration of first CR, site of relapse, and immunophenotype.1,4 Although the best treatment approach for relapsed ALL remains uncertain, there is agreement that when relapse occurs early, leukemia-free survival remains dismal. A hematopoietic stem cell transplant from a matched related or unrelated donor is an accepted form of treatment for recurrent ALL. However, the indications for transplantation are a matter of great debate.5-11 In the ideal setting, a randomized study would compare outcomes of chemotherapy and transplantation. However, randomization to these 2 conceptually very different therapies has proved difficult for physicians and families to accept.12 Therefore published reports comparing outcomes after transplantation and chemotherapy, including an earlier one from the International Bone Marrow Transplant Registry, are nonrandomized.6,11,12
The aim of this report was to identify the best treatment option for children with relapsed B-precursor ALL involving bone marrow alone or bone marrow and an extramedullary site in an era that has seen progressive intensification of front-line and relapse chemotherapeutic regimens.
Patients, materials, and methods
Data collection
Patients who received chemotherapy were enrolled in Pediatric Oncology Group (POG) clinical trials and detailed demographic and clinical data were obtained from the POG Statistical Center. Data on patients undergoing transplantation were obtained from the Center for International Bone Marrow Transplant Research (CIBMTR). The CIBMTR is a voluntary working group of more than 500 transplantation centers worldwide. Participating centers register basic information on consecutive transplantations to a Statistical Center at the Medical College of Wisconsin. Detailed demographic and clinical data are collected on a representative sample of patients in the registry using a weighted randomization scheme.
Inclusion criteria
The study included patients with relapsed B-precursor ALL, younger than 18 years at diagnosis, and who received treatment for relapsed leukemia between 1991 and 1997. All patients had bone marrow relapse (isolated or combined with extramedullary relapse as determined by morphology). Patients with isolated extramedullary site relapse were excluded. All chemotherapy recipients were enrolled in POG trials (9110, 9310, or 9411) and achieved a second CR. None of the chemotherapy recipients included in the current study received a transplant. Similar to chemotherapy recipients, all transplant recipients were in second CR and had an HLA-matched sibling donor. The decision to proceed to transplantation and all aspects of the transplantation regimen were determined by the transplantation centers. The study included 188 recipients of chemotherapy (POG 9110, n = 101; 9310, n = 58; 9411, n = 29) and 186 recipients of transplants from HLA-matched siblings.
End points
Treatment-related deaths were defined as death during a continuous remission. Relapse was defined as morphologic leukemia recurrence at any site. Leukemia-free survival was defined as survival in a state of continuous complete remission.
Treatment regimens
Induction chemotherapy for POG 9110 began with a 72-hour infusion of doxorubicin followed on day 8 by 4 weeks of daily prednisone, weekly vincristine, twice weekly native Escherichia colil-asparaginase, and triple intrathecal central nervous system prophylaxis. POG 9310 and 9411 differed from POG 9110 in doxorubicin administration and asparaginase treatment. On POG 9310 and 9411, doxorubicin was given on day 1 and not as a 72-hour infusion. Patients in POG 9310 were randomized to receive pegylated l-asparaginase weekly or every other week and those in POG 9411 were randomized to receive pegylated l-asparaginase weekly or native E colil-asparaginase 3 times a week. On all protocols, patients with testicular or central nervous system relapse received radiation to the affected organ during early continuation. Continuation therapy for all protocols was 5-week cycles of rotating drug pairs: vincristine and etoposide, doxorubicin and cytosine arabinoside, pegylated l-asparaginase and triple intrathecal central nervous system prophylaxis, cyclophosphamide and prednisone, and intermediate dose 6-mercapturine and methotrexate. Transplantation regimens are shown in Table 1.
Statistical analysis
Variables related to patient and disease characteristics were compared among the groups using the χ2 statistic for categoric variables and the Kruskal-Wallis test for continuous variables. The probabilities of overall and leukemia-free survival were calculated with the use of a left-truncated Kaplan-Meier estimator.13 For analyses of survival rates, death from any cause was considered an event, and data on patients who were alive at last follow-up were censored. For leukemia-free survival, relapse or death (ie, treatment failure) was considered an event, and data on patients who were alive were censored at last follow-up. The probabilities of treatment-related death and relapse were calculated using a left-truncated cumulative incidence function method.13 For treatment-related death, relapse was the competing event and for relapse, treatment-related death was the competing event. Data on patients who were alive were censored at last follow-up. Confidence intervals (CIs) were calculated with a log transformation.13
When comparing outcomes of 2 different treatment groups (chemotherapy versus transplantation) adjustments are required for differences in time to treatment. To address for differences in time to treatment, which result from the fact that children must survive in complete remission, commonly for 2 to 3 months to be eligible for a transplant, possibly selecting a better prognosis group, left-truncated Cox proportional hazards regression models were constructed for all outcomes of interest.14 Multivariate models were built using a stepwise forward selection, with a P value of .05 or less considered to indicate statistical significance; all variables met the proportional-hazards assumption. The primary objective was to compare outcomes after chemotherapy and transplantation. Results are expressed as relative risks (RRs), that is, the relative rate of occurrence of the event with chemotherapy as compared with transplantation.
The variable for treatment type (chemotherapy versus transplantation) was retained in all steps of model building. Other variables considered were age at diagnosis (< 1 versus 1-10 versus 11-18 years), sex, white blood cell (WBC) count at diagnosis (≤ 50 versus > 50-100 versus ≥ 100 × 109/L), and time to first relapse (< 36 versus ≥ 36 months from diagnosis). For the transplantation group, the following variables were considered: conditioning regimen (total body irradiation [TBI]–containing regimen versus non-TBI regimen), donor-recipient sex match (female donor/male recipient versus other), donor-recipient cytomegalovirus (CMV) serologic status (donor/recipient negative versus donor positive/recipient negative versus donor negative/recipient positive versus donor positive/recipient positive), and graft-versus-host disease (GVHD) prophylaxis (cyclosporine and methotrexate versus other). First-order interactions between treatment type and conditioning regimen, GVHD prophylaxis, and time to first relapse were considered in final models. P values are 2-sided. Analyses were completed with the use of PROC PHREG in SAS software, version 8.2 (SAS Institute, Cary, NC).
Results
Patients
Table 1 shows patient and disease characteristics and transplantation regimens received. All patients had B-precursor ALL. Recipients of chemotherapy and transplants differed in that chemotherapy recipients were younger at diagnosis (median age, 5 versus 8 years, P < .001) and less likely to have combined bone marrow and extramedullary relapse (19% versus 30%, P = .01). The median WBC counts at diagnosis were 10 × 109/L and 11 × 109/L in recipients of chemotherapy alone and transplants, respectively. The median time to first relapse in recipients of chemotherapy and transplants was 32 months. Eighty-two percent of transplant recipients received TBI-containing regimens. All transplant recipients received bone marrow grafts. The median follow-ups of the study populations are 103 months (range, 34-159 months) and 101 months (16-160 months) after chemotherapy and transplantation, respectively. The completeness of follow-up (the ratio of the sum of the observed follow-up time to the sum of the potential follow-up time for all patients in the study) for the study population is 93%.15
Treatment-related mortality
Death from treatment-related complications occurred in 18 of 188 recipients of chemotherapy and 24 of 186 recipients of transplants. Risks of mortality from treatment-related complications did not differ significantly after transplantation with TBI-containing regimens and chemotherapy alone (RR, 1.25; 95% CI, 0.63-2.48; P = .81). Death from treatment-related complications was significantly higher after transplantation with non-TBI regimens (RR, 3.99; 95% CI, 1.64-9.71; P = .002).
Relapse
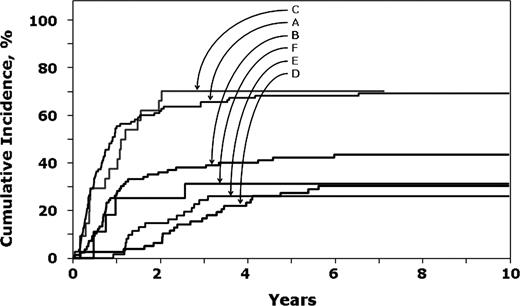
In children with an early first relapse (< 36 months), risk of second relapse was significantly lower after transplantation with a TBI-containing regimen compared to chemotherapy alone (Table 2; Figure 1). In contrast, after a late relapse (≥ 36 months), risks of second relapse were similar after transplantation with TBI-containing regimens compared to chemotherapy alone. The observed higher risk with non-TBI regimens compared to chemotherapy alone should be interpreted with caution due the small sample size for this group. In both treatment groups, a second relapse was significantly higher in older (< 10 years) children (RR, 1.71; 95% CI, 1.25-3.34; P = .01). The 8-year probabilities of a second relapse after early first relapse were 69% (95% CI, 60%-77%), 43% (95% CI, 32%-54%), and 70% (95% CI, 36%-88%) after chemotherapy alone and transplantation with TBI-containing regimens and non-TBI regimens, respectively. Corresponding probabilities in children treated for late relapse were 30% (95% CI, 21%-41%), 26% (95% CI, 16%-37%), and 31% (95% CI, 10%-55%).
Probability of second leukemia recurrence. A indicates chemotherapy recipients after an early first relapse; B, transplant recipients with TBI regimens after an early first relapse; C, transplant recipients with non-TBI regimens after an early first relapse; D, chemotherapy recipients after late first relapse; E, transplant recipients with TBI regimens after late first relapse; and F, transplant recipients with non-TBI regimens after late first relapse.
Probability of second leukemia recurrence. A indicates chemotherapy recipients after an early first relapse; B, transplant recipients with TBI regimens after an early first relapse; C, transplant recipients with non-TBI regimens after an early first relapse; D, chemotherapy recipients after late first relapse; E, transplant recipients with TBI regimens after late first relapse; and F, transplant recipients with non-TBI regimens after late first relapse.
Leukemia-free survival
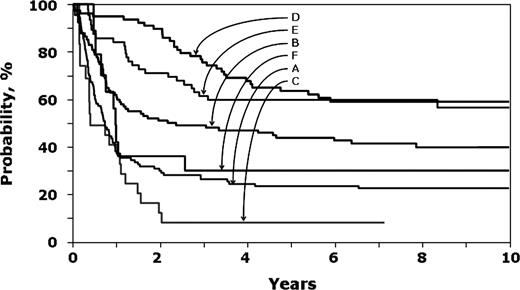
Risk of treatment failure (ie, second relapse or death) was significantly lower after transplantation with TBI-containing regimens compared to chemotherapy alone in children with early first relapse (< 36 months; Table 2; Figure 2). After late first relapse (≥ 36 months), risks were similar after transplantation with TBI-containing regimens and chemotherapy alone. The observed higher risk with non-TBI regimens compared to chemotherapy alone should be interpreted with caution due to the small sample size for this group. In both treatment groups, treatment failure was significantly higher in older children (< 10 years old; RR, 1.61; 95% CI, 1.21-2.19; P = .001). The 8-year probabilities of leukemia-free survival in children with early first relapse were 23% (95% CI, 15%-31%), 41% (95% CI, 31%-52%), and 8% (95% CI, 1%-24%) after chemotherapy alone and transplantation with TBI-containing regimens and non-TBI regimens, respectively. Corresponding probabilities after late first relapse were 59% (95% CI, 47%-69%), 60% (95% CI, 46%-71%), and 30% (95% CI, 10%-54%).
Probability of leukemia-free survival. A indicates chemotherapy recipients after an early first relapse; B, transplant recipients with TBI regimens after an early first relapse; C, transplant recipients with non-TBI regimens after an early first relapse; D, chemotherapy recipients after late first relapse; E, transplant recipients with TBI regimens after late first relapse; and F, transplant recipients with non-TBI regimens after late first relapse.
Probability of leukemia-free survival. A indicates chemotherapy recipients after an early first relapse; B, transplant recipients with TBI regimens after an early first relapse; C, transplant recipients with non-TBI regimens after an early first relapse; D, chemotherapy recipients after late first relapse; E, transplant recipients with TBI regimens after late first relapse; and F, transplant recipients with non-TBI regimens after late first relapse.
Overall mortality
Risk of overall mortality was significantly lower after transplantation with TBI-containing regimens than after chemotherapy alone in children with early relapse (< 36 months; Table 2). In children with late first relapse (≥ 36 months), risk of mortality was similar after transplantation with TBI-containing regimens and chemotherapy alone. The observed higher risk with non-TBI regimens compared to chemotherapy alone should be interpreted with caution due to the small sample size for this group. In both treatment groups, mortality was significantly higher in older children (> 10 years old; RR, 1.78; 95% CI, 1.33-2.40; P < .001). The 8-year probabilities of overall survival in children with early first relapse were 32% (95% CI, 23%-40%), 44% (95% CI, 33%-55%), and 18% (95% CI 5%-37%) after chemotherapy alone and transplantation with TBI-containing regimens and non-TBI regimens, respectively. Corresponding probabilities of overall survival in those with late first relapse were 66% (95% CI, 54%-76%), 63% (95% CI, 49%-74%), and 32% (95% CI, 23%-40%).
Causes of death
In both treatment groups, recurrent leukemia was the most common cause of death (82% and 74% after chemotherapy alone and transplantation, respectively). Nonrelapse causes of death after chemotherapy alone were due to infections (9%), hemorrhage (1%), second neoplasm (7%), and other organ failure (1%). Nonrelapse causes of death after transplantation were infections (8%), hemorrhage (2%), GVHD (1%), interstitial pneumonitis (8%), second malignancy (1%), organ failure (2%), and other causes (4%).
Subset analyses
We performed 3 subset analyses: (1) treatment outcomes limited to children with an early first relapse (< 36 months) involving only bone marrow; (2) limited to transplant recipients only; (3) limited to children aged 1 year or older at diagnosis. In the first subset analysis, children with an early first bone marrow relapse who received a TBI-based regimen for HLA-matched sibling donor transplantation had significantly lower risks of second relapse (RR, 0.51; 95% CI, 0.33-0.81; P < .001), treatment failure (RR, 0.61; 95% CI, 0.40-0.93; P = .02), and overall mortality (RR, 0.64; 95% CI, 0.42-0.90; P = .03) compared to chemotherapy recipients. Due to very small numbers of patients, transplant recipients of non-TBI regimens and an early isolated bone marrow relapse were excluded in this subset analysis. The second subset analysis was limited to transplant recipients to examine the role of conditioning regimen. Among patients who received a TBI-based conditioning regimen, risks of second relapse (RR, 0.37; 95% CI, 0.22-0.62; P < .001), treatment failure (RR, 0.36; 95% CI, 0.23-0.56; P < .001), and overall mortality (RR, 0.39; 95% CI, 0.24-0.61; P < .001) were significantly lower compared to recipients of non-TBI–based regimens and independent of duration of first remission. The third subset analysis was limited to children aged 1 to 18 years. The relative efficacy of treatment (chemotherapy versus transplantation) as described for the entire study population was unchanged with the exclusion of children younger than 1 year at diagnosis (data not shown).
Discussion
The successes attained with front-line therapy for pediatric ALL16-20 have not been reproduced in patients with recurrent leukemia.12,21,22 The relative efficacy of chemotherapy alone versus HLA-matched sibling donor transplantation in children with relapsed ALL continues to be a matter of intense debate. In this report, children with early first relapse had lower rates of second relapse and higher rates of leukemia-free and overall survival if they received an HLA-matched sibling transplant with a TBI-containing regimen compared with a nontransplant approach. In contrast, for those with late first relapse, second relapse and leukemia-free and overall survival rates were similar after chemotherapy alone and transplantation with a TBI-containing regimen. Transplant recipients who received a non-TBI regimen fared poorly; recurrence rates were higher with corresponding lower leukemia-free and overall survival rates. These findings differ from our earlier report6 where leukemia-free survival rates were higher after HLA-matched sibling transplantation than after chemotherapy alone and independent of duration of first CR.
Consistent with other reports, the duration of first CR was the most important predictor of outcome in the current study.12,21,22 We observed leukemia-free survival rates of 41% at 8 years after transplantation with TBI-containing regimens in children with early relapse compared to 23% and 8% after chemotherapy alone and transplantation with non-TBI regimens, respectively. In a subset analysis restricted to transplant recipients and consistent with other reports23 we observed that transplantation with a TBI-containing regimen resulted in significantly lower risks of relapse, treatment failure, and overall mortality compared to non-TBI regimens and independent of duration of first remission. Borgmann and colleagues11 compared outcomes after chemotherapy alone and unrelated donor transplantation and report similar event-free survival rates to that reported here and an advantage to transplantation in high-risk patients. Most recipients in the Borgmann study received bone marrow grafts from a donor matched at HLA class I by serology and class II by high resolution and TBI-containing conditioning regimens.
Taken together, these data argue in favor of transplantation with a TBI-containing regimen if a suitably matched related or alternative donor is available for children with early relapsed ALL. Among children who have a relapse late, there appears to be little advantage with transplantation because second relapse and leukemia-free survival rates are similar after transplantation with TBI-containing regimens and chemotherapy alone. Our findings are consistent with other recent reports4,11,12 but differ from some of the earlier reports.6,24,25 In the earlier reports, leukemia-free survival rates after chemotherapy were substantially lower compared to the more recent reports and may explain the observed advantage after transplantation in those with late first relapse.
Some reports indicate site of first relapse to be predictive of treatment-outcome in ALL.4,11,12,21,22 Due to small numbers of patients we considered bone marrow (isolated or combined with extramedullary relapse) together. However, because 30% of transplant recipients had a combined bone marrow and extramedullary site relapse compared to 19% in the chemotherapy only group, a subset analysis restricted to patients with early relapse and isolated bone marrow relapse was performed. The relative efficacy of transplantation with TBI regimens compared to chemotherapy alone in patients with early first relapse was unchanged, confirming that the superior results of transplantation with a TBI-containing regimen are not due to the higher proportion of patients with combined bone marrow and extramedullary site relapse.
Older age at diagnosis (> 10 years) was associated with higher risks of second relapse, treatment failure, and overall mortality. We did not observe a similar trend for children younger than 1 year at diagnosis. Few were younger than 1 year and our inability to detect differences may be explained by small numbers (n = 17). Other variables such as WBC count at diagnosis, sex, and aspects of the transplant regimen such as GVHD prophylaxis, donor-recipient CMV status, and donor-recipient sex match did not affect treatment outcomes.
Because ours is not a randomized study there are several potential biases that may have affected the outcomes described in this report. The decision to proceed to transplantation was determined by the availability of an HLA-matched sibling donor and at the discretion of the transplantation center and includes only patients who achieved a second remission. All aspects of the transplantation regimen were selected by transplantation centers. To avoid biases in time to transplantation and risk factors that may have influenced the decision to recommend transplantation over chemotherapy, we used left-truncated multivariate Cox regression models to adjust these such that children receiving chemotherapy alone were alive at least as long at the interval from first relapse to transplantation. Potential prognostic factors such as duration of first remission, age, sex, and WBC count, and aspects of the transplantation regimen such as conditioning and GVHD prophylaxis were considered in Cox regression models allowing us to adjust for key prognostic factors and thereby allowing a controlled though not randomized comparison possible. Finally, the current study population is limited to children with B-precursor ALL who achieved a second remission and the role of transplantation may differ in children with T-cell ALL or B-precursor ALL who fail to achieve a second remission.
Data on cytogenetic abnormalities were available for only a third of the study population because these children received their therapy in an era when cytogenetic testing was not mandated. Further, we do not have detailed data on treatment received prior to first relapse. Consistent with treatment standards at the time these children received a 3- or 4-drug induction regimen, antimetabolite-based intensification regimen, triple intrathecal central nervous system prophylaxis, and maintenance therapy. Despite variations in up-front treatment regimens for ALL event-free survival rates are comparable across cooperative groups. Unmeasured and unknown factors may bias the results of any observation study and ours is no exception.
In summary, HLA-matched sibling donor transplants with TBI-containing regimens offer a clear advantage in children with early relapse and B-precursor ALL who achieve a second remission. Because this advantage is not extended to those who with a late relapse, efforts should focus on limiting the role of transplantation in these groups of children. Further, transplantation should not occur with non-TBI regimens, because outcomes are clearly inferior to that after chemotherapy alone or transplantation with TBI-containing regimens.
Appendix
Participating CIBMTR transplant centers: Alberta Children's General Hospital, Calgary, AB, Canada; Auckland City Hospital, Auckland, New Zealand; British Columbia's Children's Hospital, Vancouver, BC, Canada; Cancer Care Manitoba, Winnipeg, MN, Canada; Cardinal Glennon Children's Hospital, St. Louis, MO; Carlos Haya Regional Hospital, Malaga, Spain; Centre Hospitalier Universitaire Huriez, Lille, France; Children's Hospital at Westmead, Westmead, Australia; Children's Hospital of Los Angeles, Los Angeles, CA; Children's Hospital of Philadelphia, Philadelphia, PA; Children's Memorial Hospital, Chicago, IL; Children's Mercy Hospitals and Clinics, Kansas City, MO; Children's University Hospital, Brussels, Belgium; Christchurch Hospital, Christchurch, New Zealand; Cincinnati Children's Hospital Medical Center, Cincinnati, OH; Columbus Children's Hospital, Columbus, OH; Cook Children's Medical Center, Forth Worth, TX; Duke University Medical Center, Durham, NC; Great Ormond Street Hospital for Children, London, England; Hannover Medical University, Hannover, Germany; Hospital de Pediatria, S.A.M.I.C., Buenos Aires, Argentina; Hospital for Sick Children, Toronto, Ontario, Canada; Hospital Infantil Vall d'Hebron, Barcelona, Spain; Hospital La Fe, Valencia, Spain; Hospital Privado do Cordoba, Cordoba, Argentina; Hospital Puerta de Hierro, Madrid, Spain; Hospital Santa Creu I Sant Pau, Barcelona, Spain; Huddinge University Hospital, Stockholm, Sweden; Instituto Portugues de Oncologia, Lisbon, Portugal; Institutos Medicos Antartida ICTEM, Hospital Privado, Buenos Aires, Argentina; Jean Minjoz Hospital, Besancon, France; Johns Hopkins University School of Medicine, Baltimore, MD; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia; King Faisal Specialist Hospital and Research Center-Pediatrics, Riyadh, Saudi Arabia; Leiden Pediatrics, Leiden, Netherlands; Louisiana State University Children's Hospital, New Orleans, LA; Loyola University Medical Center, Maywood, IL; McMaster University Medical Center, Hamilton, Ontario, Canada; Medical College of Virginia, Richmond, VA; Medical University of Vienna, Vienna, Austria; Miami Children's Hospital, Miami, FL; Montreal Children's Hospital, Montreal, Canada; NCI Cairo University, Cairo, Egypt; Princess Margaret Hospital for Children, Perth, Australia; Rainbow Babies and Children's Hospital, Cleveland, OH; Rocky Mountain Cancer Center-Denver, Denver, CO; Roswell Park Cancer Institute, Buffalo, NY; Royal Children's Hospital, Parkville, Australia; Royal Children's Hospital, Brisbane, Australia; Royal Free Hospital and School of Medicine, London, England; Royal Hospital for Sick Children, Glasgow, Scotland; Royal Marsden Hospital, Surrey, England; Royal Perth Hospital, Perth, Australia; Sainte-Justine Hospital, Montreal, QC, Canada; Sanatorio Allende, Cordoba, Argentina; Shands Hospital at the University of Florida, Gainesville, FL; SPb State I. Pavlov Medical University, St. Petersburg, Russia; St. Anna Children's Hospital, Vienna, Austria; St. Bartholomew's and the Royal London Hospital, London, England; St. George's Hospital Medical School, London, England;
St. James Hospital, Dublin, Ireland; St. Joseph's Hospital, Patterson, NJ; St. Louis Children's Hospital, St. Louis, MO; St. Louis Hospital, Paris, France; Stanford University Medical Center, Stanford, CA; Starship Children's Health, Auckland, New Zealand; Sydney Children's Hospital, Sydney, Australia; Tata Memorial Hospital, Parel, India; The Catholic University of Korea, Seoul, South Korea; The Chinese University of Hong Kong, Shatin, Hong Kong; The Queen Sylvia Children's Hospital, Goteburg, Sweden; University Children's Hospital, Tubingen, Germany; University Federal do Parana, Curitiba, Brazil; University Hospital, Basel, Switzerland; University Hospital, Zurich, Switzerland; University Hospital Motol, Prague, Czech Republic; University of Alabama at Birmingham, Birmingham, AL; University of Bologna Institute of Hem/Onc, Bologna, Italy; University of California Children's Hospital, San Franciso, CA; University of Copenhagen, Copenhagen, Denmark; University of Hamburg-Eppendorf, Hamburg, Germany; University of Kansas Medical Center, Kansas City, KS; University of Louisville Hospital, Louisville, KY; University of Lund, Lund, Sweden; University of Malaya Hospital, Kuala Lumpur, Malaysia; University of Minnesota, Minneapolis, MN; University of Munich, Munich, Germany; University of Nebraska Medical Center, Omaha, NE; University of North Carolina Hospitals, Chapel Hill, NC; University of Oklahoma Health Sciences Center, Oklahoma City, OK; University of Ulm, Ulm, Germany; University of Wisconsin Hospital and Clinics, Madison, WI; West Virginia University Hospitals, Morgantown, WV; Zentrum fur Knochmarktransplantation, Wiesbaden, Germany.
Participating COG institutions: Advocate Hope Children's Hosp, Oaklawn, IL; Alberta Children's Hospital, Calgary, AL, Canada; All Children's Hospital, St. Petersburg, FL; Baylor College of Medicine, Dallas, TX; Body School of Medicine at East Carolina University, Greenville, NC; Boston Floating Hospital for Infants and Children, Boston, MA; Cancer Researh Center of Hawaii, Honolulu, HI; Carolinas Medical Center, Charlotte, NC; Centre Hospitalier Universitaire de Quebec, Sainte-Foy, QC, Canada; Children's Hospital of Eastern Ontario, Ottawa, ON, Canada; Children's Hospital of the Greenville System, Greenville, SC; Children's Hospital Michigan, Detroit, MI; Children's Hospital New Orleans, New Orleans, LA; Children's Memorial Medical Center, Chicago, IL; Children's Health Care of Atlanta, Emory University, Atlanta, GA; Children's Hospital San Diego, San Diego, CA; Virginia Commonwealth University, Richmond, VA; Cook Children's Medical Center, Fort Worth, TX; Cook County Children's Hospital, Chicago, IL; Dana-Farber Cancer Institute, Boston, MA; Dartmouth Hitchcock Medical Center, Lebanon, NH; Duke University, Durham, NC; East Tennesse State University, Johnson City, TN; Eastern Maine Medical Center, Bangor, ME; Emanuel Hospital-Health Center, Portland, OR; Hackensack Medical Center, Hackensack, NJ; Hospital for Sick Children, Toronto, ON, Canada; Hurley Medical Center, Flint, MI; Inova Fairfax Hospital, Fairfax, VA; Joe DiMaggio Children's Hospital, Hollywood, FL; Johns Hopkins University, Baltimore, MD; Kaiser Foundation Research Institute, Oakland, CA; Medical University of South Carolina, Charleston, SC; Madigan Army Medical Center, Tacoma, WA; Maine Children's Cancer Program, Scarborough, ME; McGill University Health Center, Montreal, QC, Canada; Miami Children's Hospital, Miami, FL; Midwest Children's Cancer Center, Milwaukee, WI; Mount Sinai Medical School, New York, NY; Naval Medical Center, Portsmouth, VA; Nemours Children's Clinic, Jacksonville, FL; Nemours Children's Clinic, Orlando, FL; Oklahoma University, Oklahoma City, OK; Presbyterian Hospital, Charlotte, NC; Rhode Island Hospital, Providence, RI; Roswell Park Cancer Institute, Buffalo, NY; Rush-Presbyterian St Luke's Medical Center, Chicago, IL; Sacred Heart Hospital, Pensacola, FL; Schneider Children's Hospital, New Hyde Park, NY; Scott and White Memorial Hospital, Temple, TX; Swiss Pediatric Oncology Group, Bern, Switzerland; Swiss Pediatric Oncology Group, Geneva, Switzerland; Swiss Pediatric Oncology Group, Lausanne, Switzerland; St John Hospital, Grosse Pointe, MI; St. Christopher's Hospital, Philadelphia, PA; St. Vincent Children's Hospital, Indianapolis, IN; Stanford University Medical Center, Stanford, CA; Stollery Children's Hospital, Edmonton, AB, Canada; SUNY Upstate Medical University, Syracuse, NY; Sutter Medical Center, Sacramento, CA; Tampa Children's Hospital, Tampa, FL; University of Alabama, Birmingham, AL; University of Arizona, Tucson, AZ; University of Arkansas, Little Rock, AR; University of Florida, Gainesville, FL; University of Kansas, Kansas City, KS; University of Maryland, Baltimore, MD; University of Miami, Miami, FL; University of Missippi Medical Center, Jackson, MS; University of South Florida, Tampa, FL; University of South Alabama, Mobile, AL; University of Vermont, Burlington, VT; University of Virginia, Charlottesville, VA; University of California, Davis, CA; University of Missouri - Columbia, Columbia, MO; University of Texas Southwestern Medical Center, Dallas, TX; University of Texas Medical Center, Galveston, TX; University of Texas Health Sciences Center, San Antonio, TX; Via Christi Regional Medical Center, Wichita, KS; Wake Forest University School of Medicine, Winston-Salem, NC; Walter Reed Army Medical Center, Washington, DC; Warren Clinic, Tulsa, OK; Washington University, St. Louis, MO; Wilford Hall Medical Center, Lackland, TX; West Virginia University HSC, Charleston, WV; West Virginia University HSC, Morgantown, WV; Yale University, New Haven, CT.
Prepublished online as Blood First Edition Paper, February 21, 2006; DOI 10.1182/blood-2005-12-4942.
A complete list of the participating members of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research is provided in the “Appendix.”
Supported by Public Health Service Grant U24-CA76518-08 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute and U10-CA098543 from the National Cancer Institute and the Department of Health and Human Services.
M.E., E.R., M.-J.Z., M.D., B.C., W.L.C., and S.M.D. contributed equally to the conception, design, and interpretation of data and the final manuscript; T.A., A.B., and A.H. contributed to the interpretation of data and final manuscript; M.-J.Z. and C.M. did the statistical analysis; and M.E. had primary responsibility for drafting the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors declare that there are no conflicts of interest.