Imatinib (Glivec; Novartis, Basel, Switzerland), specifically inhibiting ABL kinase, has evidenced an important role in chronic myeloid and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemias (ALLs); however, resistance is increasingly encountered,1,2 primarily mediated by mutations within the kinase domain of BCR-ABL that interfere with drug binding.3,4
The second generation of tyrosine kinase inhibitors, which include BMS-354825 (Dasatinib; Bristol Myers Squibb, Princeton, NJ), also target ABL,5 but they appear able to bind also the majority of mutated forms of the protein.6
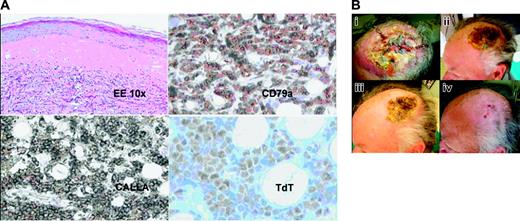
Cutaneous response to BMS-354825. (A) Skin biopsy shows cutaneous involvement by precursor B cells; neoplastic cells diffusely infiltrate the dermis. Hematoxylin-eosin staining. Low magnification, × 10. At immunohistochemistry, the lymphoblasts express B-cell markers as CD79a, precursor B-cell marker CD10-CALLA, and show nuclear staining for TdT. Images were obtained with a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) with 10 ×/0.25 NA and 40 ×/0.65 NA brightfield objectives, acquired with a JVC TK 1270 color video camera (JVC, Tokyo, Japan), and AVer E-Z Capture software (AVerMedia, Milpitas, CA). Images were processed with Paint Shop Pro 7.0 (Jase Software, Eden Prairie, MN). CD79a, CALLA, and TdT are from Dako (Glostrup, Denmark). (B) Patient with cutaneous infiltrate of acute lymphoblastic leukemia resistant to previous treatments. In panel Bi, presence of multiple dermohypodermal nodules with a central ulceration pre–BMS-354825 therapy. In panels Bii-iii the same lesion 1 and 2 weeks after starting therapy. Four weeks after treatment, the scalp lesion was completely healed with only the presence of a hypopigmentated scar (Biv). Pictures were taken using a Panasonic Lumix 2MC-FX5 (Panasonic, Osaka, Japan) digital camera and processed with Paint Shop Pro 7.0.
Cutaneous response to BMS-354825. (A) Skin biopsy shows cutaneous involvement by precursor B cells; neoplastic cells diffusely infiltrate the dermis. Hematoxylin-eosin staining. Low magnification, × 10. At immunohistochemistry, the lymphoblasts express B-cell markers as CD79a, precursor B-cell marker CD10-CALLA, and show nuclear staining for TdT. Images were obtained with a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) with 10 ×/0.25 NA and 40 ×/0.65 NA brightfield objectives, acquired with a JVC TK 1270 color video camera (JVC, Tokyo, Japan), and AVer E-Z Capture software (AVerMedia, Milpitas, CA). Images were processed with Paint Shop Pro 7.0 (Jase Software, Eden Prairie, MN). CD79a, CALLA, and TdT are from Dako (Glostrup, Denmark). (B) Patient with cutaneous infiltrate of acute lymphoblastic leukemia resistant to previous treatments. In panel Bi, presence of multiple dermohypodermal nodules with a central ulceration pre–BMS-354825 therapy. In panels Bii-iii the same lesion 1 and 2 weeks after starting therapy. Four weeks after treatment, the scalp lesion was completely healed with only the presence of a hypopigmentated scar (Biv). Pictures were taken using a Panasonic Lumix 2MC-FX5 (Panasonic, Osaka, Japan) digital camera and processed with Paint Shop Pro 7.0.
We present the case of a patient with relapsed/refractory Ph+ ALL with disease at extramedullary (cutaneous) and blood level treated with BMS-354825 who achieved complete response in a very short period of time. This 67-year-old male patient was diagnosed with Ph+ ALL (70% Ph+ metaphases, p190 transcript) CD10/CD19/CD38+, in September 2002. He was treated with induction chemotherapy and received prophylactic intrathecal methotrexate, achieving complete remission. In March 2003, he was started on maintenance treatment with 400 mg imatinib per day. In July 2004, he complained of severe headache refractory to nonsteroidal anti-inflammatory drugs; a lumbar puncture demonstrated the presence of CD10/CD19/CD38+ lymphoblastic cells. At this time bone marrow revealed no blasts; karyotype, fluorescence in situ hybridization (FISH) and nested polymerase chain reaction (PCR) results were normal. He was given standard intrathecal treatment without the use of Ommaya reservoir, and imatinib was escalated to 600 mg per day. In October 2004 restaging demonstrated remission on both central nervous system (CNS) and bone marrow; thus, imatinib was reduced to 400 mg per day. In March 2005, the patient lost cytogenetic response and presented with a large, indurated, and ulcerated lesion of the scalp. The cutaneous lesion had developed very quickly (10 days), starting with multiple dermohypodermal reddish and painful nodules that conflated into a plaque, rapidly evolving to a central ulceration. Histologic examination showed a diffuse infiltration of the dermis by monomorphic proliferation of small- to intermediate-sized cells with scanty cytoplasm, irregular round to oval nuclei, finely dispersed chromatin, and indistinct nucleoli. Immunohistochemically, these cells expressed CD79a, CD20, and CD10, and showed nuclear positivity for TdT (terminal deoxynucleotidyl transferase), which was consistent with cutaneous infiltrate of precursor B lymphoblastic leukemia (Figure 1A). No superinfection on the area was evidenced. High-dose steroids had no effect. He was started on BMS-3548257,8 on April 12, 2005, with 45% blasts in bone marrow, 8 of 20 Ph+ metaphases, and the persistence of the large lesion on the scalp (Figure 1B).
On days 8 and 15, the skin lesion was much reduced, with disappeareance of the erythema and beginning of the healing of the central ulceration (Figure 1Bii-iii). On May 16, 2005, after 4 weeks of treatment with BMS-354825, we evidenced the total remission of the scalp lesion that was completely healed with a hypopigmentated scar (Figure 1Biv), while the restaging demonstrated complete hematologic and cytogenetic response.
After 5 months of treatment the patient was doing well and in complete remission with no signs of extramedullary disease in skin or CNS. Previous reports have described chronic-phase CML patients with extramedullary (CNS and skin) blastic infiltrates during imatinib therapy, suggesting an incomplete drug penetrance9,10 at this sites. This report suggests a specific and strong activity of BMS-354825 at the cutaneous level.