Abstract
Deficient activation of apoptosis signaling pathways may be responsible for treatment failure in acute leukemia. Here, we address the impact of intact apoptosis signaling in 78 patients with pediatric precursor B-cell acute lymphoblastic leukemia (ALL) by analysis of 2 key apoptogenic events: caspase-3 activation and cytochrome c release in leukemia cells cultured in vitro. Both events correlated only in the group of patients who had a good response and patients in continuous remission, suggesting that intact apoptosis signaling is a characteristic for favorable outcome. By combining both parameters, we identified a novel indicator, cytochrome c–related activation of caspase-3 (CRAC). CRAC directly connects the extent of caspase-3 activation to cytochrome c release in single cells in an individual patient sample. In CRAC-positive patients, indicating proficient apoptosis signaling, the number of persisting leukemia cells on day 15 was significantly lower than in the CRAC-negative patient group (n = 27, mean 6.0% versus n = 36, mean 22.6%; P = .003). At a median follow-up of 31 months, disease-free survival was 84 months (95% CI = 76 to 91 months) and 66 months (95% CI = 52 to 80 months) for patients with positive and negative CRAC, respectively (P = .019). CRAC may serve as a functionally defined risk factor for treatment stratification.
Introduction
Apoptosis or programmed cell death is the physiologic process by which multicellular organisms regulate tissue homeostasis by elimination of damaged or superfluous cells.1,2 Surface receptors, proteases, the endoplasmic reticulum, and mitochondria are among the cellular compartments involved in the apoptotic process.3-6 Mitochondria integrate diverse apoptotic stimuli such as death receptor signaling, factor withdrawal, and cytotoxic stress, and regulate the final execution of the cell death program.7 A hallmark of mitochondrial apoptosis signaling is the mitochondrial release of apoptogenic factors, in particular cytochrome c.8 Upon release into the cytosol, cytochrome c forms a multimeric complex with Apaf-1 and caspase-9, termed apoptosome.9,10 As a result of this process, caspase-3 becomes either caspase-9 dependent or autocatalytically cleaved11 and in turn mediates most features of apoptosis, such as DNA fragmentation via activation of the caspase-dependent DNAse CAD and cellular disintegration.12 Several regulatory elements of apoptosis signaling converge on mitochondria. While molecules such as Bcl-2 inhibit mitochondria,13 proximal caspase activation may initiate or amplify mitochondrial apoptosis signaling either directly by caspase-214 or via caspase-mediated truncation of Bid.15 The contribution of caspase-dependent or -independent mitochondrial activation to the efficient execution of cell death is still poorly defined.16
Defects in apoptosis signaling pathways play an essential role in leukemogenesis. Since cytotoxic drugs used in anticancer chemotherapy induce the physiologic cell death program by activating apoptotic molecules,17-19 apoptosis defects have also been implicated in the development of drug resistance and treatment failure in acute leukemia.20,21 Pursuing this hypothesis, several studies have been performed to analyze the expression of apoptosis regulators with respect to their impact on treatment outcome. Molecules analyzed included death receptors,22-27 Bcl-2 family members,23,28-37 caspases,38,39 and inhibitors of apoptosis proteins (IAPs).40,41 So far, most of these studies could not establish a significant correlation to treatment response and prognosis22-24,28,30,39 or resulted in contradictory findings.32,33,36-41 Since the efficacy of apoptosis signaling may not be sufficiently represented by the expression of apoptosis molecules alone, we previously developed a novel approach to assess the functional integrity of apoptosis signaling by simultaneous measurement of 2 apoptogenic events in individual cells: caspase-3 activation and cytochrome c release.42,43 We analyzed the activation of apoptosis signaling in primary leukemia cells during apoptosis induction by the physiologic stimulus of lack of survival factors in order to identify constitutive defects in apoptosis signaling in individual leukemia samples.
Activation and mutual correlation of cytochrome c release and caspase-3 activation were quantified in 78 patient samples of precursor B-cell acute lymphoblastic leukemia (ALL). We found that an intact connection of cytochrome c release and caspase activation is important for efficient remission induction treatment and maintenance of remission in childhood precursor B-cell ALL. We identified a novel parameter, cytochrome c–related activation of caspase-3 (CRAC), reflecting proficient or deficient cytochrome c–related caspase activation in the individual patient sample with prognostic impact on treatment failure and relapse.
Patients, materials, and methods
Patient characteristics and treatment
Selection of samples for the study was based solely on the availability of frozen material, and the clinical data were blinded to the investigators until the experiments had been completed. Samples were obtained at diagnosis from 78 pediatric patients with precursor B-cell ALL (pro-B ALL, n = 3, 3.8%; common ALL, n = 50, 64.1%; and pre-B ALL, n = 25, 32.1%). The patients' age in the study group ranged from 3 months to 17 years, with a mean of 6.6 years. All patients were treated according to the ALL-BFM-95 or -2000 protocols (standard-risk group, n = 26, 33.3%; medium-risk group, n = 35, 44.9%; and high-risk group, n = 17, 21.8%). Response to induction therapy was assessed on days 8, 15, and 33 of treatment. Initial response to treatment was defined by the number of leukemic blasts in peripheral blood (PB) after one week of prednisone and one initial dose of intrathecal methotrexate: If fewer than 1000 blast cells per μL peripheral blood were detected by then, treatment response was considered adequate (good response to prednisone) on day 8 after prednisone treatment. Blast cell persistence on day 15 and nonresponse at the end of induction therapy on day 33 were defined by more than 5% blast cells in the bone marrow. Relapse was diagnosed according to the criteria of the ALL-BFM-95 and -2000 studies. Risk groups were defined according to criteria of the ALL-BFM trials, consisting of white blood cell (WBC) count, age, initial response to treatment, and cytogenetic features. Informed consent was given by the patients and/or their parents in accordance with the Declaration of Helsinki in its most recent version. Both study protocols (ALL-BFM 95 and ALL-BFM 2000) were approved by the Ethics Review Committee of the Medical School of Hannover, which served as the central review committee. Local institutional review boards at participating institutions also approved the study protocols.
Cell culture
Cryopreserved mononuclear blood cells were thawed and controlled for viability by trypan blue exclusion. Cells were cultured for 16 hours following standard culture conditions in RPMI 1640 (Life Technologies, Eggenstein, Germany) with 10% fetal calf serum (FCS; Sigma Chemicals, Deisenhofen, Germany), together with penicillin, streptomycin, and glutamine (Biochrome, Berlin, Germany) at 37°C in humidified air/5% CO2 with or without the pan-caspase inhibitor zVAD-fmk at 100-μM concentration (Alexis Biochemicals, Lausen, Switzerland).
Several reports have shown that these culture conditions provide an apoptotic stimulus for primary B-cell precursor (BCP) ALL cells similar to factor withdrawal in growth factor–dependent cell lines. In contrast to cell lines, viability of BCP ALL cells in vitro cannot be maintained by soluble factors or serum but is strongly dependent on cell-cell interaction provided by cocultured cells.44,45 Lack of cell-mediated survival signals such as in our culture conditions therefore initiates the apoptosis cascade via a “death by default” mechanism in BCP ALL cells and thereby facilitates the analysis of the cells' constitutive apoptosis program.
Cell staining and flow cytometry
In order to quantify apoptosis signaling in leukemia cells, cells were first stained with antibodies against leukemia cell surface markers CD19 (or CD10) and CD34 (peridinine chlorophyll protein– or allophycocyanin-conjugated antibodies; BD Pharmingen, Heidelberg, Germany). Thereafter, the cells were fixed with 4% PFA and permeabilized with saponine 0.2% (Sigma Chemicals) in order to measure intracellular levels of cytochrome c and active caspase-3. Caspase-3 activation was detected by binding of the antibody to the active caspase-3 fragment (anti–active caspase-3–phycoerythrin (PE) conjugated; BD Pharmingen).46 Cellular cytochrome c was assessed by intracellular staining with the anti–cytochrome c–directed antibody 7H8.2C12 (BD Pharmingen) detected by a specific secondary goat anti–mouse IgG2b fluorescein isothiocyanate (FITC)–conjugated antibody (Southern Biotechnology Associates, Birmingham, AL). Binding of the 7H8.2C12 antibody is conformation dependent and specific for mitochondria-bound cytochrome c. Release of cytochrome c into the cytosol results in a reduced signal for cellular cytochrome c as previously described.43 As negative staining control, cells were stained with isotype-matched fluorochrome-conjugated antibodies of irrelevant specificity (BD Pharmingen; and Dako, Hamburg, Germany). Flow cytometric analysis was conducted using a FACSCalibur flow cytometer and Cell Quest software (both from BD Bioscience, Heidelberg, Germany).
Quantification of cell death, cytochrome c release, and active caspase-3 fragment
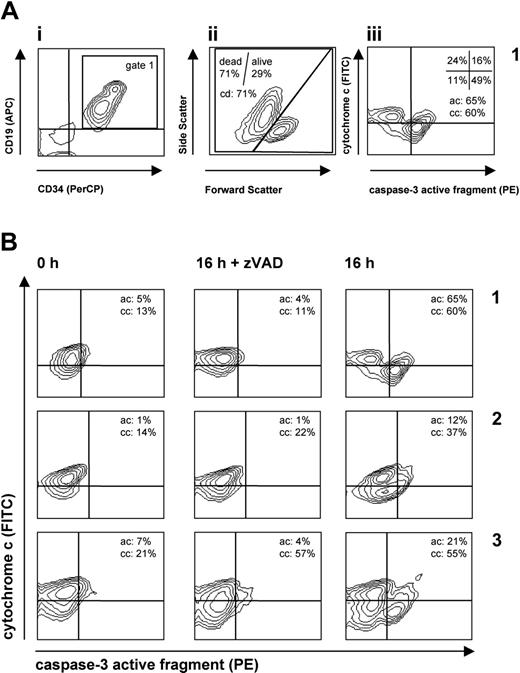
The flow cytometric gating strategy and quantification of apoptosis parameters are illustrated in Figure 1A. In brief, leukemia cells were identified by gating on events positive for leukemia markers (Figure 1Ai), and further analysis was performed on the cells within this gate. Cell death (cd) was quantified by forward/side scatter criteria (Figure 1Aii). Caspase activation (ac) and cytochrome c release (cc) were quantified in the caspase-3 versus cytochrome c plot (Figure 1Aiii). All experiments have been performed in triplicate, and mean values were used for further calculation and analysis (Figure 1B). In the calculation of the cell death and cytochrome c release, we discriminated caspase-dependent and -independent events. Caspase-independent cytochrome c release (ccindep) was calculated as the difference of the cc values after incubation for 16 hours (in the presence of zVAD) and of the cc value prior to incubation (at 0 hour). Caspase-dependent cytochrome c release (ccdep) was calculated as the difference between the cc values obtained after incubation in the absence and in the presence of zVAD. The sum of ccindep and ccdep provided the total extent of cytochrome c release (cctotal). The cell death, both caspase dependent and independent, as well as its total extent was calculated in the same manner. In contrast to the cytochrome c release and cell death, activation of caspase-3 is a totally caspase-dependent event.11 Therefore, we considered only the zVAD inhibitable caspase activation and calculated the extent of caspase-3 activation as the difference between the ac values obtained after incubation in the absence and in the presence of zVAD. Figure 1B provides examples of the described quantification procedure.
Statistical analysis
Experiments were performed in triplicate and differences tested for significance by t test. In order to characterize apoptosis in leukemic samples in the context of clinical data, continuous parameters (percentage of cell death, caspase activation, cytochrome c release) and categoric parameters (prednisone response, good/poor; CR on day 33, yes/no; relapse, yes/no; CRAC, positive/negative) were analyzed. The correlation between continuous variables was analyzed using bivariate nonparametric Spearman correlation statistics. For the analysis of correlation between 2 categoric variables, the Fisher exact test (contingency table) was applied. For analysis of a categoric parameter (eg, prednisone response, yes/no) and a continuous parameter (eg, percent caspase activation), the independent samples t test was used, which tests for differences in 2 groups on the basis of the mean and the distribution of all values. In addition, the standard error of the mean is given.
Relapse-free survival was estimated by Kaplan-Meier analysis. Differences in survival were tested for significance by the log-rank test. Differences were considered significant for P < .05. Statistical analysis was performed using SPSS 11.02 software (SPSS, Munich, Germany).
Quantification of distinct patterns of apoptosis signaling in leukemia cells. Primary leukemia cells from individual patients were analyzed before (0 h) and after 16 hours in culture with or without zVAD-fmk in triplicate. In order to quantify apoptosis signaling in leukemia cells, cells were stained with antibodies against leukemia markers CD19 (or CD10) and CD34 and against cytoplasmic caspase-3 and cytochrome c. (A) Gating strategy and quantification of apoptosis events. In order to identify the leukemia cell population and to exclude debris, a gate was set on the population positive for leukemia marker (i, gate 1). In this gate, cell death (cd) was assessed by forward/side scatter criteria (ii). In the same gate (gate 1), cytochrome c release (cc) was measured simultaneously with caspase-3 activation (ac) (iii). Cells with activated caspase-3 were quantified as percent of cells in the right (top + bottom) quadrants (ac = 65% in the example shown). Cells with released cytochrome are identified by reduction of the cytochrome c signal and calculated as percent of cells in the bottom (left + right) quadrants (cc = 60% in the example shown). (B) Distinct patterns of apoptosis signaling in 3 prototype leukemia patient samples (samples 1-3). Flow cytometric plots depict cytochrome c versus caspase-3 staining in individual samples, and mean values of triplicate measurements are indicated. From these measurements, the extent of cytochrome c release (caspase-dependent, ccdep; caspase-independent, ccindep; total, cctotal) and of caspase-3 activation (actotal) was calculated as described in “Patients, materials, and methods.” The following values are for examples A, B, and C: for ccindep, –2% (t test not significant compared with control 0 h), 8% (P < .01), and 36% (P < .01), respectively; for ccdep, 49% (P < .01), 15% (P < .01), and –2% (not significant), respectively; for cctotal (ccindep + ccdep), 47% (P < .01), 23% (P < .01), and 34% (P < .01), respectively; and for actotal, 61% (P < .01), 11% (P < .01), and 17% (P < .01), respectively. Calculated differences between percentages of actotal and cctotal (CRAC; “Results”) are +14%, –12%, and –17%, thus indicating proficient (case A) and deficient (cases B-C) apoptosis signaling in leukemia cells.
Quantification of distinct patterns of apoptosis signaling in leukemia cells. Primary leukemia cells from individual patients were analyzed before (0 h) and after 16 hours in culture with or without zVAD-fmk in triplicate. In order to quantify apoptosis signaling in leukemia cells, cells were stained with antibodies against leukemia markers CD19 (or CD10) and CD34 and against cytoplasmic caspase-3 and cytochrome c. (A) Gating strategy and quantification of apoptosis events. In order to identify the leukemia cell population and to exclude debris, a gate was set on the population positive for leukemia marker (i, gate 1). In this gate, cell death (cd) was assessed by forward/side scatter criteria (ii). In the same gate (gate 1), cytochrome c release (cc) was measured simultaneously with caspase-3 activation (ac) (iii). Cells with activated caspase-3 were quantified as percent of cells in the right (top + bottom) quadrants (ac = 65% in the example shown). Cells with released cytochrome are identified by reduction of the cytochrome c signal and calculated as percent of cells in the bottom (left + right) quadrants (cc = 60% in the example shown). (B) Distinct patterns of apoptosis signaling in 3 prototype leukemia patient samples (samples 1-3). Flow cytometric plots depict cytochrome c versus caspase-3 staining in individual samples, and mean values of triplicate measurements are indicated. From these measurements, the extent of cytochrome c release (caspase-dependent, ccdep; caspase-independent, ccindep; total, cctotal) and of caspase-3 activation (actotal) was calculated as described in “Patients, materials, and methods.” The following values are for examples A, B, and C: for ccindep, –2% (t test not significant compared with control 0 h), 8% (P < .01), and 36% (P < .01), respectively; for ccdep, 49% (P < .01), 15% (P < .01), and –2% (not significant), respectively; for cctotal (ccindep + ccdep), 47% (P < .01), 23% (P < .01), and 34% (P < .01), respectively; and for actotal, 61% (P < .01), 11% (P < .01), and 17% (P < .01), respectively. Calculated differences between percentages of actotal and cctotal (CRAC; “Results”) are +14%, –12%, and –17%, thus indicating proficient (case A) and deficient (cases B-C) apoptosis signaling in leukemia cells.
Results
Activation of apoptosis signaling pathways in primary leukemia cells
Primary leukemia samples from 78 patients were analyzed for constitutive response to the lack of cellular survival factors as a proapoptotic stimulus. We focused on 2 well-established critical steps in the apoptosis cascade: cytochrome c release as an indicator of mitochondrial apoptogenic function and caspase-3 activation as the most important effector caspase integrating upstream signals. The apoptosis parameters measured in this study and their assignment to the corresponding steps of pathway activation are indicated in Figure 2. Analysis of apoptosis, cytochrome c release, and caspase activation in individual cells revealed several distinct patterns of pathway activation (Figure 1B). In one fraction of samples exemplified by patient 1, factor withdrawal induced simultaneous release of cytochrome c and caspase-3 activation seen as a single prominent population of cells in the lower-right quadrant. A different pattern was observed in leukemia samples represented by samples 2 and 3, in which cytochrome c was released without concomitant caspase-3 activation, resulting in a predominant population of cells in the lower-left quadrant. In order to address the question whether cytochrome c release is caspase dependent or independent, caspase-3 activation and cytochrome c release were analyzed under conditions of caspase inhibition by the pan-caspase inhibitor zVAD-fmk. As shown in Figure 1B, caspase-3 activation could be completely blocked by the caspase inhibitor, while cytochrome c release was either dependent on caspase activation (sample 1), partially dependent (sample 2), or completely independent of caspase activation (sample 3). Taken together, different patterns of pathway activation could be identified by simultaneous quantification of caspase-3 activation and cytochrome c release in individual samples.
Cell death parameters and treatment response
Next we correlated the results obtained in the functional assays to clinical data such as long-term survival and initial response to treatment assessed by clearance of leukemia cells from peripheral blood or bone marrow on days 8, 15, or 33 of treatment. The distribution of values for cell death (cd), caspase activation (ac), and cytochrome c release (cc) was compared in the patients grouped according to good or poor treatment response (Table 1). The independent samples t test was applied, which tests for differences in 2 groups on the basis of the mean and the distribution of all values. In order to further illustrate the distribution of the values, the standard error of the mean is provided in Table 1. No significant differences for cell death or cytochrome c release parameters with respect to initial treatment response or long-term survival could be detected in the patient groups. Caspase activation was higher in patients who did not encounter relapse (P = .010) and in patients with a blast cell proportion of less than 5% in the bone marrow on day 15 (mean, 27.8% vs 18.2%; P = .013). However, there were no differences between patients with a good response and those with a poor response on days 8 and 33.
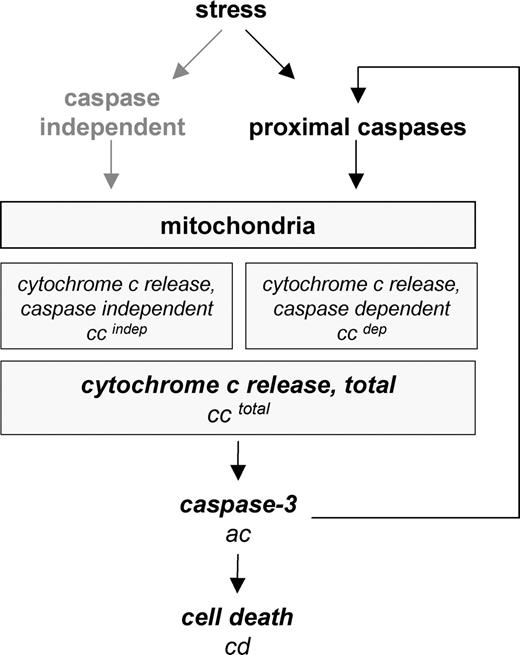
Parameters analyzed in the study and their attribution to distinct steps of apoptosis signal transduction. Mitochondria are activated with or without involvement of caspases. Caspase-mediated mitochondrial activation may occur either by amplification of the apoptotic signal by proximal caspase activation (eg, via caspase-8 and t-Bid) or by direct mitochondrial activation through initiator caspases (eg, caspase-2). Release of cytochrome c is followed by activation of effector caspases such as caspase-3 through the formation of the apoptosome. Assessed parameters are in italics.
Parameters analyzed in the study and their attribution to distinct steps of apoptosis signal transduction. Mitochondria are activated with or without involvement of caspases. Caspase-mediated mitochondrial activation may occur either by amplification of the apoptotic signal by proximal caspase activation (eg, via caspase-8 and t-Bid) or by direct mitochondrial activation through initiator caspases (eg, caspase-2). Release of cytochrome c is followed by activation of effector caspases such as caspase-3 through the formation of the apoptosome. Assessed parameters are in italics.
Good response to antileukemic treatment is associated with an intact correlation of cytochrome c release and caspase-3 activation
Since single apoptosis parameters were only weakly linked with initial response and long-term survival, we analyzed whether caspase-3 activation occurred together with cytochrome c release. We hypothesized that a concomitant activation of caspase-3 and cytochrome c release indicating intact apoptosis signaling is associated with good response to treatment. In the whole patient group, both parameters, caspase-3 activation and total cytochrome c release, were significantly correlated to each other (Table 2), indicating that, in general, caspase-3 activation and cytochrome c release are functionally linked. We next analyzed whether this association could also be detected in the different response groups. Simultaneous caspase-3 activation and cytochrome c release were identified in all patient groups with good response to remission induction treatment (days 8, 15, and 33) as well as in the patients who did not encounter relapse (Table 2, left column). Of interest, the link between caspase-3 activation and cytochrome c release was completely lost in the initially poor-responding patient groups at all 3 time points as well as in relapse patients. Thus, an intact link of cytochrome c release and caspase-3 activation indicating a functional apoptosome is a discriminating feature for patient groups with good response to treatment.
Based on different activation of apoptosis signaling pathways, cytochrome c release is either independent of or dependent on caspase activation. We therefore analyzed cytochrome c release under the condition of caspase inhibition by zVAD-fmk. We were especially interested whether the prognostic value of an intact link between cytochrome c release and caspase-3 activation is true for caspase-dependent or -independent cytochrome c release. Caspase-dependent, but not caspase-independent, cytochrome c release was found to be significantly correlated to caspase-3 activation in the patient groups with a good response (Table 2, middle and right column). Compared with the analysis of total cytochrome c release (Table 2, middle and left column), higher coefficients of correlation (rs) and higher levels of significance were found. Thus, we found an intact connection of caspase-dependent cytochrome c release to caspase-3 activation to be a general feature of successful treatment.
Efficient cytochrome c–related caspase activation predicts good response to initial treatment and relapse-free survival
Analysis of the prognostic impact of cytochrome c–related caspase activation requires the quantification of cytochrome c release and caspase-3 activation in individual patient samples. We identified a new parameter describing the efficient or deficient activation of caspase-3 in the presence of released cytochrome c (cytochrome c–related activation of caspase-3, CRAC) by calculating the difference of caspase-3 activation and cytochrome c release. In case of proficient apoptosis signaling, the values for cytochrome c release and caspase activation would be equal or caspase-3 activation would be higher than cytochrome c release. This results in a value of zero or higher for CRAC. Deficiency in the caspase activation despite cytochrome c release results in negative CRAC. The prototype samples in Figure 1B may serve to illustrate the calculation and meaning of CRAC. In sample 1, caspase-3 is easily activated as reflected by positive CRAC (+14%). In contrast, in samples 2 and 3 with incomplete caspase-3 activation upon cytochrome c release, CRAC is negative (B: –12%, C: –17%), indicating deficient apoptosis signaling.
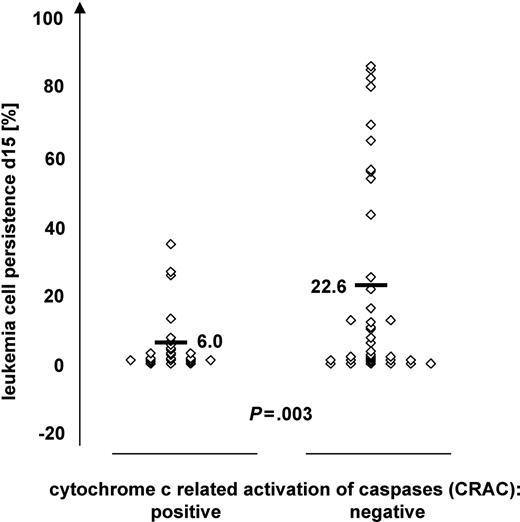
Patients were divided into a CRAC-positive (n = 35) and CRAC-negative (n = 43) group. Pretherapeutic characteristics such as sex, age, WBC count, immunophenotype, and cytogenetics were found to be equally distributed in the 2 groups, indicating that CRAC is independent of these known risk factors (Table S1A-B, available on the Blood website; see the Supplemental Table link at the top of the online article). With regard to treatment response characteristics (Table S1C), almost all prednisone poor responders at day 8 (10 of 12) and patients who did not achieve complete remission at day 33 (4 of 4) revealed CRAC-negative values (Fisher exact test, P = .056 and P = .123, respectively). The similar clear trend toward association of CRAC negativity with worse treatment response was observed for BM blast counts at day 15 (19 of 27 patients with blast count > 5%, P = .078). Moreover, using the data on blast counts as continuous variables (available only for BM at day 15), rather than arbitrary cut-offs for treatment response (1000 blasts/μL at day 8 in PB or 5% blasts at days 15 and 33 in BM), we observed strong quantitative differences between CRAC-positive and -negative cases. As shown in Figure 3, the CRAC-negative group had a significantly higher percentage of leukemia cells in bone marrow on day 15 compared with CRAC-positive patients (mean, 22.6 vs 6.0; P = .003).
In addition to the initial response to treatment, we analyzed the impact of CRAC on long-term survival. Assignment of the patients according to CRAC resulted in a proportion of 91.4% of relapse-free survival in CRAC-positive patients in contrast to 67.4% for patients with negative CRAC. Fourteen of 17 patients who encountered relapse were found to have negative CRAC (Fisher exact test, P = .013). In Kaplan-Meier analysis (median follow-up, 31.4 months), the mean survival of patients positive or negative for CRAC was 83.7 months (SE = 3.9; CI = 76.1 to 91.4) and 65.8 months (SE = 7.0; CI = 52.0 to 79.6), respectively (Table 3). The log-rank test revealed a significant difference in survival distribution (P = .019).
We also analyzed different risk groups of patients based on clinical characteristics at diagnosis, initial response to treatment, and cytogenetic risk factors. The distribution of patients with CRAC-positive and -negative values, respectively, was 14 versus 12 in the standard-risk, 17 versus 18 in the intermediate-risk, but 4 versus 13 in the high-risk group (Table 3). The predominance of CRAC-negative patients in the high-risk group probably reflects the connection of CRAC to initial treatment response, since the majority of high-risk patients were assigned to this group for poor response to initial treatment. In the 3 risk groups, the CRAC value also discriminated patients with relapse-free survival from those who encountered relapse. Due to a low patient number in the subgroups, these results did not reach significance. However, the constant pattern of superior survival in the CRAC-positive group suggests a potential additional prognostic value of CRAC.
Discussion
In the present study, we analyzed the function of apoptosis signaling pathways in primary leukemia cells in the context of response to antileukemic treatment and long-term survival. The aim of this study was to identify proficient or deficient apoptosis signaling by induction of apoptosis in vitro and subsequent analysis of the decisive steps of cytochrome c release and caspase-3 activation.
Classification of patients according to CRAC values in relation to leukemia cell persistence in bone marrow on day 15 of induction treatment. Patients (n = 63) were grouped according to CRAC, indicating proficient (CRAC positive, n = 27) or deficient (CRAC negative, n = 36) cytochrome c–related caspase activation. The percentage of leukemia cells in bone marrow after 15 days of remission induction treatment is given on the y-axis. Bars indicate mean values; P value of independent samples t test.
Classification of patients according to CRAC values in relation to leukemia cell persistence in bone marrow on day 15 of induction treatment. Patients (n = 63) were grouped according to CRAC, indicating proficient (CRAC positive, n = 27) or deficient (CRAC negative, n = 36) cytochrome c–related caspase activation. The percentage of leukemia cells in bone marrow after 15 days of remission induction treatment is given on the y-axis. Bars indicate mean values; P value of independent samples t test.
Different cellular functions of leukemia cells have been analyzed for the establishment of novel prognostic factors. A high proliferative capacity of leukemia cells assessed by in vitro coculture experiments on stroma cells has been proposed to identify leukemias with poor prognosis.47 A less complex functional assay is the widely used cellular cytotoxicity assay based on the capacity of leukemia cells to reduce formazan (methyl-thiazoltetrazolium, MTT) after in vitro drug treatment of leukemia samples.48 This cellular cytotoxicity assay integrates diverse properties of leukemia samples such as cell survival, cell proliferation, and mitochondrial function of the cells and displays some correlation to clinical outcome.49 Both assays, however, do not assess molecular mechanisms and therefore cannot directly contribute to the elucidation of molecular signal transduction in primary leukemia cells. An approach to investigate the function of apoptosis signaling pathways is the measurement of caspase activation after induction of apoptosis by electroporation of leukemia cells with apoptogenic molecules such as cytochrome c and caspase-8.50 However, the electroporation procedure itself leads to a high rate of cell death and is not suitable in particular for ALL samples.
In our study, we made use of the well-established finding that culture of primary ALL cells with standard conditions without stroma and cytokines rapidly induces apoptosis. On the molecular level, extracellular signals by integrins and fibronectin mediate intracellular antiapoptotic signals such as caspase inhibition and survivin expression.51,52 Lack of these cell-mediated survival signals initiates the apoptosis cascade via a death by default mechanism in BCP ALL cells and thereby facilitates the analysis of the cells' constitutive apoptosis program.
In order to test the functional integrity of a core apoptosis signaling pathway, we have previously developed and evaluated a method for the simultaneous detection of activated caspase-3 and the release of cytochrome c by flow cytometry.42,43 By combination of these techniques, we were able to analyze and, more importantly, to quantify potential defects in apoptosis signal transduction on a single-cell level in patient samples cultured in vitro.
In our analysis of single apoptosis parameters, only caspase-3 activation correlated to initial treatment response on day 15 and to long-term survival. The differences in caspase activation were statistically significant, but of relatively low discriminative value (Table 1; day 15: 27.8% versus 18.2%; relapse: 27.1% versus 14.7%). In addition, of 3 initial response parameters (prednisone response on day 8, blast cell persistence on day 15, and achievement of remission on day 33) only the bone marrow blast cell count on day 15 was associated with caspase activation. In line with our data, one study on caspase-3 expression demonstrated an association of cleaved caspase-3 and long-term survival,38 while others could not establish a connection between caspase activation and initial response to treatment39 or long-term survival.53 This indicates that caspase activation as a single parameter is only poorly associated with response to treatment.
Of interest, we did not find any prognostic impact of cytochrome c release as single parameter, indicating that complete mitochondrial resistance as described in Bcl-2–overexpressing54 or BclXL-overexpressing55 cells in experimental systems does not contribute to treatment failure or relapse in B-cell precursor ALL. In line with this, expression of molecules regulating mitochondrial apoptosis signaling such as Bax and Bcl2 was not associated with treatment outcome in ALL.23,30
Intact cytochrome c release indicates an intact mitochondrial system, whereas caspase-3 activation indicates an intact downstream effector system integrating mitochondrial-dependent and -independent effector caspase activation. The quantification of cytochrome c release in relation to caspase-3 activation in all leukemia samples revealed a constant correlation of these pathways in all favorable clinical subgroups such as treatment response on days 8, 15, and 33 and long-term remission. In contrast to this, the unfavorable groups (poor response to initial treatment and relapse patients) displayed a complete absence of this correlation (Table 2). Thus, an intact connection of cytochrome c release to caspase activation is of advantageous prognostic relevance for treatment outcome.
Deficient activation of caspase-3 by “apoptogenic” mitochondria may involve IAP and Smac proteins. The release of Smac from mitochondria is required for full processing of downstream caspases in some cell types. In particular, cleavage of caspase-3 is inhibited by IAPs in Bcl-2–overexpressing cells due to deficient release of Smac.56-58 Either deficient release of Smac or overexpression of IAPs could account for deficient caspase-3 activation despite cytochrome c release. Of interest, high expression of XIAP in adult acute myeloid leukemia (AML) is discussed as an adverse prognostic factor.40 The effect of disturbances in these different molecular mechanisms involved in the activation of caspase-3 by mitochondria is assessed in our functional assays. The prognostic relevance, as observed in our study, underlines the pivotal role for mitochondria-related caspase activation for successful antileukemic treatment and suggests that adverse outcome is associated with deregulation of this pathway by different mechanisms.
By analysis of caspase-3 activation and cytochrome c, we addressed the core of the apoptosis cascade, where signals converging on mitochondria are transduced by caspase-9 to the main executioner caspase, caspase-3. Other parts of the apoptosis cascade initiate or amplify the mitochondrial apoptosis signal. Initiator caspases such as caspase-2 have been shown to directly activate mitochondria.14 Activation of the effector caspase-3 may cleave initiator caspase-8, which in turn produces t-Bid acting on mitochondria and thereby amplifies mitochondrial apoptosis signaling.59-61 Due to the lack of antibodies discriminating active caspase fragments from the inactive proforms, activation of the initiator caspases-2, -9, and -8 cannot directly be analyzed in flow cytometry. We therefore addressed the issue of upstream and downstream mitochondrial signaling in our functional studies on caspase dependency of cytochrome c release by analysis of inhibition of cytochrome c release through ZVAD-fmk. The fact that cytochrome c release can be inhibited by ZVAD-fmk, at least in part, suggests that the induction of the mitochondrial signal by caspases, either proximal such as caspase-2 or amplification of the mitochondrial signal via caspases-9, -3, -8, and t-Bid is operative in primary leukemia cells. In addition, the correlation of caspase-dependent and -independent cytochrome c release to clinical response groups revealed that caspase-dependent, but not -independent, cytochrome c release is associated with good treatment response. Hence, caspase-related signaling pathways amplifying mitochondrial apoptosis signaling seem to be essential for treatment response and achievement of relapse-free survival.
In this study, we further identified cytochrome c–related activation of caspase-3 (CRAC) as a new parameter indicating the efficient or deficient activation of caspase-3 in the presence of released cytochrome c. This parameter was found to discriminate patients with good and poor response to initial treatment independently from established, pretherapeutic risk factors. Moreover, CRAC discriminates patients with long-term survival from patients who encounter relapse. Of most interest, this pattern was also found in different treatment groups (low, intermediate, and high risk). Due to low patient numbers in each subgroup, the results did not gain statistical significance. However, these findings suggest that CRAC provides additional prognostic information independently of initial response and known cytogenetic risk groups. This issue will be addressed in detail in a prospective study within the ALL-BFM study group.
Taken together, we identified cytochrome c–related activation of caspase-3 as a relevant molecular mechanism of apoptosis signaling, which determines treatment response and long-term survival in precursor B-cell ALL in children. The novel parameter CRAC, indicating a functional intact apoptosis signaling system, is a potential prognostic factor for future treatment stratification and may serve to identify novel therapeutic target structures.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-08-3305.
Supported by grants from the European Union, Deutsche Forschungsgemeinschaft, and Bundesministerium für Bildung und Forschung within the Kompetenznetz Pädiatrische Onkologie.
The online version of this article contains a data supplement.
K.-M.D. and K.S. contributed equally to this work.
L.H.M. and L.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.