Deletions and/or mutations of p53 are relatively rare and late events in the natural history of B-cell chronic lymphocytic leukemia (B-CLL). However, it is unknown whether p53 signaling is functional in B-CLL and if targeted nongenotoxic activation of the p53 pathway by using nutlin-3, a small molecule inhibitor of the p53/MDM2 interaction, is sufficient to kill B-CLL cells. In vitro treatment with nutlin-3 induced a significant cytotoxicity on primary CD19+ B-CLL cells, but not on normal CD19+ B lymphocytes, peripheral-blood mononuclear cells, or bone marrow hematopoietic progenitors. Among 29 B-CLL samples examined, only one was resistant to nutlin-3–mediated cytotoxicity. The induction of p53 by nutlin-3 in B-CLL samples was accompanied by alterations of the mitochondrial potential and activation of the caspase-dependent apoptotic pathway. Among several genes related to the p53 pathway, nutlin-3 up-regulated the steady-state mRNA levels of PCNA, CDKN1A/p21, GDF15, TNFRSF10B/TRAIL-R2, TP53I3/PIG3, and GADD45. This profile of gene activation showed a partial overlapping with that induced by the genotoxic drug fludarabine. Moreover, nutlin-3 synergized with both fludarabine and chlorambucil in inducing B-CLL apoptosis. Our data strongly suggest that nutlin-3 should be further investigated for clinical applications in the treatment of B-CLL.
Introduction
The tumor suppressor p53 coordinates a complex network of cellular proteins evolved to protect cells from malignant transformation.1 The activation of p53 is tightly regulated by the murine double minute 2 (MDM2) gene,2 whose expression is regulated in part by a p53-responsive promoter. In turn, MDM2, which is an E3 ubiquitin ligase for p53 and itself, controls p53 half-life via ubiquitin-dependent degradation. Additionally, MDM2 protein binds the p53 N-terminal transactivation domain and negatively regulates tumor suppressor function by compromising transcriptional regulation. In response to cellular stress, the p53-MDM2 interaction is disrupted, p53 undergoes posttranslational modifications on multiple sites,1,3 and also, MDM2 undergoes modifications enhancing its autoubiquitination and degradation.4 Recently, potent and selective small molecule inhibitors of p53-MDM2 interaction, the nutlins, have been reported.5,6 These nongenotoxic compounds bind MDM2 in the p53 binding pocket with high selectivity and can release p53 from negative control leading to effective stabilization of p53 and activation of the p53 pathway.5,6 On the other hand, nutlins do not bind to p53 protein and do not interfere with its activities.
B-cell chronic lymphocytic leukemia (B-CLL) is a clinically heterogeneous disease characterized by the accumulation of CD19+/CD5+ B lymphocytes with significant resistance to apoptosis and, therefore, prolonged survival both in vivo and in vitro.7,8 Although one-third of patients never require treatment, in the other patients the disease progresses at a variable rate and requires treatment regimens, which employ purine analogs (eg, fludarabine) or alkylating agents (eg, chlorambucil), monoclonal antibodies, or combinations thereof.8 Since none of these therapies is curative, continued preclinical studies on innovative therapeutic strategies are warranted. In particular, the identification of new agents that interfere with the survival of B-CLL cells by promoting their apoptosis is one critical approach to improve therapeutic outcome. Taking into account that p53 deletions and/or mutations in B-CLL are restricted to 10% to 15% of patients and are associated with decreased survival and clinical resistance to chemotherapeutic treatment,9 we have investigated the effect of nutlin-35,6 to probe the functionality of the p53 pathway in primary B-CLL samples in comparison with normal CD19+ B lymphocytes, peripheral-blood mononuclear cells (PBMCs), bone marrow mononuclear cells (BMMCs), and purified CD34+ hematopoietic progenitor cells.
Patients, materials, and methods
Patients and cell isolation
Peripheral-blood samples were collected in heparin-coated tubes from healthy human blood donors and patients with B-CLL (Table 1) following informed consent obtained in accordance with the Declaration of Helsinki, and with approval obtained from the institutional review board of the University of Udine (Udine, Italy) for these studies. The diagnosis of B-CLL was made by peripheral-blood morphology and immunophenotyping. PBMCs from healthy blood donors and from patients with B-CLL were isolated by gradient centrifugation with lymphocyte-cell separation medium (Cedarlane Laboratories, Hornby, ON, Canada). T lymphocytes, NK lymphocytes, granulocytes, and monocytes were negatively depleted with immunomagnetic microbeads (MACS microbeads; Miltenyi Biotech, Auburn, CA), with a purity more than 90% of resulting CD19+ B lymphocytes, as assessed by flow cytometry using specific FITC- or PE-conjugated monoclonal antibodies (MoAbs; Becton Dickinson, San Jose, CA). Freshly purified CD19+ B lymphocytes from patients with B-CLL were used immediately in all in vitro experiments with nutlin-3 or chemotherapeutic drugs and aliquots of these samples were frozen in RPMI 1640/50% FBS/10% DMSO before performing further characterization (such as fluorescence in situ hybridization [FISH] and ZAP-70 analyses). In some experiments, when aliquots of frozen B-CLL cells were thawed and retested for response to nutlin-3, the values of nutlin-3–specific apoptosis were comparable to those obtained with freshly isolated B-CLL cells.
Rai stage, absolute lymphocyte count, lymphocyte doubling time, and treatment status at the time of cell acquisition were abstracted from clinical records. Most of the patients had been without prior therapy, whereas other patients (patient nos. 12, 18, 22, 23, 24, 25, 26, 27, 28; Table 1) were considered to have active disease since they required initiation of therapy within 2 months of donating cells. However, these patients had not been treated for at least 3 weeks prior to blood processing for this study. B-CLL samples were also characterized by interphase FISH, performed as previously described,10 and by Western blot analysis to evaluate the expression levels of ZAP-70 protein.
BM specimens were obtained from healthy donors by aspiration with Jamshidi needle. Each sample was gently run through a 22-gauge needle in order to homogenize the marrow particles. Cell suspensions were fractionated on Lymphocyte-H (Cedarlane Laboratories) by centrifugation at 500g for 20 minutes. Cells were washed twice with PBS 1X and normal CD34+ cells were separated to more than 95% purity by positive-selection magnetic-bead sorting using a VarioMACS device (Miltenyi Biotech) and immediately used for the in vitro studies.
Culture treatments and assessment of cell viability
For the in vitro toxicity assays, immediately after purification, CD19+ B-CLL cells, normal CD19+ B cells, normal PBMCs, normal BMMCs, and normal CD34+ cells were resuspended at a cell density of 1 × 106 cells/mL in RPMI or IMDM medium supplemented with 10% FCS, l-glutamine, and penicillin/streptomycin (Gibco BRL, Grand Island, NY). For cell treatment, the following reagents, individually or in combination, were used: nutlin-3 (0.01 μM-30 μM; obtained from either Sigma-Aldrich, St Louis, MO or from Cayman Chemical, Ann Arbor, MI), the proteasome inhibitor clastolactacystin β-lactone (25 μM; Biomol, Plymouth Meeting, PA), the cell-permeable form of fludarabine (F-ARA-AMP des-phosphate) and chlorambucil, both obtained from Sigma-Aldrich and used at concentrations of 0.1 μM to 10 μM. No differences in the ability to induce p53 accumulation and B-CLL apoptosis were observed between nutlin-3 obtained from Sigma-Aldrich and that obtained from Cayman Chemical.
At various time points after treatments, cell viability was assessed by trypan blue-dye exclusion. MTT (3-(4,5-dimethilthiazol-2yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay (Roche Diagnostics, Indianapolis, IN) was performed in most experiments for data confirmation. Induction of apoptosis was evaluated by annexin V–FITC/propidium iodide (PI) staining (Immunotech, Marseille, France) and analyzed by using a FACScan flow cytometer (Becton Dickinson). To avoid nonspecific fluorescence from dead cells, live cells were gated tightly using forward and side scatter. To measure mitochondrial membrane potential (Δψm), cells were loaded with MitoTracker Red CMXRos (300 μM) and MitoTracker Green (100 μM; both from Molecular Probes, Eugene, OR) for 1 hour at 37°C. The Δψm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence). All experiments were conducted in duplicate or triplicate.
Short-term colony assay
Hematopoietic colony formation was analyzed using standard methylcellulose colony assay (StemCell Technologies, Vancouver, BC, Canada). After an overnight incubation with 10 μM of nutlin-3, 10 μM fludarabine, or an adequate amount of control vehicle, BMMCs were plated in triplicate at 1 × 105 cells density in 35-mm Petri dishes in 1.1 mL of a methylcellulose semisolid medium (Methocult H4434; StemCell Technologies) for the assessment of their clonogenic potential as previously described.11
Reverse transcriptase–polymerase chain reaction (RT-PCR) and cDNA microarray
Total RNA was extracted from CD19+ B-CLL cells or normal CD19+ B cells by using the Qiagen RNeasy mini kit (Qiagen, Hilden, Germany) according to the supplier's instructions. The quality of the total RNA preparation was verified by agarose gel and, when necessary, further purification was performed with the RNeasy cleanup system (Qiagen) to remove chromatin DNA. Amplification for p53 and MDM2 gene expression in both CD19+ B-CLL or CD19+ normal B cells was performed with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) by using SYBR Green–based technology and the following primers: p53-forward, CTG GGA CGG AAC AGC TTT GA, p53-reverse, CCT TTC TTG CGG AGA TTC TCT TC; MDM2-forward, GCT GGA GTG CAG TGG CGT GAT, MDM2-reverse, GAT GAC TGT AGG CCA AGC TAA TTG. After GAPDH normalization, p53 and MDM2 expression levels were compared between the leukemic and normal CD19+ B-lymphocyte samples.
In other experiments, RNA was extracted from 4 B-CLL patient samples with or without 24-hour treatment with 10 μM nutlin-3 or 10 μM fludarabine. Total RNA (3 μg) was transcribed into cDNA using the GEArray AmpoLabeling-LPR Kit (Superarray Bioscience, Frederick, MD). An in vitro linear polymerase reaction was then performed to generate biotinylated cDNA. Labeled cDNA was hybridized with customized cDNA microarrays, containing arrays of genes representative of several different pathways frequently altered during the progression of cancer together with housekeeping genes (the list of the genes is available elsewhere12,13 ). Hybridization was revealed by alkaline phosphatase–conjugated streptavidin, using a chemiluminescent detection kit (Superarray Bioscience). Signal intensity was measured for each microarray, the minimal intensity was used for background subtraction, and the values were normalized to the median signal value for each array. Expression levels in leukemic samples treated with nutlin-3 or fludarabine were compared with the matched control (untreated) leukemic samples. Data were filtered for the genes whose expression level increased or decreased by at least 2-fold, that is, filtering the ratio for values more than or equal to 2.0 or less than or equal to 0.5. Microarray results were validated by SYBR Green real-time PCR detection method, using the SuperArray Bioscience RT2 Real-Time Gene Expression Assays that include specific validated primer sets and PCR master mixes (Superarray Bioscience). All samples were run in triplicate.
Western blot analysis
Western blot was performed on approximately 3 × 106 to 5 × 106 cells/sample. To obtain cell lysates, cell suspensions were mixed with a lysing buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, and protease inhibitors (protease inhibitor cocktail P8340; Sigma-Aldrich). Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of proteins for each sample were migrated in 10% PAGE, blotted onto nitrocellulose filters, blocked with a 3% suspension of dried skimmed milk in PBS, and incubated with the following antibodies: anti–ZAP-70 (Upstate, Lake Placid, NY), anti-MDM2, anti-p53, anti-PARP, and anti–caspase-3 (all from Santa Cruz Biotechnology, Santa Cruz, CA), anti–phosphorylated (Ser15)-p53 (Cell Signaling Technology, Beverly, MA), and anti–β-actin (Sigma-Aldrich) as loading control. Filters were washed and further incubated for 1 hour at room temperature with peroxidase-conjugated anti–mouse or anti–rabbit IgG (Sigma-Aldrich). Reactions were revealed with the ECL Western blotting detection reagent (Amersham, Arlington Heights, IL). The level of ZAP-70 protein in each patient sample was defined based on densitometric analyses by ImageQuant software (Molecular Dynamics), after β-actin normalization. Multiple film exposures were used to verify the linearity of the samples analyzed and to avoid saturation of the film. Expression of ZAP was considered absent/low when the densitometric values were not significantly over the film background (Figure 3).
Assessment of the effect of combination treatment
For selected patient samples, we investigated the effect of combining nutlin-3 with fludarabine or chlorambucil in B-CLL cells in vitro. B-CLL cells were treated with serial dilutions of nutlin-3 (0.1 μM to 10 μM) or the active moiety of fludarabine (0.1 μM to 10 μM) or chlorambucil (0.1 μMto 10 μM) individually or in combination using a constant ratio (nutlin-3/fludarabine or nutlin-3/chlorambucil, 1:1) for 48 hours. Results were analyzed using CalcuSyn software program (Biosoft, Cambridge, United Kingdom), which uses the method of Chou and Talalay14 to determine whether combined treatment yields greater effects than expected from summation alone. A combination index (CI) of 1 indicates an additive effect, a CI above 1 indicates an antagonistic effect, and a CI below 1 indicates a synergistic effect.14
Statistical analysis
The mean, median, minimum, and maximum values were calculated for each group of data obtained from B-CLL samples and from healthy controls. Box plots were used to show the median, minimum, and maximum values and 25th to 75th percentiles. The results were evaluated by using analysis of variance with subsequent comparisons by Student t test and with the Mann-Whitney rank-sum test. Correlation coefficients were calculated by the Spearman method. Statistical significance was defined as a P value of less than .05.
Results
The steady-state mRNA levels of p53 are decreased in B-CLL cells with respect to normal B lymphocytes
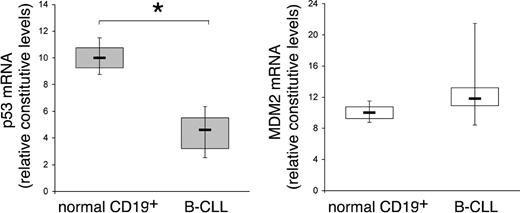
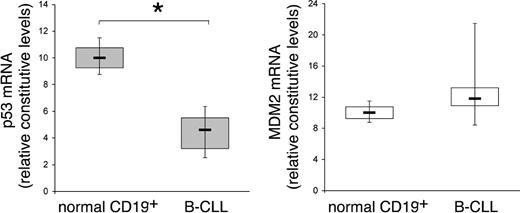
In recent years, genetic factors have been established as important predictors of disease progression and survival in patients with B-CLL, and they have implications for the risk-adapted clinical management of B-CLL.15 Among these, 17p deletion, a genetic aberration that modifies the expression of p53, represents an independent poor prognostic factor in CLL.16 In addition, growing evidence has documented hypermethylation in the promoter region of TP53 and a decrease in the transcription of this gene in a subset of B-CLL samples without p53 deletions and/or mutations.17,18 On these bases, the constitutive steady-state mRNA levels of p53 and MDM2 were quantitatively evaluated by RT-PCR in B-CLL samples (n = 12) obtained at the diagnosis, in comparison to normal CD19+ B lymphocytes (n = 5) (Figure 1). RNA was extracted from freshly purified B-CLL PBMCs containing an excess of B-leukemic cells (> 90% of CD19+/CD5+ B cells) and from purified normal B-lymphocyte samples (> 90% CD19+). The mRNA levels of p53 were significantly (P < .01) lower in B-CLL samples with respect to normal B lymphocytes (Figure 1). On the other hand, at variance to other hematologic malignancies in which overexpression of MDM2 has been reported,19 no significant modifications in the mRNA levels of MDM2 were observed between B-CLL cells and normal B lymphocytes (Figure 1).
The MDM2 antagonist nutlin-3 induces the accumulation of p53 protein in B-CLL cells as well as in primary normal cells
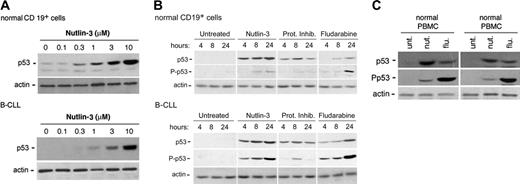
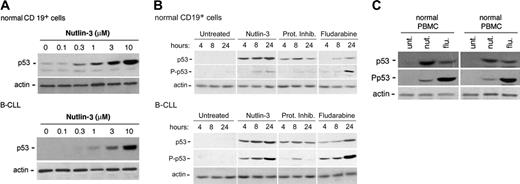
Consistent with the fact that wild-type 53 protein has a short half-life (10-20 minutes) and is present in a latent state in the absence of stress,1,2 at Western blot analysis the amount of p53 in cell lysates obtained from untreated B-CLL samples and normal CD19+ B lymphocytes was barely detectable (Figure 2A). However, in spite of the low steady-state levels of p53 mRNA observed in B-CLL samples (Figure 1), a 24-hour treatment with nutlin-3 induced a dose-dependent accumulation of p53 protein in both normal and leukemic cells (Figure 2A). In kinetics experiments, nutlin-3 (10 μM) induced a detectable accumulation of p53 already after 4 hours, which progressively increased at 8 hours and 24 hours in both normal and leukemic B cells (Figure 2B). The proteasome inhibitor clasto-lactacystin, used for comparison, induced a rapid p53 increase (at 4-8 hours), followed by a decline at 24 hours in both cell types (Figure 2B). On the other hand, the genotoxic drug fludarabine induced a much-delayed accumulation of p53, evident only after 24 hours. Of interest, in nutlin-3– and clasto-lactacystin–treated samples, the levels of p53 Ser15 phosphorylation were barely detectable in normal B cells. On the other hand, a progressive increase of phosphorylated p53 concomitant to the progressive stabilization and accumulation of p53 was observed in nutlin-treated B-CLL samples, whereas clasto-lactacystin modestly induced p53 Ser15 phosphorylation only at 8 hours (Figure 2B). Of note, fludarabine potently induced p53 Ser15 phosphorylation in both normal and leukemic B cells, in keeping with the ability of genotoxic agents to induce p53 accumulation primarily through phosphorylation of p53 on multiple sites, including Ser15.3
Comparison of p53 and MDM2 steady-state mRNA levels between B-CLL cells and normal CD19+ B lymphocytes. RNA from samples of purified normal CD19+ B lymphocytes (n = 5) and from B-CLL cells (n = 12) were quantitatively analyzed by p53 and MDM2 RT-PCR after normalization to the level of GAPDH mRNA. Each sample was determined in duplicate. Horizontal bars are median; upper and lower edges of box are 75th and 25th percentiles; lines extending from box are 10th and 90th percentiles. *Significance at P < .01.
Comparison of p53 and MDM2 steady-state mRNA levels between B-CLL cells and normal CD19+ B lymphocytes. RNA from samples of purified normal CD19+ B lymphocytes (n = 5) and from B-CLL cells (n = 12) were quantitatively analyzed by p53 and MDM2 RT-PCR after normalization to the level of GAPDH mRNA. Each sample was determined in duplicate. Horizontal bars are median; upper and lower edges of box are 75th and 25th percentiles; lines extending from box are 10th and 90th percentiles. *Significance at P < .01.
p53 induction and Ser15 phosphorylation in B-CLL and in normal cells upon treatment with nutlin-3. Induction of p53 and levels of Ser15 phosphorylated-p53 (P-p53) were assessed by Western analysis. (A) Dose-dependent induction of p53 in normal CD19+ B and B-CLL cells after 24 hours of incubation with increasing doses of nutlin-3. Representative examples of 4 independent experiments are shown. (B) Normal CD19+ B and B-CLL cells were either left untreated or incubated with nutlin-3 (10 μM), or the proteasome inhibitor (Prot Inhib) clasto-lactacystin (25 μM), or fludarabine (10 μM) for the indicated time. Representative examples of 3 independent experiments are shown. (C) Normal PBMCs and BMMCs were either left untreated or incubated with nutlin-3 (10 μM) or fludarabine (10 μM) for 24 hours. Representative examples of 3 BMMC or 5 PBMC independent experiments are shown.
p53 induction and Ser15 phosphorylation in B-CLL and in normal cells upon treatment with nutlin-3. Induction of p53 and levels of Ser15 phosphorylated-p53 (P-p53) were assessed by Western analysis. (A) Dose-dependent induction of p53 in normal CD19+ B and B-CLL cells after 24 hours of incubation with increasing doses of nutlin-3. Representative examples of 4 independent experiments are shown. (B) Normal CD19+ B and B-CLL cells were either left untreated or incubated with nutlin-3 (10 μM), or the proteasome inhibitor (Prot Inhib) clasto-lactacystin (25 μM), or fludarabine (10 μM) for the indicated time. Representative examples of 3 independent experiments are shown. (C) Normal PBMCs and BMMCs were either left untreated or incubated with nutlin-3 (10 μM) or fludarabine (10 μM) for 24 hours. Representative examples of 3 BMMC or 5 PBMC independent experiments are shown.
In parallel, the ability of nutlin-3 to induce p53 accumulation was evaluated in other normal cell types: unfractionated PBMCs and BMMCs. As shown in Figure 2C, Western blot analysis confirmed that treatment with nutlin-3 (10 μM) led to a significant increase of p53 accumulation in both PBMCs and BMMCs, accompanied by barely detectable levels of Ser15 p53, in comparison with fludarabine-treated samples.
Nutlin-3 induces a dose-dependent cytotoxicity on B-CLL cells
To investigate the effect of nutlin-3 on B-CLL cell viability, B-CLL samples obtained from patients free of therapy (Table 1, patient nos. 1 to 11 and nos. 13 to 16) were incubated in vitro with increasing concentrations of nutlin-3 and assayed for viability by trypan blue dye exclusion at 24 hours (data not shown) and 48 hours (Table 2). In the absence of exogenous cytokines, B-CLL cells did not proliferate ex vivo during this time frame and the number of viable cells remained relatively constant, never dropping below 85% (range 0.85 × 106 cells/mL-1.15 × 106 cells/mL) of the cell number seeded at time 0 (1 × 106 cells/mL). The nutlin-3 concentration required to induce death in 50% of B-CLL cells (IC50) relative to control was 10.4 μM at 48 hours of culture. In parallel, nutlin-3 cytotoxicity was evaluated on normal cells (Table 2). At 48 hours, the IC50 values for all normal cell types examined (CD19+ B cells, PBMCs, BMMCs, purified CD34+ cells) were greater than 30 μM. In this respect, it should be noted that the specificity of nutlin-3 in interrupting the MDM2-p53 interaction is lost at concentrations greater than 10μM.5 Remarkably, the difference of cytotoxicity between B-CLL cells and all normal cell types investigated (CD19+ B cells, PBMCs, BMMCs, purified CD34+ cells) was statistically significant at nutlin-3 concentrations of 3 μM(P < .05) and 10 μM(P < .01).
On the basis of these results, the concentration of 10 μM nutlin-3 was selected for further studies on B-CLL samples.
Nutlin-3 triggers the apoptotic pathway in most B-CLL samples
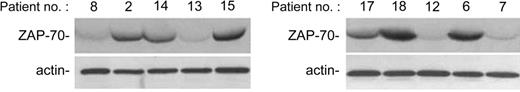
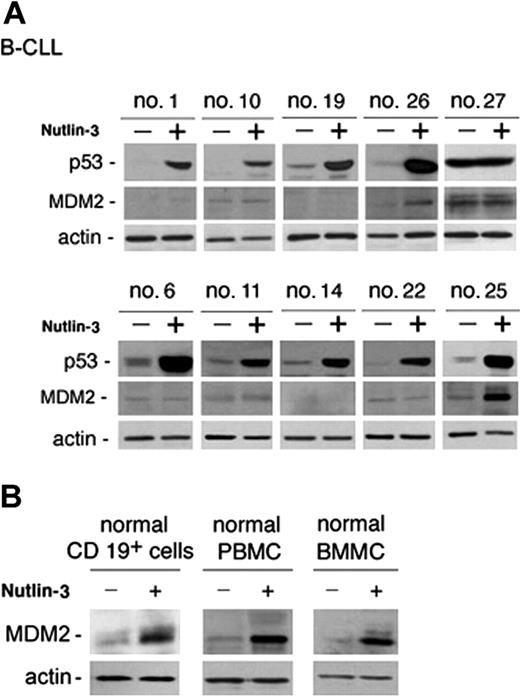
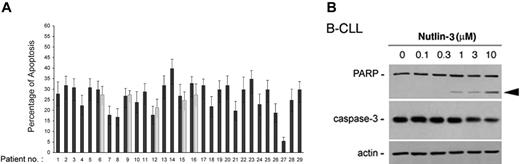
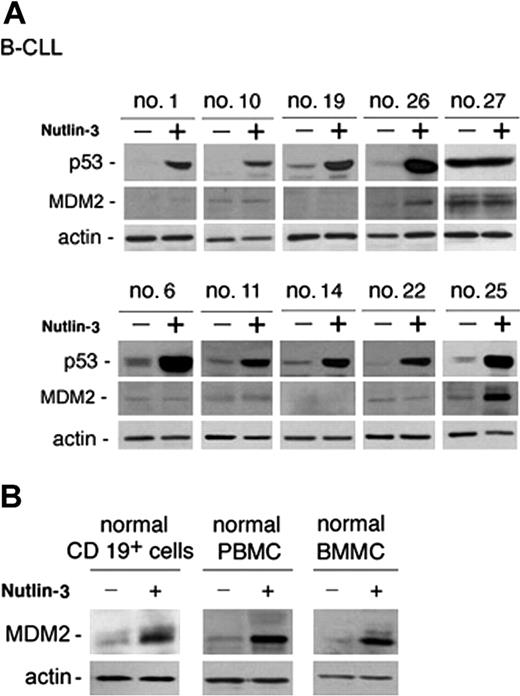
Analysis of B-CLL cell viability in response to treatment with nutlin-3 (10 μM) for 48 hours was next extended to all patients with B-CLL who entered this study and it is reported for each patient in Table 1. The majority (28 of 29) of B-CLL patient samples tested demonstrated susceptibility to nutlin-3 cytotoxicity, although reduction of cell viability was variable from sample to sample (percentage of cell viability compared with untreated controls: 56 ± 12.7, mean ± SD; range 35%-92%; Table 1). The in vitro sensitivity to nutlin-3 was analyzed also in relationship with relevant biologic/clinical characteristics of patients with B-CLL, such as the levels of ZAP-70, which represents an important negative prognostic factor.20 The B-CLL cells examined in this study were classified as ZAP-70 absent/low–expressing or ZAP-70 intermediate/high–expressing samples (Table 1) on the basis of Western blot analyses, as exemplified in Figure 3 for 10 B-CLL samples. Of note, the susceptibility to nutlin-3 cytotoxicity was comparable in samples with intermediate/high ZAP-70 expression (ie, lower percent cell viability compared with control: 51 ± 9, mean ± SD) and with absent/low ZAP-70 expression (60 ± 13.8, mean ± SD). Moreover, samples obtained from patients with cytogenetic abnormalities (nos. 17, 18, 24, 27 of Table 1), potentially affecting the p53 pathway activation (deletion of 17p and 11q revealed by FISH), still displayed responsiveness to nutlin-3, with the exception of patient no. 27. Interestingly, at Western blot analysis, patient no. 27 was the only sample examined showing a high level of p53 in untreated cultures (Figure 4A), which is a characteristic feature of mutated p53.21 In this sample, nutlin-3 did not affect p53 level, whereas it efficiently, although variably, upregulated p53 protein levels in the other B-CLL samples examined (Figure 4A). Whereas p53 was efficiently induced by nutlin-3 in all B-CLL samples examined except no. 27, MDM-2 was induced by nutlin-3 in a minority of B-CLL samples, as exemplified in Figure 4A for 10 B-CLL samples. The inability to up-regulate MDM-2 protein expression was confined to B-CLL, since all normal cell types investigated (CD19+ B cells, PBMCs, and BMMCs) showed a clearly detectable induction of MDM-2 in response to treatment with 10 μM nutlin-3 (Figure 4B).
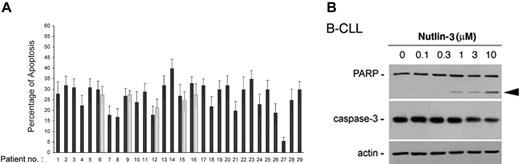
To determine whether the cytotoxicity of nutlin-3 toward B-CLL cells was caused by apoptosis, a series of independent approaches were used. Exposure of B-CLL samples to nutlin-3 (10 μM) for 24 hours resulted in a variable but significant (P < .05) increase in the percentage of annexin-V–positive cells with respect to control cultures (Figure 5A). For 5 B-CLL patient samples, the effect of nutlin-3 was re-evaluated also on cells that had been previously frozen, obtaining comparable results (Figure 5A, gray bars). Moreover, treatment of B-CLL cells with nutlin-3 (10 μM) resulted in the loss of Δψm, an early feature of the mitochondrial apoptotic pathway (data not shown). In additional experiments, the amounts of procaspase-3 and the cleavage of its major substrate, the DNA repair enzyme poly-ADP-ribose polymerase (PARP), were examined by Western blot. As shown in Figure 5B, nutlin-3 treatment of B-CLL cells induced a dose-dependent decrease of procaspase-3 paralleled by a progressive PARP cleavage.
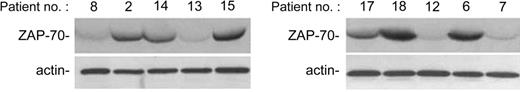
Analysis of ZAP-70 expression in B-CLL patient cells. ZAP-70 expression has been evaluated by Western blot on CD19+ purified B-CLL cells. Representative examples of patients characterized by absent/low (nos. 8, 13, 12, 7) and intermediate/high (nos. 2, 14, 15, 17, 18, 6) ZAP-70 levels are shown.
Analysis of ZAP-70 expression in B-CLL patient cells. ZAP-70 expression has been evaluated by Western blot on CD19+ purified B-CLL cells. Representative examples of patients characterized by absent/low (nos. 8, 13, 12, 7) and intermediate/high (nos. 2, 14, 15, 17, 18, 6) ZAP-70 levels are shown.
p53 and MDM2 induction in B-CLL and in normal cells in response to nutlin-3. B-CLL cells (A) and normal CD19+ B lymphocytes, PBMCs, and BMMCs (B) were incubated with or without nutlin-3 (10 μM) for 24 hours before analysis of p53 and MDM2 by Western blot. (A) Representative samples from patients characterized by absent/low (nos. 1, 10, 19, 26, and 27) and intermediate/high (nos. 6, 11, 14, 22, 25) ZAP-70 levels, showing a variable induction of p53 and a low frequency of MDM2 induction (nos. 26, 25), are reported. High constitutive and unmodified level in patient no. 27 indicates p53 mutation. (B) Representative results of 3 to 6 independent experiments are shown.
p53 and MDM2 induction in B-CLL and in normal cells in response to nutlin-3. B-CLL cells (A) and normal CD19+ B lymphocytes, PBMCs, and BMMCs (B) were incubated with or without nutlin-3 (10 μM) for 24 hours before analysis of p53 and MDM2 by Western blot. (A) Representative samples from patients characterized by absent/low (nos. 1, 10, 19, 26, and 27) and intermediate/high (nos. 6, 11, 14, 22, 25) ZAP-70 levels, showing a variable induction of p53 and a low frequency of MDM2 induction (nos. 26, 25), are reported. High constitutive and unmodified level in patient no. 27 indicates p53 mutation. (B) Representative results of 3 to 6 independent experiments are shown.
Nutlin-3 induces the transcription of a cluster of p53 target genes in B-CLL cells
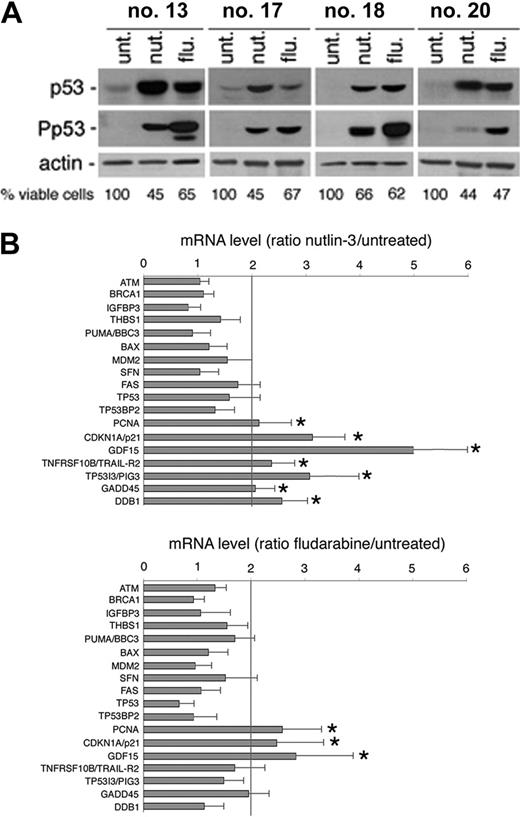
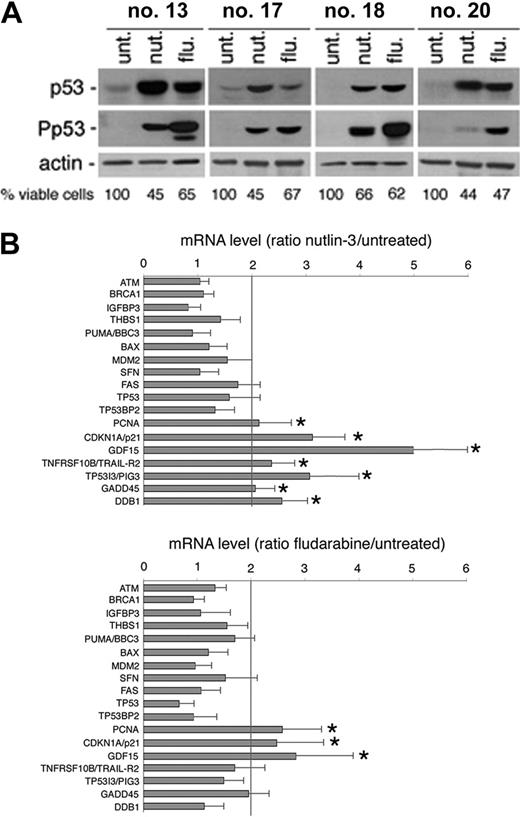
We next used gene-expression profiling to determine which p53-related genes were affected by exposure to nutlin-3. Gene expression was analyzed in 4 B-CLL patient samples, using total RNA extracted after 24 hours of treatment with nutlin-3. Also in this set of experiments, fludarabine was used for comparison, not only because it represents an effective treatment in early and advanced B-CLL disease,22,23 but also taking into account that a major role of p53 in mediating the cytotoxic activity of fludarabine has been shown in some studies,9,24 but not in others.21,25 As expected, exposure to either nutlin-3 or fludarabine (10 μM each) for 24 hours induced variable p53 protein accumulation in all of the 4 B-CLL samples examined (Figure 6A), with fludarabine significantly more powerful than nutlin-3 in promoting Ser15 phosphorylation of p53, as also shown in Figure 2B-C.
Among potential p53 transcriptional target genes,24,25 nutlin-3 significantly increased the mRNA steady-state levels of PCNA, CDKN1A/p21, GDF15, TNFRSF10B/TRAIL-R2, TP53I3/PIG3, GADD45, and DDB1 (Figure 6B; Table 3). Overall, fludarabine was less efficient than nutlin-3 in activating p53 target genes, although it showed some overlap being able to upregulate the steady-state levels of PCNA, CDKN1A/p21, and GDF15 (Figure 6B; Table 3). The up-regulation of the p53-induced genes indicated by the array data were validated and confirmed by real-time PCR (Figure 6B; Table 3).
The cytotoxic activity of nutlin-3 in B-CLL cells is enhanced by combination with fludarabine or chlorambucil
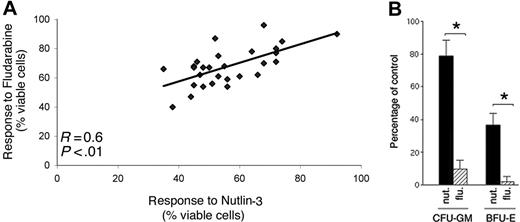
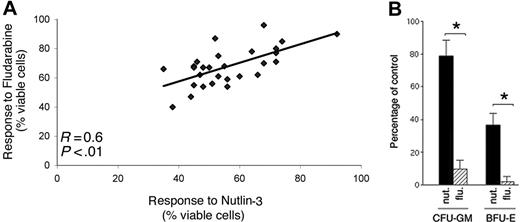
In agreement with the gene-expression profile shown in Figure 6B, with those studies proposing that fludarabine cytotoxicity was due at least in part to activation of the p53 pathway,9,24 a significant (P < .01) positive correlation was observed between fludarabine- and nutlin-3–mediated cytotoxicity in B-CLL samples (Figure 7A). Of interest, the overall cytotoxicity of nutlin-3 (10 μM) after 48 hours of culture was greater than that of fludarabine (10 μM) on the same samples (percentage of cell viability compared with untreated controls, expressed as means ± SD: 56 ± 12.7 for nutlin-3 versus 67.8 ± 13.5 for fludarabine, P < .01). However, when the cytotoxicity of nutlin-3 and fludarabine (10 μM each) was examined on normal BM clonogenic progenitor cells, fludarabine almost completely inhibited both granulocytic-macrophagic (CFU-GM) and erythroid (BFU-E) progenitors, whereas 79% ± 12% of CFU-GMs and 38% ± 9% BFU-Es still survived after treatment with nutlin-3 (Figure 7B).
Nutlin-3 induces cytotoxicity toward B-CLL cells via apoptosis. After exposure to nutlin-3 for 24 hours, B-CLL samples were analyzed by flow cytometry, after staining with annexin V–FITC/PI (A) and by Western blot on cell lysates (B). (A) Induction of apoptosis in nutlin-3–treated B-CLL samples, calculated with respect to control cultures (vehicle alone). For selected samples (nos. 6, 9, 12, 15, 16), aliquots of frozen B-CLL cells were thawed and retested for response to nutlin-3 (▦). Results are expressed as mean plus or minus SD of assays each performed in triplicate. (B) Western blot analysis for PARP and procaspase-3 after 24 hours of treatment with increasing doses of nutlin-3 of representative B-CLL cultures. The proform of PARP (115 kDa) and the cleaved form (80 kDa; arrowhead) are shown. The dose-dependent PARP cleavage in B-CLL cultures is concurrent with the decrease in the procaspase-3. Error bars indicate SD.
Nutlin-3 induces cytotoxicity toward B-CLL cells via apoptosis. After exposure to nutlin-3 for 24 hours, B-CLL samples were analyzed by flow cytometry, after staining with annexin V–FITC/PI (A) and by Western blot on cell lysates (B). (A) Induction of apoptosis in nutlin-3–treated B-CLL samples, calculated with respect to control cultures (vehicle alone). For selected samples (nos. 6, 9, 12, 15, 16), aliquots of frozen B-CLL cells were thawed and retested for response to nutlin-3 (▦). Results are expressed as mean plus or minus SD of assays each performed in triplicate. (B) Western blot analysis for PARP and procaspase-3 after 24 hours of treatment with increasing doses of nutlin-3 of representative B-CLL cultures. The proform of PARP (115 kDa) and the cleaved form (80 kDa; arrowhead) are shown. The dose-dependent PARP cleavage in B-CLL cultures is concurrent with the decrease in the procaspase-3. Error bars indicate SD.
p53-dependent target gene activation in response to nutlin-3 and fludarabine. Samples from 4 patients with B-CLL were treated with nutlin-3 and fludarabine (both used at 10 μM) for 24 hours. The levels of p53 and Ser15 phosphorylated-p53 (P-p53) (A) as well as p53-related gene expression (B) were assessed by Western blot and cDNA microarray analysis, respectively. (A) The effect of nutlin-3 and fludarabine treatments on cell viability is also reported for each patient analyzed. (B) Ratios represent nutlin-3 or fludarabine values divided by untreated values. Results are expressed as means plus or minus SD of the 4 B-CLL samples analyzed. *Genes significantly upmodulated by the treatments (above the cut-off of 2-fold of induction).
p53-dependent target gene activation in response to nutlin-3 and fludarabine. Samples from 4 patients with B-CLL were treated with nutlin-3 and fludarabine (both used at 10 μM) for 24 hours. The levels of p53 and Ser15 phosphorylated-p53 (P-p53) (A) as well as p53-related gene expression (B) were assessed by Western blot and cDNA microarray analysis, respectively. (A) The effect of nutlin-3 and fludarabine treatments on cell viability is also reported for each patient analyzed. (B) Ratios represent nutlin-3 or fludarabine values divided by untreated values. Results are expressed as means plus or minus SD of the 4 B-CLL samples analyzed. *Genes significantly upmodulated by the treatments (above the cut-off of 2-fold of induction).
In additional sets of experiments, the effect of combining nutlin-3 with fludarabine was investigated. B-CLL cells were cultured with increasing concentrations of nutlin-3 alone (0.1 μM to 10 μM), fludarabine alone (0.1 μM to 10 μM), or increasing concentrations of a constant ratio of nutlin-3 to fludarabine (1:1) for 48 hours. When data were analyzed by the method of Chou and Talalay,14 synergy of the 2 agents (values < 1) was observed in 11 of 15 B-CLL samples (Table 4). Similarly, the combination of nutlin-3 with chlorambucil, an alkylating drug commonly used in the treatment of B-cell malignancies,8 induced synergistic cytotoxicity in 4 of 6 B-CLL samples tested (Table 5).
Discussion
B-CLL is the most common type of leukemia in the western world, accounting for around 30% of all leukemias. The clinical course of B-CLL shows a marked heterogeneity from an indolent type, without need for treatment for decades, to a rapid progressive disease that requires immediate therapy.8 Clinical stage at diagnosis (ie, Rai and Binet stages) remains a strong predictor for survival. Additional prognostic parameters, including lymphocyte doubling time, immunophenotype, and cytogenetics, have been identified.8 Although more than 50% of human primary tumors exhibit abnormalities in the p53 gene,26 deletions and/or mutations of the TP53 gene occur in about 10% to 15% in B-CLL. They become more frequent as the disease progresses and predict aggressive disease that will be unresponsive to chemotherapy.16
Comparative effect of nutlin-3 and fludarabine on leukemic B-CLL cells and on normal BM clonogenic progenitors. (A) Positive and significant correlation between nutlin-3 and fludarabine cytotoxicity in B-CLL cultures (n = 29), measured after 48 hours of treatment. (B) Effect of nutlin-3 and fludarabine on normal clonogenic progenitors. Results are expressed as the mean of 3 independent experiments each performed in triplicate plus or minus SD of the number of colonies in the presence of 10 μM nutlin-3 or 10 μM fludarabine compared with the number in control cultures. Asterisk indicates significance at P < .01.
Comparative effect of nutlin-3 and fludarabine on leukemic B-CLL cells and on normal BM clonogenic progenitors. (A) Positive and significant correlation between nutlin-3 and fludarabine cytotoxicity in B-CLL cultures (n = 29), measured after 48 hours of treatment. (B) Effect of nutlin-3 and fludarabine on normal clonogenic progenitors. Results are expressed as the mean of 3 independent experiments each performed in triplicate plus or minus SD of the number of colonies in the presence of 10 μM nutlin-3 or 10 μM fludarabine compared with the number in control cultures. Asterisk indicates significance at P < .01.
Consistent with previous findings suggesting that other aberrations of p53 gene expression may contribute to p53 dysfunction in B-CLL,17,18 we have shown that the basal steady-state mRNA levels of TP53 are significantly decreased in B-CLL cells with respect to normal B lymphocytes. However, in spite of the decreased p53 mRNA levels, the p53 pathway could be activated by treatment with nutlin-3 in the great majority of B-CLL samples examined, including some taken from patients displaying poor prognostic features. In particular, nutlin-3 was effective also in B-CLL samples showing a low doubling time and high ZAP-70 expression. These findings are noteworthy since ZAP-70 was found highly expressed in a subset of B-CLL and closely associated with an unmutated configuration of the immunoglobulin heavy-chain variable region (IgVH) genes.20 Patients with B-CLL with strong ZAP-70 expression have unfavorable biologic features with rapid clinical progression, require treatment, and have short survival times.27,28
In considering the potential therapeutic application of nutlin-3 to B-CLL, it should be emphasized that the therapeutic activity of any of the currently used cytotoxic drugs for that subset of B-CLL patients requiring treatment,8 is a trade-off between efficacy and adverse effects, namely aspecific genotoxic collateral damage. In this respect, we have demonstrated in this study that fludarabine showed higher cytotoxicity than equimolar concentrations of nutlin-3 on hematopoietic clonogenic progenitors. Therefore, non-genotoxic activation of the p53 pathway is an attractive therapeutic strategy for B-CLL. Interestingly, while this manuscript was in preparation, 2 studies have demonstrated that nutlins are effective in inducing cytotoxicity in other hematologic malignancies, such as acute myeloid leukemia29 and multiple myeloma.30
Notably, nutlin-3 induces cytotoxicity toward B-CLL cells at concentrations that are minimally toxic toward normal B lymphocytes, PBMCs, and BM hemopoietic progenitor cells. Moreover, it is noteworthy that nutlin-3 induced the transcriptional activation of MDM2 in all normal cell types examined (B lymphocytes, PBMCs, and BMMCs) but only in a minority of B-CLL cells. Although we do not have an explanation for this differential behavior between primary normal and leukemic cells, the poor activation of MDM-2 by nutlin-3 in B-CLL cells might be beneficial in a therapeutic perspective contemplating the use of nutlin-3, taking into consideration that MDM-2 acts as a negative feedback regulator of p53.2
A second unexpected finding of our study was that the stabilization and accumulation of p53 induced by nutlin-3 in leukemic cells was accompanied by a variable increase in Ser15 phosphorylation. Because Ser15 is a site typically phosphorylated in response to DNA damage,3 the present results cannot rule out the possibility that nutlin-3 has a second mechanism of action that involves DNA damage in B-CLL cells. In this respect, it should be noted that the role of phosphorylation in p53 activation and function is still controversial. In fact, Ser15 phosphorylation has been shown to enhance the transactivation activity of p53 by enhancing the interactions with the coactivator CBP, whereas it does not affect the interaction between p53 and MDM2.31,32 However, studies using mouse mutants with substitutions of Ser15, Ser18, or Ser23 have shown that these residues are not essential for p53 activation,33-35 and Thomson et al36 have recently demonstrated that nutlin-3a induces p53 accumulation and transcriptional activity in the absence of phosphorylation in 2 colon cancer cell lines.
In apparent contrast with the findings of Thomson et al,36 we have documented a variable induction of p53 Ser15 phosphorylation in B-CLL samples, in response to nutlin-3, although at significantly lower levels compared with genotoxic agents. This discrepancy can easily be reconciled, taking into account the different cell models used in our study and the Thomson study and that potential upstream kinases able to phosphorylate p53, such as phosphatidylinositol-3 kinase family members, have been found spontaneously elevated in B-CLL cells.37 Moreover, consistent with the findings of Thomson et al,36 we have found that a strong induction of Ser15 p53 phosphorylation by fludarabine in B-CLL did not correlate with an increased transcriptional activity of p53 or with an increased cytotoxic activity with respect to nutlin-3.
Another interesting observation of our study is that nutlin-3 enhanced the cytotoxic effects of both fludarabine and chlorambucil. This is particularly noteworthy taking into account that treatment with fludarabine has been shown to increase the complete remission rate, enhance progression-free survival, and increase the median duration of the clinical response but not the overall survival in patients with CLL, as compared with treatment with chlorambucil alone or combination chemotherapy. Moreover, analyzing the gene-expression profile of B-CLL in response to nutlin-3 and fludarabine, we could demonstrate the upregulation of several genes that have been involved in mediating the apoptotic response of p53.26,38 However, since p53 induces apoptosis via both transcription-dependent and transcription-independent mechanisms,39,40 a direct apoptogenic role of p53 at the mitochondria level following nutlin-3 treatment cannot be excluded.
Taken together, our data demonstrate that selective p53 activation with nutlin-3 variably induces apoptosis in both low- and high-risk subtypes of B-CLL patient cells. Therefore, additional preclinical evaluation of nutlin-3, used as a single agent or in combination with fludarabine, for the treatment of B-CLL appears warranted.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-11-4465.
Supported by the Italian Fondo per l'Incentivazione della Ricerca di Base (FIRB) grants (P.S., G.Z.), by an Associazione Italiana per la Ricerca sul Cancro (AIRC) grant (G.Z.), and by the CAN2005 Project “Comitato dei Sostenitori” (P.S.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.