Abstract
Because of their frequent expression in a wide spectrum of malignant tumors but not in normal tissue except testis, cancer testis antigens are promising targets. However, except for HOM-TES-14/SCP1, their expression in malignant lymphomas is rare. SCP1 (synaptonemal complex protein 1) has been shown to elicit antibody responses in the autologous host, but no T-cell responses against HOM-TES-14/SCP1 have been reported. Using the SYFPEITHI algorithm, we selected peptides with a high binding affinity to major histocompatibility complex class 2 (MHC 2) molecules. The pentadecamer epitope p635-649 induced specific CD4+ T-cell responses that were shown to be restricted by HLA-DRB1*1401. The responses could be blocked by preincubation of T cells with anti-CD4 and antigen-presenting cells with anti–HLA-DR, respectively, proving the HLA-DR–restricted presentation of p635-649 and a CD4+ T-cell–mediated effector response. Responding CD4+ cells did not secrete interleukin-5 (IL-5), indicating that they belong to the TH1 subtype. The natural processing and presentation of p635-649 were demonstrated by pulsing autologous and allogeneic dendritic cells with a protein fragment covering p635-649. Thus, p635-649 is the first HOM-TES-14/SCP1–derived epitope to fulfill all prerequisites for use as a peptide vaccine in patients with HOM-TES-14/SCP1–expressing tumors, which is the case in two thirds of peripheral T-cell lymphomas.
Introduction
A prerequisite for immunotherapeutic approaches for the treatment of cancer is the existence of molecules that are either exclusively or preferentially expressed by malignant, but not normal, cells and that have the capacity to induce a specific immune response in the tumor-bearing host. Of the different types of such tumor-specific or tumor-associated antigens (for a review, see Preuss et al1 ), the so-called shared tumor-specific antigens are the most attractive targets because they are expressed in a broad spectrum of different human neoplasms. Shared tumor-specific antigens include the cytotoxic T-lymphocyte (CTL)–reactive MAGE,2 BAGE,3 and GAGE4,5 families, as well as HOM-MEL-40/SSX2,5 the other SSX family members,6 NY-ESO-1,7 HOM-TES-14/SCP1,8 CT-7,9 and HOM-TES-85,10 all of which have been defined using SEREX, the serological identification of antigens by recombinant expression cloning.11 It is enigmatic that the expression of all the so-called shared tumor antigens in humans that have been molecularly defined to date by cellular and serologic techniques is restricted to different types of cancers and normal testis. Therefore, the term “cancer testis antigens” (CTA)7,12 or “cancer germline antigens”12 has been coined for them, and “cancer testis genes” has been coined for their encoding genes.
Even though the SEREX approach has considerably enlarged the pool of molecularly defined cancer testis antigens,13 the ability of SEREX-defined antigen to induce T-cell responses has been demonstrated for only a few of them, namely NY-ESO-114-16 and SSX2,17-20 but not for SCP1, which has been shown to be the most frequently expressed CTA in a variety of neoplasms of different origin.21-24 This holds particularly true for malignant lymphomas, in which other CTAs are rarely expressed. HOM-TES-14/SCP1 expression is most frequent in peripheral T-cell lymphomas; two thirds of patients have been shown to express this antigen.24 Therefore, SCP1 is an attractive target structure for immunotherapeutic approaches in T-cell lymphomas. However, because the SCP1 gene contains an open reading frame as large as 3 kb, SCP1 protein vaccines are difficult and expensive to produce, and only vaccines consisting of immunogenic peptides or protein fragments containing the relevant peptides appear to be clinically feasible. Prerequisite for such vaccines is the identification of epitopes within the sequence of the SCP1 antigen that can induce a T-cell response when presented in the context of major histocompatibility complex (MHC) molecules. Because increasing evidence from human and animal studies demonstrates that CD4+ T cells play a central role in initiating and maintaining host immune responses against cancer, we aimed at screening the HOM-TES-14/SCP1 antigen for MHC class 2–restricted epitopes. To this end, we used the SYFPEITHI algorithm, which predicts the binding affinities of peptides to 6 different HLA-DR subtypes—HLA-DRB1*0101, HLA-DRB1*0301, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*1101, and HLA-DRB1*1501—with a high prevalence among the white population. We now report on the identification and functional analysis of the first HOM-TES-14/SCP1–derived peptide that induces CD4+ responses in an MHC class 2–restricted fashion. Our results show that the pentadecamer p635-649 is a valuable tool for immunotherapeutic approaches targeting HOM-TES14/SCP1.
Patients, materials, and methods
The study was approved by the local ethics review committee (Ethikkommission der Aerztekammer des Saarlandes) and was performed in accordance with the Declaration of Helsinki. Recombinant DNA work was performed with the permission, and according to the regulations, of local authorities (Regierung des Saarlandes).
Patients and healthy controls
Seven unselected patients were included in this study irrespective of their anti–HOM-14/SCP1 serology and HLA-DR subtype. All patients and healthy controls gave written informed consent. The patients were studied at the time of diagnosis and had not received any chemotherapy or radiotherapy.
Selection of peptides
Peptides were derived from the previously published HOM-TES-14/SCP1 sequence (Swissprot accession no. Q15431).25 The SYFPEITHI algorithm (www.syfpeithi.de) was used to predict SCP1-derived peptides with a high probability to bind to the 6 DRB1 subtypes (*0101, *0301, *0401, *0701, *1101, and *1501). These subtypes have a high prevalence among the white population. SYFPEITHI predicted 14 pentadecamer peptides to have a high promiscuous binding to these 6 HLA-DRB1 molecules (Table 1). The 13-mer PADRE, a pan DR-binding peptide, was used as negative control.26 A mix of 7 pentadecamers derived from pp65 of the human cytomegalovirus (CMV) and predicted to bind to the HLA-DR subtypes of interest (p32, p117, p243, p269, p299, p510, and p524) was used as positive control.19 Peptides were synthesized according to the Fmoc/tBu strategy, as described.27 Purity was greater than 90%, as assessed by high-performance liquid chromatography (HPLC) and mass spectrometry. All peptides were dissolved completely in a mixture of water and dimethyl sulfoxide (DMSO).
Production of recombinant proteins
Because of the length of the SCP1 protein, it was not possible to produce a full-length SCP1 protein. SCP1-derived fragment p630-817 was amplified by polymerase chain reaction (PCR). For expression as GST fusion protein, the PCR product was cloned into the GEX4T1 vector (Amersham Pharmacia Biotech AB; Uppsala, Sweden) and was expressed in Escherichia coli BL21 cells that were induced by 1 mM isopropylthiogalactoside (IPTG) for 4 hours. After sonification, GST-SCP1/630-817 was purified by glutathione affinity chromatography followed by thrombin cleavage and benzamidine column purification, as described in the GST Gene Fusion System manual (Amersham Pharmacia Biotech AB, Frankfurt, Germany). SSX2 was expressed in E coli SG13009 strain as full-length protein with a His-tag at the NH2 terminus using pQE9 (QIAGEN, Hilden, Germany). The induction of recombinant protein synthesis and subsequent purification by Ni2+ column chromatography was performed according to the manufacturer's instructions. Purified proteins reacted with a polyclonal rabbit anti-SCP1 antiserum, which was obtained by immunizing a rabbit with SCP1 fragment p630-817 and the anti-SSX monoclonal antibody E3AS,28 respectively, as shown by Western blot analysis. Purity of the recombinant proteins was at least 90%, as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Generation of SCP1-derived, epitope-specific CD4+ T cells
Peripheral-blood mononuclear cells (PBMCs) and serum samples were obtained from 7 patients and several healthy blood donors and were isolated by Ficoll-Hypaque PLUS separation (Amersham Pharmacia Biotech AB). For in vitro priming, the unseparated PBMCs were suspended in serum-free medium (Cambrex, Verviers, Belgium) supplemented with 2 mM Lglutamine (Gibco, Invitrogen, Karlsruhe, Germany) and 1% penicillin/streptomycin (Gibco) and were divided into 3 fractions. Two of these fractions (with equal numbers of PBMCs) were pulsed with different peptide pools, each with 7 SCP1-derived peptides (2 μg/mL for each peptide). As positive control for the in vitro stimulation, the third fraction of the PBMCs was pulsed with a mix of 7 peptides derived from the pp65 antigen of the human CMV. Pulsing was performed for 90 to 120 minutes at 37°C. Thereafter, the pulsed cells were washed once with serum-free medium. For cultivation, the pulsed cells were suspended in T-cell medium (X-Vivo 15 [Cambrex] supplemented with 10% human AB serum) and 5 ng/mL IL-7 (R&D Systems GmbH, Wiesbaden, Germany) and were distributed at a density of 2 × 106 PBMCs/mL into the wells of a 48-well clone plate (Nunc, Wiesbaden, Germany). Twenty-four hours after (re)stimulation, 20 U/mL recombinant human (rh) IL-2 (rhIL-2; R&D Systems GmbH) was added. According to this protocol, the unseparated PBMCs were used as antigen-presenting cells (APCs) and as a source for effector cells. The remaining PBMCs were divided into aliquots and frozen until use as APCs for restimulation. After 7 days, the number of CD4+ T cells was assessed by flow cytometry, and the cells were restimulated with autologous PBMCs that had been irradiated with 30 Gy and pulsed with the appropriate peptides under the same conditions described for the initial priming, at a CD4+/APC ratio of 1:1. On day 14, the first ELISPOT assay was performed.
Establishment of epitope-specific CD4+ T-cell clones and lines
CD4+ T cells secreting interferon-γ (IFN-γ) in response to peptide stimulation were isolated by IFN-γ–based magnetic cell enrichment using a cytokine secretion assay (Miltenyi Biotec, Bergisch Gladbach, Germany) and were cloned by limiting-dilution culture, according to a modified protocol for the generation of CD8+ T-cell clones.29 RPMI 1640 (Gibco) supplemented with 10% human AB serum (Cambrex), 50 U/mL rhIL-2, and anti-CD3 antibody (clone OKT-3) at 30 ng/mL was used as medium for cloning. Allogeneic irradiated PBMCs (30 Gy) and allogeneic lymphoblastoid cell lines (LCLs) (120 Gy) were used as stimulator and feeder cells, respectively. Specifically reacting clones were expanded with restimulation performed after 14 days under identical conditions.
Establishment of LCLs
PBMCs (1 × 107) were incubated in 2.5 mL complete RPMI 1640 medium (Gibco) supplemented with 2.5 mL Epstein-Barr virus (EBV) containing (more than 103 transforming units/mL) B95-8 culture supernatant in humidified air at 37°C and 5% CO2. Cells were supplemented with fresh medium (complete RPMI 1640) if required. After 2 to 3 weeks, the EBV-transformed B cells (LCLs) appeared as clusters in the culture medium. For expansion, the culture was split (1:3 to 1:5) twice a week. All LCLs used for this study strongly expressed HLA-DR molecules, as assessed by flow cytometry.
Generation of DCs
Monocyte-derived dendritic cells (DCs) were generated from PBMCs. In brief, PBMCs were isolated from 60 to 70 mL EDTA (ethylenediaminetetraacetic acid) blood by density gradient centrifugation with Ficoll-Hypaque Plus (Amersham Pharmacia Biotech AB). CD14+ monocytes were separated by magnetically activated cell sorting (MACS) with CD14+ microbeads (Miltenyi Biotec) according to the manufacturer's instructions. The enriched CD14+ fraction was cultured in X-Vivo 15 medium (Cambrex) containing 1% human AB serum (Cambrex). For DC maturation, 800 U rhGM-CSF and 1000 U rhIL-4 were added. On day 5, all nonadherent cells were collected and transferred to new wells with fresh cytokine-supplemented medium, and the respective antigens were added at the indicated concentrations. All cytokines were purchased from R&D Systems GmbH. DCs were harvested on day 6 and were used as APCs in the ELISPOT assays at 1 × 103 cells per well. DC maturation stage was assessed by the reactivity of the DCs with a panel of monoclonal antibodies and analyzed by flow cytometry using a FACScan (Becton Dickinson, Heidelberg, Germany). Mature DCs had the phenotype CD14–, CD1a+, CD83+, CD86+++, HLA-DR+++, and MHC class 1+++ (data not shown).
Flow cytometric characterization of cells
For phenotypic characterization of effector cells and APCs, the cells were stained according to the supplier's instructions using the following antibodies: anti–HLA-DR/PE (clone L243), anti–hCD4/fluorescein isothiocyanate (FITC), anti–hCD4/peridin chlorophyll protein (PerCP) (both from clone SK3), anti-hCD8/phycoerythrin (PE) (clone SK1), anti-hCD45RA (clone L48; all obtained from Becton Dickinson), and anti-hCCR7/PE (clone 150503; R&D Systems GmbH). After staining the cells were resuspended in CellWash solution (Becton Dickinson), and 2 to 3 × 104 cells were analyzed using a FACScan 80214 (Becton Dickinson).
Blocking of T-cell responses
To prove the DR restriction of the respective T-cell responses, the T-cell receptor/MHC class 2 interaction was blocked using antibodies against HLA-DR (clone L243) or HLA-DP (clone B7/21; both from Becton Dickinson). To demonstrate the CD4+ phenotype of the responding T cells, the effector cells were blocked using an anti-CD4 antibody (clone MT310; DakoCytomation, Hamburg, Germany). Peptide/antigen-pulsed APCs or effectors were suspended in assay medium and incubated with the appropriate antibody (5 μg/mL) for 30 minutes on ice. Subsequently, the cells were washed, resuspended at the indicated concentrations in assay medium, and transferred to the test plate. None of the antibodies exhibited any cytotoxic activity at the concentrations used for the blocking experiments, as demonstrated by the absence of inhibition of MHC class 1–mediated T-cell responses by L243, B7/21, and MT310 (data not shown).
ELISPOT assay
In addition to the first ELISPOT assay on day 14, 2 more IFN-γ ELISPOT assays were performed on days 21 and 28, respectively, using poolstimulated bulk T cells as effectors to demonstrate specific reactivity to autologous APCs (PBMCs, irradiated with 60 Gy, 5 × 104/well) pulsed with each single peptide (2 μg/mL) of the respective peptide pool. In the case of a peptide-specific T-cell response, the bulk T-cell population was cloned. The resultant CD4+ T-cell clones were used for repeat assays with various allogeneic or autologous APCs (irradiated LCLs at 3 × 104/well or DCs at 1 × 104/well, respectively). Assays were performed in nitrocelluloselined 96-well plates (MAHAN 45; Millipore, Bedford, MA). Wells were precoated with anti–IFN-γ capture antibody at the dilution recommended in the supplier's instructions (Mabtech, Nacka, Sweden). To block unspecific binding, the wells were then incubated with 10% human AB serum. Next, the APCs were added after suspension in serum- and cytokine-free X-Vivo 15 medium (assay medium). Finally, effector cells were harvested, washed once to remove cytokines, resuspended in assay medium, and added at the indicated numbers. For bulk cultures, 2.5 × 104 or 1.5 × 104 effector CD4+ T cells/well were used; when CD4+ T-cell clones were tested, 1000 CD4+ T cells/well were used. Effector cells and APCs were coincubated for 14 to 16 hours at 37°C. Thereafter, the supernatants were collected and frozen until analyzed for IL-5 content by enzyme-linked immunosorbent assay (ELISA) (see “IL-5 ELISA”). To visualize the IFN-γ spots, the plates were washed thoroughly. Then biotinylated IFN-γ detection antibody (Mabtech) was added to each well diluted according to the supplier's instructions. The plates were incubated for 2 hours at 37°C. Again, plates were washed thoroughly. To enhance the sensitivity of the IFN-γ detection, plates were then incubated for 1 hour with alkaline phosphatase-conjugated streptavidin (Roche Diagnostics, Mannheim, Germany) diluted 1:2000 in phosphatebuffered saline (PBS). After a final washing, the IFN-γ spots were stained using the AP Conjugate Substrate Kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Spots were counted using the Bioreader-3000 Pro (Biosys, Karben, Germany). All tests were run in triplicate, and mean values and standard deviations were determined.
IL-5 ELISA
Supernatants of the IFN-γ ELISPOT assays, which had been stored frozen, were thawed and analyzed for IL-5 production using the DuoSet ELISA kit for human IL-5 (R&D Systems GmbH) with strict adherence to the supplier's instructions.
HLA-DR typing
HLA-DR subtyping of PBMCs and LCLs was performed by highresolution HLA-DR using sequence-specific oligonucleotide (SSO)–and sequence-specific primer (SSP)–PCR, respectively, according to protocols based on the 12th and 13th International Histocompatibility Workshops (http://www.ihwg.org/protocols/protocol.htm).30,31
Analysis of SCP1 mRNA expression
Fresh biopsy specimens were frozen within 15 to 20 minutes of surgical excision. The transcription of SCP1 mRNA was checked by reverse transcription (RT)–PCR using the primers SCP-1 1344-sense gt gag ctg gaa gag atg act aag ctt ac and SCP1 1705-antisense c ttc ttg ctg att ctt gag ttc tag ggt c (all primers from MWG Biotech, Ebersberg, Germany), as described previously.24
Detection of serum antibodies against SCP1
Serum antibodies against HOM-TES-14/SCP1 were assessed using SEREX, as described previously.8
Statistical analysis
All results are based on tests that were repeated at least twice. Only representative results are shown in this paper. The significance of the ELISPOT results was determined by a 2-sided Student t test.
Results
Identification of a SCP1-derived CD4+ T-cell–stimulating epitope
The SYFPEITHI algorithm predicted 14 peptides derived from HOM-TES-14/SCP1 with high binding probability to 6 different HLA-DRB1 subtypes (Table 1). To streamline the screening for T-cell–stimulating SCP1-derived epitopes, these 14 peptides were grouped into 2 pools of 7 peptides each (pool 1: peptides p244, p320, p338, p430, p482, p635, p684; pool 2: peptides p742, p821, p828, p837, p848, p901, p942). The 2 pools were used separately for in vitro stimulation of the PBMCs from 7 patients. After 21 days and 2 restimulations (Figure 1) with the respective peptide pools, the bulk T-cell cultures were tested by IFN-γ ELISPOT assay for reactivity against autologous PBMCs that had been pulsed with each peptide. As can be seen from Figure 1A-B, PBMCs from patient 497 developed a significant and specific response against p635-649, comparable to the anti-CMV response that served as a positive control. The reactivity against p635-649 increased after each additional restimulation with the peptide. After 28 days, 1.5 × 104 CD4+ T cells induced 150 spots, indicating that approximately 1% of the in vitro–stimulated bulk population (150 spots/1.5 × 104 CD4+ T cells) reacted specifically to p635-649 (Figure 2). As can be seen from Table 2, patient (Pt) 497 was the only patient with detectable anti-SCP1 antibody reactivity in his serum. No T-cell reactivity was detected in the PBMCs of the remaining 6 patients against any of the 14 SCP1-derived peptides tested. Five of these 6 patients were tested for anti-SCP1 antibody reactivity, and results for all 5 were negative.
T-cell response against the HOM-TES-14/SCP1–derived peptides. (A) Autologous PBMCs (5 × 104/well) were used as APCs to stimulate bulk T cells of 7 patients. T cells (2.5 × 104 CD4+ T cells/well) from 1 of 7 of these patients showed a significant response against peptide p635-349 after stimulation for 21 days with PBMCs loaded with the first pool of 7 SCP1-derived peptides (P < .001). The p635-649–specific response was of a magnitude similar to that of T cells that had been prestimulated with a pool of 7 peptides derived from the pp65 antigen of the human CMV under the same conditions. (B) No significant response was observed after stimulation with 7 additional HOM-MEL-14/SCP1–derived peptides constituting the second pool. In the absence of effector cells, the autologous APCs did not secrete IFN-γ.
T-cell response against the HOM-TES-14/SCP1–derived peptides. (A) Autologous PBMCs (5 × 104/well) were used as APCs to stimulate bulk T cells of 7 patients. T cells (2.5 × 104 CD4+ T cells/well) from 1 of 7 of these patients showed a significant response against peptide p635-349 after stimulation for 21 days with PBMCs loaded with the first pool of 7 SCP1-derived peptides (P < .001). The p635-649–specific response was of a magnitude similar to that of T cells that had been prestimulated with a pool of 7 peptides derived from the pp65 antigen of the human CMV under the same conditions. (B) No significant response was observed after stimulation with 7 additional HOM-MEL-14/SCP1–derived peptides constituting the second pool. In the absence of effector cells, the autologous APCs did not secrete IFN-γ.
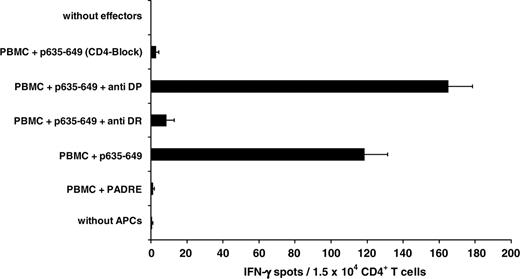
Demonstration of the HLA-DR restriction of the CD4+-mediated T-cell response against the HOM-TES-14/SCP1–derived peptide p635-649. The HLA-DR restriction of the anti–p635-649 T-cell response (1.5 × 104 CD4+ T cells/well) was demonstrated by ELISPOT assay by significant (P < .01) blocking of the response by incubating the APCs with anti-DR antibody (clone L243) after p635-649 stimulation for 28 days. Treatment of p635-649–pulsed autologous PBMCs (5 × 104/well) with anti-DP antibody had no effect. Similarly, the reaction was significantly (P < .01) blocked after incubation of the effector cells with anti-CD4 antibody (clone MT310).
Demonstration of the HLA-DR restriction of the CD4+-mediated T-cell response against the HOM-TES-14/SCP1–derived peptide p635-649. The HLA-DR restriction of the anti–p635-649 T-cell response (1.5 × 104 CD4+ T cells/well) was demonstrated by ELISPOT assay by significant (P < .01) blocking of the response by incubating the APCs with anti-DR antibody (clone L243) after p635-649 stimulation for 28 days. Treatment of p635-649–pulsed autologous PBMCs (5 × 104/well) with anti-DP antibody had no effect. Similarly, the reaction was significantly (P < .01) blocked after incubation of the effector cells with anti-CD4 antibody (clone MT310).
Demonstration of HLA-DR restriction of the CD4+-mediated response to p635-649
The DR restriction of the T-cell response against SCP1-derived p635-649 was demonstrated by blocking the response with the HLA-DR–specific antibody L243 (Figure 2). Restriction by HLA-DP was excluded by demonstrating that the HLA-DP–specific antibody B7/21 did not interfere with this response. The response to p635-649 was mediated by CD4+ T cells because treatment with anti-CD4 resulted in a significant inhibition of the reaction (Figure 2).
Establishment and characterization of T-cell lines and clones with specificity for p635-649
The p635-649–reactive T cells were enriched using IFN-γ–based MACS separation. The MACS-enriched fraction was submitted to limited-dilution culture, and 3 different p635-649–specific CD4+ T-cell clones and 10 lines could be established. The fraction of CD4+ T cells was less than 80% before MACS separation and ranged from 81% to 90% in the various T-cell lines and from 96% and 99% in the T-cell clones (data not shown).
All T-cell lines and clones were shown to recognize p635-649 specifically. Clone 60 was chosen for further analysis. T cells of clone 60 were highly activated, as indicated by their strong expression of HLA-DR shown by flow cytometry (Figure 3A-B). According to the Lanzavecchia classification scheme,32 clone 60 cells were in the stage of effector memory T-helper cells because they were negative for CCR7 and CD45RA (Figure 3C). No IL-5 was detected by ELISA (detection limit less than 25 pg/mL) in the supernatant of the T cells responding to p635-649 in the IFN-γ ELISPOT assay, whereas IFN-γ secretion was detectable in cells of clone 60 cells after stimulation with APCs pulsed with as little as 30 nM p635-649 (Figure 4). The fact that the CD4+ T cells in the bulk culture and in 2 different CD4+ T-cell lines and clones tested produced IFN-γ, but no IL-5, on stimulation with SCP1-derived p635-649 indicates that the T cells responding to p635-649 belonged to the TH1 subset.
Dissection of DR restriction with high resolution
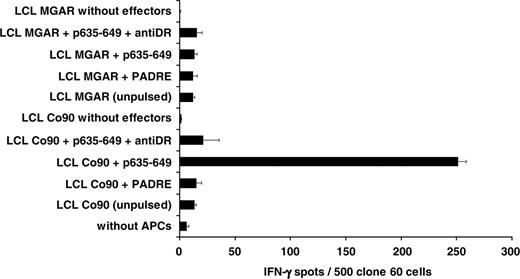
To determine the HLA-DR subtype restricting the T-cell response against p635-649, allogeneic APCs with only partial HLA-DR identity were used in the ELISPOT assays. To exclude an unspecific reaction caused by T-cell reactivity against molecules other than the peptide under investigation presented by the respective (allogeneic) LCL, it was mandatory to use T cells exclusively with p635-649–specific reactivity. Therefore, T cells derived from the p635-649–specific CD4+ T-cell clone 60 were used for these experiments. For use as allogeneic APCs, 3 partially HLA-DR–identical LCLs were established and pulsed with 2 μg/mL p635-649 or a control peptide. Pulsed LCLs were used to stimulate cells derived from the p635-649–specific clone 60, and the observed reactions were blocked with an anti–HLA-DR antibody. As shown in Figure 5, the T cells of clone 60 (HLA-DRB1*1401/1501; HLA-DRB3*0202; HLA-DRB5*0101) responded to p635-649–pulsed LCLs from donor Co90 in a DR-restricted way (HLA-DRB1*1101/1401; HLA-DRB3*0202). In contrast, no reactivity was observed against the p635-649–pulsed MGAR cells (HLA-DRB1*1501; HLA-DRB5*0101). These results indicate that the T-cell response against p635-649 was mediated by HLA-DRB1*1401 and/or HLA-DRB3*0202. To further narrow down the responsible HLA-DR restriction, the allogeneic LCL Co7 (HLA-DRB1*1101; HLA-DRB3*0202) was pulsed with p635-649 and used as an APC, which shared only the HLA-DRB3*0202 subtype with the T cells from patient 497, clone 60. Because Co7 LCL did not stimulate T cells from clone 60, the response of the T cells from clone 60 against p635-649 is restricted by HLA-DRB1*1401. A summary of the experiments dissecting the restriction against p635-649 is shown in Figure 6.
Characterization of the cells of p635-649–specific clone 60. (A) Expression of HLA-DR molecules on the surfaces of the CD4+-gated cells before in vitro stimulation with SCP1-derived peptides. (B) Expression of HLA-DR on the CD4+-gated cells of clone 60 (which made up 96% of this clone) demonstrated the strong activation of these T cells on stimulation with p635-649. (C) Phenotype of the CD4+-gated cells of clone 60 (CD45 RA–/CCR7–) corresponded to the effector-memory type, according to the classification scheme suggested by Sallusto et al.32
Characterization of the cells of p635-649–specific clone 60. (A) Expression of HLA-DR molecules on the surfaces of the CD4+-gated cells before in vitro stimulation with SCP1-derived peptides. (B) Expression of HLA-DR on the CD4+-gated cells of clone 60 (which made up 96% of this clone) demonstrated the strong activation of these T cells on stimulation with p635-649. (C) Phenotype of the CD4+-gated cells of clone 60 (CD45 RA–/CCR7–) corresponded to the effector-memory type, according to the classification scheme suggested by Sallusto et al.32
Natural processing and presentation of the HOM-TES-14/SCP1–derived peptide p635-649
The natural processing and presentation of p635-649 were demonstrated by adding the recombinant protein fragment p630-817, derived from the HOM-MEL-14/SCP1 antigen, to the culture medium used to generate DCs. Full-length SSX2 antigen served as a negative control. Figure 7 shows the specific response of T cells from clone 60 against the SCP1 fragment p630-817, processed and presented by allogeneic DCs. The response showed a strong correlation with the concentration of the protein fragment used in the medium for DC generation. The reactivity against the processed epitope derived from the protein fragment p630-817 could be blocked by preincubation of the APCs with anti–DR-antibody, proving once more the HLA-DR restriction of the presented epitope after processing of the HOM-TES-14/SCP1 fragment p630-817 by DCs.
Discussion
Immunotherapy using the unique portions of the surface immunoglobulin on each B-cell lymphoma (idiotypes) has been shown to be a promising approach for the treatment of follicular lymphoma.33-35 Similar vaccine approaches appear to be even more warranted for T-cell lymphoma, which has a worse prognosis than B-cell lymphoma.36 The fact that most patients with T-cell lymphoma achieve complete remission but have a high relapse rate makes lowering the relapse rate a major therapeutic goal. The period of remission after intensive chemotherapy opens a time window when immunotherapeutic approaches should be most effective because the tumor burden of patients in remission is comparatively low. However, in contrast to B-cell lymphomas, vaccine strategies have not been widely tested in T-cell lymphomas. To a large extent this is because of the lack of target structures expressed by T-cell lymphomas that are suitable for vaccine development.
Dose-response curve of clone 60 cells against p635-649. Titration of peptide p635-649 used for pulsing allogeneic APCs (LCL Co90 at a concentration of 3 × 104/well) showed a response of 1000 clone 60 cells/well with concentrations of as little as 30 nM peptide.
Dose-response curve of clone 60 cells against p635-649. Titration of peptide p635-649 used for pulsing allogeneic APCs (LCL Co90 at a concentration of 3 × 104/well) showed a response of 1000 clone 60 cells/well with concentrations of as little as 30 nM peptide.
Subtype-specific dissection of the HLA-DR restriction of the p635-649 induced T-cell response. Using allogeneic LCLs (3 × 104/well) with different HLA-DR subtypes as APCs for cells (500/well) of the p635-649–specific CD4%+ T-cell clone 60 allowed determining the HLA-DR subtype-specific restriction of the anti–p635-649 reaction. The HLA-DR set-up of the 3 LCLs derived from Co90, Co7, and MGAR, respectively (Table 3), identified HLA-DRB1*1401 as the restricting subtype because in the allogeneic setting only LCLs from Co 90 induced significant IFN-γ production (P < .01) in response to the HOM-TES-14/SCP1–derived epitope p635-649 in T cells from clone 60.
Subtype-specific dissection of the HLA-DR restriction of the p635-649 induced T-cell response. Using allogeneic LCLs (3 × 104/well) with different HLA-DR subtypes as APCs for cells (500/well) of the p635-649–specific CD4%+ T-cell clone 60 allowed determining the HLA-DR subtype-specific restriction of the anti–p635-649 reaction. The HLA-DR set-up of the 3 LCLs derived from Co90, Co7, and MGAR, respectively (Table 3), identified HLA-DRB1*1401 as the restricting subtype because in the allogeneic setting only LCLs from Co 90 induced significant IFN-γ production (P < .01) in response to the HOM-TES-14/SCP1–derived epitope p635-649 in T cells from clone 60.
HOM-TES-14/SCP1 was originally detected by its ability to induce a specific antibody response in a patient with seminoma,8 but antibodies against HOM-TES-14/SCP1 have also been repeatedly demonstrated in the sera of patients with cutaneous T-cell lymphomas37 and other lymphomas.38 Most important, HOM-TES-14/SCP1 has been shown to be the most frequently expressed cancer testis antigen in cutaneous39 and in nodal peripheral T-cell lymphomas.24
Summary of T-cell responses of clone 60 effector cells against APCs with partial HLA-DR identity. This strategy allowed a definite dissection of the DR restriction of the T-cell response against the HOM-TES-14/SCP1 derived epitope p635-649. NE indicates not expressed; Yes indicates a significant and specific IFN-γ release in response to p635-649; No indicates that no significant response was observed in the context of the respective HLA-DR configuration (see also Figure 5).
Summary of T-cell responses of clone 60 effector cells against APCs with partial HLA-DR identity. This strategy allowed a definite dissection of the DR restriction of the T-cell response against the HOM-TES-14/SCP1 derived epitope p635-649. NE indicates not expressed; Yes indicates a significant and specific IFN-γ release in response to p635-649; No indicates that no significant response was observed in the context of the respective HLA-DR configuration (see also Figure 5).
Natural processing and presentation of the HOM-TES-14/SCP1–derived peptide p635-649. To provide evidence for the natural processing of SCP1-derived peptide p635-649, a fragment was synthesized spanning the residues p630 to p817 and including p635-649. Generating DCs in medium with increasing concentrations of p630-687 induced a concentration-dependent T-cell response of clone 60 cells (1000 cells/well), which could be blocked by anti–HLA-DR. Full-length SSX2 antigen was used as a control (P < .001). Additional pulsing of the DCs with peptide p635-649 boosted the T-cell reaction significantly (P = .05), causing an increase of spot numbers by approximately 35%.
Natural processing and presentation of the HOM-TES-14/SCP1–derived peptide p635-649. To provide evidence for the natural processing of SCP1-derived peptide p635-649, a fragment was synthesized spanning the residues p630 to p817 and including p635-649. Generating DCs in medium with increasing concentrations of p630-687 induced a concentration-dependent T-cell response of clone 60 cells (1000 cells/well), which could be blocked by anti–HLA-DR. Full-length SSX2 antigen was used as a control (P < .001). Additional pulsing of the DCs with peptide p635-649 boosted the T-cell reaction significantly (P = .05), causing an increase of spot numbers by approximately 35%.
Despite the frequent expression of HOM-TES-14/SCP1 and the repeated demonstration of serum antibodies against this cancer testis antigen, the development of specific vaccine strategies targeting this antigen has been hampered by the fact that SCP1 has an open-reading frame of 3 kb, making the production of a whole-protein vaccine technically difficult and expensive. A prerequisite for the development of vaccines consisting of antigenic protein fragments or peptides is the exact identification of the epitopes that induce T-cell responses. Because of the extraordinary length of the entire SCP1 protein, the identification of T-cell–stimulating epitopes by synthesizing and testing overlapping peptides covering the whole antigenic protein15 or screening HLA-DR transgenic mice40 with such peptides would also be hindered by the enormous numbers of PBMCs or mice required. Instead, we used the SYFPEITHI algorithm to screen the entire sequence of the HOM-TES14/SCP1 antigen in silico. SYFPEITHI predicted 14 pentadecamer peptides to have a high binding probability to the HLA-DRB1 subtypes *0101, *0301, *0401, *0701, *1101, and *1501. According to HLA-DR typing performed a posteriori, 3 of 6 of the selected HLA-DRB1 subtypes (*0101, *0301, *1101) were not expressed by the PBMCs of the 7 patients included in this study. This fact and the result that the anti–p635-649 T-cell response was mediated by HLA-DRB1*1401, which is not included in SYFPEITHI to date, justify our strategy of reverse T-cell immunology for the identification of MHC class 2 binding peptides that does not restrict the analysis to patients selected for HLA-DR subtypes with a known high binding affinity to the respective peptide predicted by the SYFPEITHI algorithm because the SYFPEITHI score does not (yet) cover all known HLA-DR subtypes. Rather, for the present study and in light of the well-known promiscuous binding pattern of MHC class 2 peptides, it was advisable to include patients with unknown HLA status for the primary identification of CD4+ T-cell–stimulating epitopes. Although the HLA-DRB1*1401 restriction of the CD4+-mediated response to p635-649 has been established without any doubt by our experiments, a more promiscuous binding of this peptide to additional HLA-DR subtypes, as has been demonstrated for many other HLA-DR–restricted antigenic peptides,16,19,41-43 can be demonstrated or excluded only by analysis of a larger series of patients.
The HOM-TES-14/SCP1–derived epitope p635-649 induced an extraordinarily strong T-cell response mediated by peripheral CD4+ that was comparable in strength to the anti-CMV response in patient Pt 497. The dissection of this p635-649–induced T-cell response demonstrated that p635-649 was presented in the context of HLA-DRB1*1401. All other combinations for an interaction between the T-cell receptor on the CD4+ cells of patient 497 and MHC molecules of other classes or subtypes could be excluded by using APCs from allogeneic donors who shared only one haplotype with the p635-649–responding T cells. Although the in silico prediction of the potential HLA class 2 ligand from any protein sequence based on the identification of appropriately positioned anchor residues specific for a given HLA class 2 allele44 allowed for the identification of peptides with high binding affinity, many such peptides were shown not to be presented by tumor or APCs, as demonstrated by the poor recognition of cells expressing the corresponding protein of interest endogenously. Hence, a crucial step for the application of this strategy of “reverse T-cell immunology” is the demonstration that the generated T-cell lines can recognize target cells that have processed the antigenic protein and can present the antigenic peptide. The natural processing of p635-649 was proven in this study by the demonstration that T cells prestimulated with p635-649 recognized autologous and allogeneic dendritic cells pulsed with the protein fragment p630-817, which covers the p635-649 peptide. Thus, p635-649 fulfills all requirements for a CD4+ T-cell stimulating vaccine for patients with HOM-MEL-40/SCP1–positive malignancies.
After NY-ESO-114-16,40-43,45-47 and SSX-2,17-20,48 HOM-TES-14/SCP1 is only the third of 16 cancer testis genes13 originally detected by SEREX because of their ability to induce antibody response in the autologous host10 for which coexisting T-cell reactivity has been demonstrated by reverse T-cell immunology.
MHC peptide complexes mediate key functions in adaptive immunity. In a classical view, MHC class 1 molecules present peptides from intracellular source proteins, whereas MHC class 2 molecules present antigenic peptides from exogenous and membrane proteins. Nevertheless, substantial cross-talk between these 2 pathways has been observed. Thus, the MHC class 2 peptide p635-649 could be shed from SCP1-expressing tumor cells, taken up, and processed by professional APCs, particularly dendritic cells, and presented to the autologous CD4+ in vivo, reflecting the experimental setting chosen for this study in vitro. Alternatively, MHC class 2 peptides derived from intracellular source proteins might be presented by the tumor cells themselves, a phenomenon promoted by autophagy.49
In humans, evidence of a role for CD4+ T cells in antitumor immunity comes from the study of tumor-infiltrating lymphocytes, which revealed the presence of CD8+ and CD4+ T cells at the tumor site50 and from the detection of tumor antigen-specific antibodies in the sera of patients with malignancies of different origin (for a review, see Sahin et al51 ). Specific CD4+ T-cell responses against different human tumor antigens have been described,52-56 some of which directly mediate the lysis of tumor cells in vitro.57 In addition, an antiangiogenic effect of TH1 cells has been observed.58 However, the major role of CD4+ T-cell immunity is believed to be in the regulation and coordination of different immune effector functions. It is well documented that the priming of a cytotoxic T-lymphocyte response is significantly enhanced by CD4+ cells specific for the same antigen,59 and CD4+ TH cells might play an important functional role in the maintenance of a vaccine-induced antitumor immunity.60 Less is known about the significance of CD4+ cells for the initiation of specific antibody responses.
Because the requirement of cognate CD4+ help for optimal induction of antitumor CD8+ cytotoxic T cells has been convincingly demonstrated61 and vaccination with specific T-helper epitopes has been shown to result in protective immunity against MHC class 2–negative tumor cells, MHC class 2–restricted peptide vaccines aim primarily at establishing or augmenting CD4+ T-cell help for the induction and maintenance of antitumor CD8+ T cells. The only prerequisite for the use of CD4+-stimulating epitope as a tumor vaccine is the demonstration of its natural processing by (professional) APCs and specific MHC class 2–mediated recognition by CD4+ cells. Therefore, CD4+-stimulating peptide vaccines should also do their job for MHC class 2–negative tumors or tumors that do not process the peptide naturally. Whether CD4+ cells have in addition an effector function by indirect mechanisms against MHC class 2–negative tumors, through macrophage activation, or by direct mechanisms against MHC class 2–positive tumors in vivo is unclear. Although peptide-specific CD4+-mediated cytotoxicity against melanoma cells in vitro has been demonstrated,57 MHC class 2–restricted killing in vivo has only recently been demonstrated in an animal model62 during viral infection, but not for tumor-associated antigens. It would have been of interest to test whether the SCP1-derived p635-649–reactive clone recognizes T–non-Hodgkin lymphoma (T-NHL) cell lines that express SCP1. However, though most T-NHLs express MHC class 2 antigens (as was the case in our series24 ), no such cell line expressing the HLA-DR B1*1401 subtype is available to date; therefore, the question of the natural processing of p635-649 by tumor cells and the cytotoxic potential of clone 60 could not be addressed experimentally in this study.
Interestingly, in this study, the only patient demonstrating a CD4+ T-cell response against p635-649 was also the only one with a detectable antibody response against HOM-TES-14/SCP1, supporting claims that CD4+ responses against tumor antigens are not observed in antibody-negative patients.63 However, most of these claims do not withstand statistical scrutiny. In series of larger numbers of unselected patients, we could not confirm such an association between antibody and CD4+ responses against 2 other cancer testis antigens, HOM-MEL-40/SSX-2 and NY-ESO-1.16,19
In summary, with the definition of HLA class 2 binding peptides of the cancer testis antigen HOM-TES-14/SCP1, it is now feasible to investigate the specific role of CD4+ T cells induced by HLA class 2 binding peptides and its correlation to cellular or humoral immune responses against HOM-TES-14/SCP1–expressing malignancies. Moreover, p635-649 is the first HOM-TES-14/SCP1–derived epitope that fulfills all requirements for a CD4+ T-cell stimulating vaccine. Whether the use of such peptides, either alone or as part of a polyvalent peptide vaccine cocktail consisting also of appropriate MHC class 1–restricted epitopes, will result in better immunologic and clinical responses of the vaccinated patients can now be tested in clinical studies.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2005-04-1487.
Supported by Deutsche Forschungsgemeinschaft (DFG) grant PF-135/7-1, Biomed II of the European Union, Helmholtz-Gemeinschaft Deutscher Forschungzentren e.V. (Virtuelles Institut grant VH-VI-108), the Cancer Research Institute, and Deutsche Krebshilfe grant 10-2189-St 2.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.