Abstract
Cytotoxic T lymphocytes (CTLs) specific for an HLA-A2–presented peptide epitope of the Wilms tumor antigen-1 (WT1) can selectively kill immature human leukemia progenitor and stem cells in vitro. In this study we have used retroviral gene transfer to introduce a WT1-specific T-cell receptor (TCR) into T lymphocytes obtained from patients with leukemia and from healthy donors. TCR-transduced T cells kill leukemia cells in vitro and display WT1-specific cytokine production. Intravenous injection of TCR-transduced T cells into nonobese diabetic–severe combined immunodeficiency (NOD/SCID) mice harboring human leukemia cells resulted in leukemia elimination, whereas transfer of control T cells transduced with an irrelevant TCR was ineffective. The data suggest that adoptive immunotherapy with WT1-TCR gene–modified patient T cells should be considered for the treatment of leukemia.
Introduction
Allogeneic stem cell transplantation (SCT) has been used for the treatment of patients with leukemia and, more recently, renal-cell carcinoma.1-3 There is clear evidence that the beneficial graft-versustumor (GVT) effect of allogeneic SCT is T-cell mediated, since T-cell depletion of the stem-cell population prior to transplantation resulted in the loss of antitumor activity in patients with leukemia.4 It is also evident that alloantigens are involved in GVT responses since no beneficial antileukemia effects were seen after SCT between genetically identical twins.2 This indicated that minor and major histocompatibility antigens are the main targets of GVT responses. The strong immunogenicity of alloantigens can explain why the GVT immunity is more potent than antitumor immunity of autologous T cells.
Most identified tumor-associated antigens are also expressed at low levels in normal tissues5,6 or, due to the autoimmune regulator (AIRE) transcription factor,7 in the thymus. Hence, high-avidity autologous T cells are often deleted, rendered anergic, or inhibited by antigen-specific regulatory T cells. In contrast, T cells specific for alloantigens are not affected by any of these tolerance mechanisms and are therefore capable of mounting high-avidity immune responses. The major side effect of adoptive immunotherapy with allogeneic T cells is due to the wide expression pattern of T-cell–recognized alloantigens. The minor and major histocompatibility antigens are expressed in patient malignant cells as well as in normal tissues, triggering unwanted tissue damage that can lead to fatal graft-versus-host disease.2 In order to improve the specificity of allogeneic T-cell therapy, several groups have identified minor histocompatibility antigens that are expressed in hematopoietic cells only.8-10 Following allogeneic SCT, the infusion of donor-derived cytotoxic T lymphocytes (CTLs), specific for hematopoietic-specific minor antigens, may lead to immune attack against recipient hematopoietic cells, including leukemic cells, whereas nonhematopoietic tissues should be unaffected.
We have exploited anti–major histocompatibility allo-reactivity to improve the avidity of CTL responses against tumor-associated antigens.11 Anti–major histocompatibility complex (anti-MHC) class I allo-responses are heterogeneous, with some CTLs recognizing MHC directly independent of the peptide content, whereas most CTLs recognize specific peptides derived from normal cellular proteins.12,13 Since T-cell tolerance is MHC restricted,14,15 allogeneic T cells can mount high-avidity responses against MHC-presented epitopes derived from normal cellular proteins. We have used this observation and isolated from HLA-A2– donors' high-avidity CTLs specific for HLA-A2–presented peptides of tumor-associated antigens such as cyclin-D1, murine double minute 2 (MDM2), and Wilms tumor antigen-1 (WT1).16-18 WT1 is a transcription factor that is overexpressed in many solid cancers19 and in leukemia cells,20 with a restricted expression in normal tissues including hematopoietic progenitor and stem cells. Detailed in vitro characterization of WT1-specific CTLs showed that they killed leukemia cell lines and granulocyte macrophage colony-forming unit (CFU-GM) progenitor and leukemia-initiating stem cells isolated from patients, whereas the function of normal CD34+ cells was not affected by these CTLs.17,21,22 The therapeutic use of allogeneic CTLs, however, is limited to individual patients who have established donor chimerism following allogeneic hematopoietic stem cell transplantation. In the absence of such donor-specific tolerance, donor-derived CTLs would readily be rejected.
In this study we have explored a generic strategy to use the specificity of high-avidity, allo-A2–restricted CTLs for immunotherapy of all HLA-A2+ patients. The WT1-specific T-cell receptor (TCR) was isolated from allo-restricted CTLs and introduced into CTLs of HLA-A2+ donors using retroviral gene transfer. The gene-transduced T cells acquired WT1 peptide specificity and showed stable long-term TCR expression. We also demonstrate for the first time that TCR-transduced human CTLs are functionally active in vivo. Adoptive transfer of WT1-TCR–transduced CTLs leads to the elimination of leukemia cells in the nonobese diabetic–severe combined immunodeficiency (NOD/SCID) model.
Patients, materials, and methods
Cell lines
T2 is a transporter associated with antigen processing (TAP)–deficient human HLA-A2+ cell line that can be efficiently loaded with exogenous peptides.23 The BV173 cell line was established from the peripheral blood of a male patient with chronic myeloid leukemia (CML) in blast crisis.24 The cell line 697 was established from the bone marrow of a 12-year-old boy with acute lymphoblastic leukemia.25 The human megakaryoblastic leukemia cell lines LAMA81 and Kyo-1 were kind gifts from Drs J. Apperley and F. Dazzi. All cells were maintained in RPMI plus 10% fetal calf serum (FCS) and incubated at 37°C.
Synthetic peptides and HLA-A2/peptide-complex tetramer
The following peptides used in this study were synthesized by ProImmune (Oxford, United Kingdom): pWT126 (RMFPNAPYL) and pWT235 (CMTWNQMNL) are HLA-A2–binding peptides derived from human WT1 and pMDM100 (YAMIYRNL) is an H2-Kb–binding peptide of murine MDM2. The pWT126 and pMDM100 peptides were dissolved in phosphate-buffered saline (PBS) and pWT235 was dissolved in dimethyl sulfoxide (DMSO) prior to being diluted in PBS to give a concentration of 2 mM, and all were stored at –20°C. Recombinant phycoerythrin (PE)–labeled HLA-A2 class I tetramers were obtained from ProImmune.
Retroviral TCR constructs and transduction of TCR genes into human PBMCs
The WT1-specific TCR α and β genes were isolated from the allo-restricted pWT126-specific human CTL line 77.17 To clone the TCR genes, total RNA was extracted from CTL line 77, reverse transcribed into cDNAs as described.26 To identify which variable gene segment is used in our CTLs, the cDNAs were amplified by using a consensus primer that binds to both variable α and β genes in combination with a set of constant primers as described.27 The isolated TCR Vα1.5 or Vβ2.1 gene was then cloned into pMP71 retroviral vector28 by using the Not1 and EcoR1 restriction sites. As control TCRs, we used the α and β genes isolated from the murine CTL clone 3F3B, specific for the H2-Kb–restricted peptide pMDM100.29 Retroviral transduction of TCR genes were carried out as described18 with some modifications. Briefly, 2 × 106 amphotropic packaging cells were seeded into a T25 tissue culture flask and 24 hours later transiently transfected with retroviral TCR constructs using calcium phosphate precipitation. In preparation for transduction, human peripheral blood mononuclear cells (PBMCs) were activated using anti-CD3 antibody (20 ng/mL) and interleukin 2 (IL-2; 300 U/mL) for 2 days. Activated T cells (3 × 106) were then resuspended in 3 mL of normal growth medium plus 3 mL of virus supernatant harvested from transfected packaging cells and plated in 6-well plates coated with fibronectin. Plates were incubated at 37°C in a 5% C02 and 24 to 48 hours after transduction expression of TCR transgenes was analyzed by flow cytometry analysis.
Purification and expansion of TCR-transduced T cells
Bulk transduced T cells were expanded and restimulated weekly in 24-well plates using 5 × 105 TCR-transduced cells, 2 × 105 T2 cells coated with pWT126 peptide as stimulators, 2 × 106 irradiated (35 Gy) PBMCS as feeders, and 10 U/mL IL-2. To enrich Vβ2+ T cells, expanded populations were sorted by magnetic bead selection or fluorescence-activated cell sorter (FACS) sorting. For magnetic bead selection, cells were stained with anti–human Vβ2-PE antibody, followed by incubation with anti-PE microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) and magnetic column purification according the manufacturer's instructions. Purified Vβ2+ T cells were either used directly for functional assays or expanded and further purified with anti-CD8 microbeads to obtain Vβ2+/CD8+ T cells. For FACS sorting, TCR-transduced T cells were stained with anti–human Vβ2-PE antibody (Ab) in conjunction with anti–human CD8-allophycocyanin (APC) and CD4-FITC (fluorescein isothiocyanate) monoclonal Abs (mAbs). Sorting was done with a FACS-Vantage (Becton Dickinson, Cowley, Oxford, United Kingdom). Sorted Vβ2+ CD8+ T cells were then expanded in 24-well plates as described above. Control TCR-transduced (Vα5 and Vβ7, specific for pMDM100) T cells were stained with anti–murine Vβ7-PE antibody then sorted with anti-PE microbeads (Miltenyi Biotech) for TCR-positive (Vβ7+) T cells or expanded by stimulation with pMDM100-coated T2-Kb cells as described for WT1-TCR–transduced T cells.
Purification of hematopoietic CD34+ cells
Approval was obtained from the joint University College London/University College London Hospital Committee on the Ethics of Human Research for these studies. Informed consent was provided according to the Declaration of Helsinki. As a source of leukemic CD34+ cells, bone marrow or peripheral blood was obtained from patients with acute myeloid leukemia (AML) or CML. Samples were diluted 1:2 in RPMI medium and enriched for mononuclear cells by density-gradient centrifugation (Lymphoprep 1.077 g/mL; Nycromed Pharma AS, Oslo, Norway). The recovered mononuclear fraction was subject to magnetic bead selection for isolation of CD34+ cells using a CD34 microbeads kit (Miltenyi Biotec) following the manufacturer's instructions. The purity of the cell population ranged from 80% to 95% as estimated by FACS analysis using anti–human CD34-PE mAb (Miltenyi Biotec).
CTL assays
CTL assays were performed as described.17 Briefly, 106 T2 cells were incubated at 37°C for 1 hour in 200 μL assay medium (RPMI 1640 containing 5% heat-inactivated FCS) with 100 μM synthetic peptide. Peptide-coated T2 cells or tumor cells were labeled with 51chromium for l hour, washed, and added to serial 2-fold dilutions of effector cells in round-bottom, 96-well plates to obtain a total volume of 200 μL/well. Assay plates were incubated at 37°C, 5% CO2, and after 4 hours, 100 μL supernatant was harvested and counted using a Wallac Gamma Counter (Wallac, Milton Keynes, United Kingdom). The specific killing was calculated by the equation (experimental release–spontaneous release)/(maximum release–spontaneous release) × 100%.
IFN-γ secretion assays
TCR-transduced T cells (5 × 104) were stimulated with 5 × 104 leukemia cells or peptide-coated T2 cells (1:1 ratio) in triplicate in a 96-well plate. After 24 hours incubation, the supernatant was harvested and tested in an interferon γ (IFN-γ) enzyme-linked immunosorbent assay (ELISA) using a human IFN-γ determination kit (AMS Biotechnology, Abingdon, United Kingdom) according to the manufacturer's instructions. Data were analyzed using excel software after the generation of a standard curve.
In vivo tumor protection experiments
Three-month-old NOD/SCID mice were inoculated with 5 × 106 human leukemia BV173 cells intravenously. The following day, mice were given 20 × 106 pWT126-TCR–transduced Vβ2+ T cells intravenously; the control group of mice were given 20 × 106 of control (pMDM100 specific) TCR-transduced Vβ7+ human T cells intravenously. Three independent experiments were performed. FACS analysis showed that the composition of the cell population in 2 experiments using CD8-enriched WT1-TCR– transduced T cells was 86% CD8+ (14% CD4+), 54% CD8+/Vβ2.1+, 26% CD8+/tetramer+ cells. In one experiment, Vβ2.1-enriched cells were used with the following composition: 32% CD8+/Vβ2.1+, 62% CD4+/Vβ2.1+. The composition of the control TCR-transduced population was 68% CD8+, 32% CD4+ cells and 89% CD8+, 11% CD4+ cells in independent experiments. Mice were maintained on irradiated food and water and monitored for signs of ill health. After 3 to 5 weeks, when mice showed signs of health deterioration, all animals were killed and bone marrow and spleen cells were harvested for FACS analysis to detect human leukemia cells using triple staining with anti–human HLA class I–FITC, CD45-PE, and CD8-APC mAbs.
Results
Transfer of the WT1-specific TCR into T cells of patients and healthy donors
The TCR used in this study was cloned from the WT1-specific CTL line 77. This allo-restricted CTL line is specific for the WT1-derived, HLA-A2–presented peptide pWT126, and it displayed in vitro cytotoxicity against CD34+ progenitor cells isolated from leukemia patients but not from healthy donors.17 Molecular analysis revealed that the TCR of the CTL line 77 consisted of a Vα1.5/Vβ2.1 heterodimer. The cloned TCR genes were inserted separately into the retroviral vector pMP71. PBMCs of healthy donors or of leukemia patients were activated with anti-CD3 antibodies and IL-2, followed by double infection with retroviral vectors containing the Vα1.5 and Vβ2.1 chains. FACS staining with anti-Vβ2.1 antibodies consistently showed an increase in the percentage of Vβ2.1 expressing CD8+ and CD4+ T cells compared with mock-transduced T-cell populations expressing endogenous Vβ2.1 TCR chains (Figure 1A-B). Antigen stimulation resulted in the expansion of Vβ2.1+ CD8+ T cells (Figure 1C), and these further-expanded Vβ2.1+ CD8+ T cells specifically bound A2/pWT126 tetramer (Figure 1D) but not control tetramer (Figure 1E). We also successfully introduced the WT1-specific TCR genes into T cells from patients with CML or AML (Figure 1 F-G). Similarly, when stimulated with pWT126 peptide–loaded T2 cells, the proportion of Vβ2.1+ CD8+ T cells also increased, and these WT1-TCR–transduced patient T cells can also bind A2/pWT126 tetramer (Figure 1H-I) but not control tetramer (Figure 1J).
Surface expression of pWT126-specific TCR on transduced primary human T lymphocytes. Human T lymphocytes retrovirally transduced with genes encoding the pWT126-specific TCR (Vα1.5/Vβ2.1) chains or mock transduced were triple stained with anti–human CD3-FITC or CD8-APC antibodies and anti–human Vβ2-PE antibody or HLA-A2/pWT126 tetramers. All panels display gated CD3+ T cells. Panel A shows staining of mock-transduced T cells and panel B the pWT126 TCR-transduced T cells 2 days after transduction. After 2 rounds of antigen-specific stimulation, the percentage of T cells binding Vβ2 and CD8 antibodies increased (C). After additional in vitro expansion, tetramer staining revealed that T cells specifically bound the HLA-A2/pWT126 tetramer (D) but not an A2/EBV (Epstein-Barr virus) control tetramer (E). Similarly, T cells of a patient with CML (F) and AML (G) showed a high percentage of Vβ2+/CD8+ T cells after pWT126 TCR transduction and 2 rounds of antigen-specific stimulation (control TCR-transduced T cells contained 2% and 3% Vβ2+/CD8+ T cells, respectively). Tetramer staining of the transduced T cells of the patient with CML (H) and AML (G) showed that they bound the pWT126 tetramer but not the EBV tetramer (J shows the CML patient cells; similar lack of staining was seen with AML patient cells). Numbers indicate the percentage of cells in the quadrants.
Surface expression of pWT126-specific TCR on transduced primary human T lymphocytes. Human T lymphocytes retrovirally transduced with genes encoding the pWT126-specific TCR (Vα1.5/Vβ2.1) chains or mock transduced were triple stained with anti–human CD3-FITC or CD8-APC antibodies and anti–human Vβ2-PE antibody or HLA-A2/pWT126 tetramers. All panels display gated CD3+ T cells. Panel A shows staining of mock-transduced T cells and panel B the pWT126 TCR-transduced T cells 2 days after transduction. After 2 rounds of antigen-specific stimulation, the percentage of T cells binding Vβ2 and CD8 antibodies increased (C). After additional in vitro expansion, tetramer staining revealed that T cells specifically bound the HLA-A2/pWT126 tetramer (D) but not an A2/EBV (Epstein-Barr virus) control tetramer (E). Similarly, T cells of a patient with CML (F) and AML (G) showed a high percentage of Vβ2+/CD8+ T cells after pWT126 TCR transduction and 2 rounds of antigen-specific stimulation (control TCR-transduced T cells contained 2% and 3% Vβ2+/CD8+ T cells, respectively). Tetramer staining of the transduced T cells of the patient with CML (H) and AML (G) showed that they bound the pWT126 tetramer but not the EBV tetramer (J shows the CML patient cells; similar lack of staining was seen with AML patient cells). Numbers indicate the percentage of cells in the quadrants.
Specificity of TCR-transduced T cells. (A) Cytotoxicity of TCR-transduced T cells against T2 target cells pulsed with pWT126 and the control A2-binding peptide pWT235. (B) Peptide titration of purified TCR-transduced Vb2+CD8+ T cells compared with the parental CTL line 77 from which the TCR genes were isolated. (C) Cytotoxicity of TCR-transduced T cells against HLA-A2+ leukemia lines (697, BV173, and LAMA81). HLA-A2– control Kyo-1 leukemia cells and T2 control targets. We have previously shown that all of these leukemia cell lines express WT1. (D) TCR-transduced T cells show antigen-specific cytokine production upon stimulation with T2 cells pulsed with pWT126 and pWT235 control peptide, or with A2+ leukemia cell lines and A2– control cells. Error bars indicate standard deviation of triplicates.
Specificity of TCR-transduced T cells. (A) Cytotoxicity of TCR-transduced T cells against T2 target cells pulsed with pWT126 and the control A2-binding peptide pWT235. (B) Peptide titration of purified TCR-transduced Vb2+CD8+ T cells compared with the parental CTL line 77 from which the TCR genes were isolated. (C) Cytotoxicity of TCR-transduced T cells against HLA-A2+ leukemia lines (697, BV173, and LAMA81). HLA-A2– control Kyo-1 leukemia cells and T2 control targets. We have previously shown that all of these leukemia cell lines express WT1. (D) TCR-transduced T cells show antigen-specific cytokine production upon stimulation with T2 cells pulsed with pWT126 and pWT235 control peptide, or with A2+ leukemia cell lines and A2– control cells. Error bars indicate standard deviation of triplicates.
TCR-transduced CTLs display pWT126 specificity
The specificity of the TCR-transduced T-cell populations was tested in cytotoxicity assays. As shown in Figure 2A, the human TAP-deficient T2 cells were killed after coating with the pWT126 peptide but not after coating with a control peptide. Peptide titration experiments revealed that the avidity of the TCR-transduced T cells was similar to that of the parental CTL line 77. Both CTL populations were able to kill target cells coated with 10– to 10–3 nM peptide concentration. In several experiments the original CTLs were slightly more sensitive than the TCR-transduced CTL populations (Figure 2B).
Next, the ability of the TCR-transduced CTLs to kill tumor cells was tested against a set of human leukemia cell lines that were previously shown to express WT1.17 TCR-transduced CTLs efficiently killed 3 HLA-A2+ leukemia lines, whereas an A2– control leukemia line was not killed (Figure 2C). This A2-restricted killing activity was similar to that reported for the parental CTL line 77.17 The TCR-transduced CTLs also produced IFN-γ when stimulated with T2 cells coated with pWT126 or with A2+ leukemia cell lines (Figure 2D).
Having demonstrated pWT126-specific activity of TCR-transduced T cells of healthy donors, we explored whether TCR gene transfer can convert patient lymphocytes into pWT126-specific CTLs. As shown in Figure 3, the TCR-transduced T cells of a CML or AML patient showed effective killing against pWT126-coated T2 target cells and against the A2+ WT1+ BV173 leukemia cell line (Figure 3A,C). This indicates that patient T cells are not defective and can be turned into highly effective CTLs after WT1-TCR gene transfer. Patient T cells transduced with a control TCR did not show any specific killing of peptide-coated T2 targets or BV173 leukemia cells (Figure 3B,D).
When we compared TCR-transduced patient T cells with those of healthy donors in an IFN-γ assay, they all showed similar patterns of IFN-γ secretion. As shown in Figure 3E, both healthy donor and patient T cells produced IFN-γ when stimulated with T2 cells coated with pWT126 or with A2+ WT1+ BV173 leukemia cells, and no response was seen after stimulation with pWT235 control peptides.
TCR-transduced patient T cells show WT1-specific killing activity and IFN-γ production.TCR-transduced T cells of a patient with CML (A) and AML (C) are able to kill T2 cells coated with pWT126 (pWT235 served as control) and the A2+ WT1+ leukemia line BV173. (B,D) T cells from the same CML and AML patients transduced with an irrelevant TCR (specific for Kb + pMDM100) failed to kill any of these targets. (E) TCR-transduced patient T cells show antigen-specific IFN-γ production upon stimulation with T2 cells coated with pWT126 peptide and the A2+ WT1+ leukemia line BV173. Peptide pWT235 served as control. Error bars indicate standard deviation of triplicates.
TCR-transduced patient T cells show WT1-specific killing activity and IFN-γ production.TCR-transduced T cells of a patient with CML (A) and AML (C) are able to kill T2 cells coated with pWT126 (pWT235 served as control) and the A2+ WT1+ leukemia line BV173. (B,D) T cells from the same CML and AML patients transduced with an irrelevant TCR (specific for Kb + pMDM100) failed to kill any of these targets. (E) TCR-transduced patient T cells show antigen-specific IFN-γ production upon stimulation with T2 cells coated with pWT126 peptide and the A2+ WT1+ leukemia line BV173. Peptide pWT235 served as control. Error bars indicate standard deviation of triplicates.
TCR-transduced CTLs kill leukemia cells of CML and AML patients
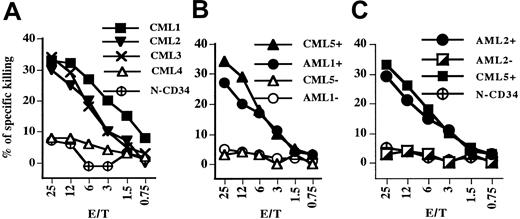
To determine if TCR-transduced CTLs of patients and of healthy donors were able to recognize fresh leukemia cells, cytotoxicity assays were carried out using CD34+ cells purified from leukemia patients as targets. In some experiments, CD34– cells of the same patients were used as control targets, since WT1 is not expressed in mature hematopoietic cells. Figure 4A shows that TCR-transduced CTLs of a healthy donor displayed cytotoxicity against CD34+ cells of 3 HLA-A2+ leukemia patients (CML1-3) but not against the HLA-A2– CD34+ cells of CML patient 4. Importantly, the TCR-transduced CTLs did not kill CD34+ cells isolated from a healthy HLA-A2+ donor. Figure 4B demonstrates that the cytotoxicity of TCR-transduced normal CTLs was directed against the CD34+-cell population of patients AML1 and CML5 but not against mature CD34– hematopoietic cells of the same patients. The relative low level of cytotoxicity against CD34+ targets is similar to that seen previously with the CTL line 77 and is likely to reflect the heterogeneity of the CD34+ population. This population contains immature leukemia stem cells, committed progenitors, and also more differentiated hematopoietic cells, with WT1 expression levels decreasing as cells differentiate.30-32 Selective CTL killing of immature CD34+ cells is supported by the finding that CTL line 77, despite displaying low-level cytotoxicity against bulk CD34+ cells, efficiently eliminated the immature stem cells that initiate leukemia in NOD/SCID mice.22
TCR-transduced T cells from healthy and CML donors can kill purified CD34+ cells of CML and AML patients. (A) The cytotoxicity of TCR-transduced CTLs of a healthy donor was tested against freshly isolated CD34+ cells of A2+ CML patients (CML1-3), an A2– CML4 control patient, and against fresh CD34+ cells isolated from an A2+ healthy donor (N-CD34). (B) TCR-transduced CTLs of a healthy donor were tested against CD34+ and CD34– cells purified of the A2+ patients CML5 and AML1. Filled symbols represent CD34+ cells and open symbols represent CD34–. (C) TCR-transduced CTLs of a CML patient were tested against CD34+ (•) and CD34– ◪) cells isolated from the A2+ AML2 patient, CD34+ cells from the A2+ CML5 patient, and fresh CD34+ cells from an A2+ healthy donor (N-CD34).
TCR-transduced T cells from healthy and CML donors can kill purified CD34+ cells of CML and AML patients. (A) The cytotoxicity of TCR-transduced CTLs of a healthy donor was tested against freshly isolated CD34+ cells of A2+ CML patients (CML1-3), an A2– CML4 control patient, and against fresh CD34+ cells isolated from an A2+ healthy donor (N-CD34). (B) TCR-transduced CTLs of a healthy donor were tested against CD34+ and CD34– cells purified of the A2+ patients CML5 and AML1. Filled symbols represent CD34+ cells and open symbols represent CD34–. (C) TCR-transduced CTLs of a CML patient were tested against CD34+ (•) and CD34– ◪) cells isolated from the A2+ AML2 patient, CD34+ cells from the A2+ CML5 patient, and fresh CD34+ cells from an A2+ healthy donor (N-CD34).
The analysis of TCR-transduced CTLs of a CML patient showed that their cytotoxicity against CD34+ target cells was similar to that of TCR-transduced healthy donor CTLs (Figure 4C). The patient CTLs killed CD34+ cells of HLA-A2+ patients CML5 and AML2 but not CD34– cells of patient AML2 or CD34+ cells of a healthy HLA-A2+ donor.
TCR-transduced CTLs eliminate human leukemia cells in NOD/SCID mice
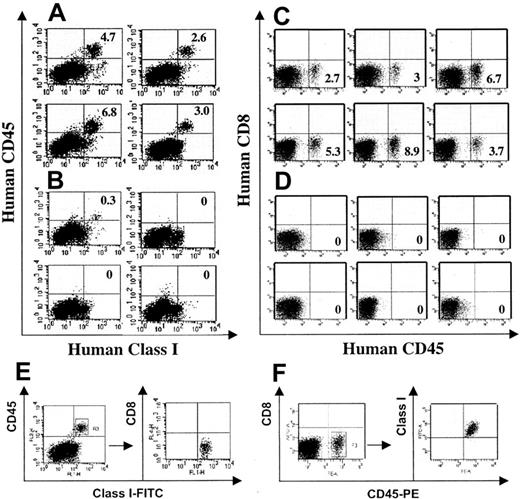
We next used WT1-TCR–transduced human T cells for adoptive immunotherapy experiments in NOD/SCID mice. Mice were challenged intravenously with 5 × 106 human BV173 leukemia cells, and 2 × 107 T cells transduced with the WT1-TCR or a control TCR were adoptively transferred after 24 hours. Three to 5 weeks after the T-cell transfer, mice were killed to assess the burden of BV173 leukemia cells in the bone marrow. Tumor formation outside the bone marrow was not observed in these mice. Bone marrow cells were stained with antibodies specific for human CD8, CD45, and HLA class I. Figure 5 shows the summary of 2 independent experiments illustrating that BV173 leukemia cells, identified by their HLA class I+, CD45+, CD8– phenotype, were detectable in most mice that were treated with control TCR-transduced T cells (Figure 5A,C), whereas leukemia cells were undetectable in most mice treated with WT1-TCR–transduced T cells (Figure 5B,D). Table 1 shows that BV173 cells were detectable at low levels in only 2 of 17 mice treated with WT1-TCR–transduced T cells, but 13 of 14 mice treated with control TCR-transduced T cells showed a higher level of BV173 leukemia cells. Representative examples of 3-color analysis are shown in Figure 5E-F, indicating that the human cells in the bone marrow of NOD/SCID were CD8–.NoCD8+ cells were detectable in the bone marrow or in the spleen of leukemia-free or leukemiabearing mice, indicating that human CTLs transduced with the WT1-TCR or control TCR did not survive in the NOD/SCID environment.
TCR-transduced human T cells eliminate human leukemia cells in NOD/SCID mice. Immunodeficient NOD/SCID mice were inoculated intravenously with 5 × 106 BV173 leukemia cells, and the following day 20 × 106 TCR-transduced Vβ2+ T cells or control TCR-transduced T cells were injected intravenously. After 3 weeks (A,B) or after 5 weeks (C,D), mice were killed and the number of leukemia cells in the bone marrow and the spleen (not shown) was determined. Cells were stained with anti–human HLA class I–FITC, CD45-PE, and CD8-APC mAbs and analyzed using a FACSCalibur (Becton Dickinson) (A,B) or FACSCanto (Becton Dickinson) (C,D). (A) Shown is the HLA class I and CD45 staining of bone marrow cells of mice treated with control TCR-transduced T cells and panel B shows the analysis of mice treated with Vβ2+ TCR-transduced T cells (each panel represents one animal). Both control TCR- and WT1-TCR–transduced T cells were derived from the same donor. (C,D) Shown is the CD45-PE and CD8-APC staining of a repeat experiment. The 2 groups of BV173-challenged mice were treated with T cells transduced with a control TCR (C) or T cells transduced with the WT1-TCR (D). Both control TCR- and WT1-TCR–transduced T cells were derived from the same donor but different donor from panels A and B. A representative 3-color analysis is shown in panels E and F, demonstrating that the engrafted cells were CD45+/HLA class I+/CD8–.
TCR-transduced human T cells eliminate human leukemia cells in NOD/SCID mice. Immunodeficient NOD/SCID mice were inoculated intravenously with 5 × 106 BV173 leukemia cells, and the following day 20 × 106 TCR-transduced Vβ2+ T cells or control TCR-transduced T cells were injected intravenously. After 3 weeks (A,B) or after 5 weeks (C,D), mice were killed and the number of leukemia cells in the bone marrow and the spleen (not shown) was determined. Cells were stained with anti–human HLA class I–FITC, CD45-PE, and CD8-APC mAbs and analyzed using a FACSCalibur (Becton Dickinson) (A,B) or FACSCanto (Becton Dickinson) (C,D). (A) Shown is the HLA class I and CD45 staining of bone marrow cells of mice treated with control TCR-transduced T cells and panel B shows the analysis of mice treated with Vβ2+ TCR-transduced T cells (each panel represents one animal). Both control TCR- and WT1-TCR–transduced T cells were derived from the same donor. (C,D) Shown is the CD45-PE and CD8-APC staining of a repeat experiment. The 2 groups of BV173-challenged mice were treated with T cells transduced with a control TCR (C) or T cells transduced with the WT1-TCR (D). Both control TCR- and WT1-TCR–transduced T cells were derived from the same donor but different donor from panels A and B. A representative 3-color analysis is shown in panels E and F, demonstrating that the engrafted cells were CD45+/HLA class I+/CD8–.
Discussion
TCR gene transfer is an attractive technology for production of antigen-specific T cells for adoptive immunotherapy.18,33-43 Cloned TCR genes can serve as generic reagents for treatment of patients with malignancies expressing the TCR-recognized antigen. The TCR described in this study, recognizing a WT1 epitope presented by HLA-A2, would be suitable for treatment of HLA-A2+ patients with WT1-expressing malignancies such as leukemia, breast cancer, lung cancer, colon cancer, and other less common solid cancers.19,20,44 One round of retroviral transduction is usually sufficient to produce sufficient antigen-specific T cells for adoptive immunotherapy without further in vitro selection. We found that antigen stimulation of bulk TCR-transduced T cells led to the preferential expansion of transduced CTLs, with stable TCR expression and functional activity of CTLs maintained in vitro for over 1 year (data not shown). This suggests that adoptive transfer of TCR-transduced CTLs into patients may provide lasting tumor protection and immunologic memory. This is consistent with a previous report demonstrating that adoptive transfer of bulk murine T cells transduced with a TCR specific for influenza nucleoprotein led to antigen-specific T-cell expansion in mice challenged with nucleoprotein-transfected tumor cells and to long-term survival of transferred CTLs.35 Thus successful TCR introduction into a small fraction of patient T cells may be sufficient to establish long-term antigen-specific immunity.
We have shown previously that the allo-restricted WT1-specific CTL line 77 was able to kill in vitro stem cells that give rise to leukemia in NOD/SCID mice. We have now extended these observations to a therapeutic setting using the BV173 leukemia cell line. The NOD/SCID model is well validated for studies of the in vivo biology of human leukemia and is probably best suited to explore therapeutic options in a preclinical setting. In 3 independent experiments, the adoptive transfer of WT1-TCR–transduced T cells showed therapeutic efficacy. In 13 of 14 of mice receiving control treatment the bone marrow contained 2.6% to 8.9% human leukemia cells, whereas only 2 of 17 mice treated with T cells expressing the WT1-TCR contained low levels (0.3% and 0.6%) of human leukemia cells. It is likely, however, that human TCR-transduced T cells will not function optimally in the NOD/SCID environment. For example, in our experiments the adoptively transferred human T cells were not detectable in mice analyzed 3 to 5 weeks after T-cell transfer, which is in contrast to the long-term survival and memory development of TCR-transduced murine T cells adoptively transferred into syngeneic recipients. The observed short-term T-cell survival in our experiments may be due to poor interaction of human cells with murine MHC molecules, cytokines, and costimulatory molecules resulting in the lack of signals required for in vivo T-cell survival. It is therefore possible that smaller cell doses might be effective in humans. We have used 20 × 106 T cells in NOD/SCID mice, representing approximately 800 × 106 T cells/kg, a dose that would be unrealistic for human trials.
We were able to transfer the WT1-TCR into T cells isolated from CML and AML patients to produce WT1-specific CTL populations. Transduced patient T cells displayed HLA-A2–restricted cytotoxicity against leukemia cell lines and against fresh CD34+ cells isolated from leukemia patients. This strongly suggests that patient T cells are functionally active and that WT1-TCR gene transfer is likely to enable them to recognize autologous leukemia cells. The high-avidity WT1-specific TCR, isolated from the allo-reactive repertoire of an HLA-A2– individual, is of human origin and is therefore unlikely to trigger immune responses that may lead to the rejection of TCR-transduced T cells in patients. This proof-of-principle study provides a generic strategy whereby the alloreactive TCR repertoire can be exploited to equip patient CTLs with tumor-reactive high-avidity TCRs that are absent from the autologous repertoire. The survival and efficacy of TCR-transduced human T cells is likely to be greater in patients than in the murine model experiments used in this study.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-01-0146.
Supported by the Leukaemia Research Fund. R.G. is supported by the Dinwoodie Trust. A.T. is a Senior Wellcome Trust fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.