Abstract
We conducted a phase 1/2 trial of high-dose 90Y-ibritumomab tiuxetan in combination with high-dose etoposide (VP-16) 40 to 60 mg/kg (day -4) and cyclophosphamide 100 mg/kg (day -2) followed by autologous stem cell transplantation (ASCT) in 31 patients with CD20+ non-Hodgkin lymphoma (NHL). Patients underwent dosimetry (day -21) with 5 mCi (185 MBq) 111In-ibritumomab tiuxetan following 250 mg/m2 rituximab, followed a week later by 90Y-ibritumomab tiuxetan to deliver a target dose of 1000 cGy to highest normal organ. Bone marrow biopsy was done on day -7 to estimate radiation dose and stem cells were reinfused when the radiation dose was estimated to be less than 5 cGy. The median 90Y-ibritumomab tiuxetan dose was 71.6 mCi (2649.2 MBq; range, 36.6-105 mCi; range, 1354.2-3885 MBq). Histology included follicular lymphoma (n = 12), diffuse large B-cell (n = 14), and mantle cell (n = 5). The median number of prior chemo-therapy treatments was 2. The treatment was well tolerated. The median times to reach an absolute neutrophil count greater than 500/μL and platelet count more than 20 000/μL were 10 days and 12 days, respectively. There were 2 deaths and 5 relapses. At a median follow-up of 22 months, the 2-year estimated overall survival and relapse-free survival rates are 92% and 78%, respectively. We conclude that high-dose 90Y-ibritumomab tiuxetan can be combined safely with high-dose etoposide and cyclophosphamide without an increase in transplant-related toxicity or delayed engraftment. (Blood. 2005;106:2896-2902)
Introduction
Despite the use of aggressive combination chemotherapy regimens, approximately 30% to 40% of the patients with aggressive non-Hodgkin lymphoma (NHL) do not achieve a complete remission (CR) or they have a relapse after attaining a remission.1 High-dose chemotherapy or chemo/radiotherapy followed by autologous stem cell transplantation (ASCT) has been shown to induce long-term disease control in about 10% to 50% of patients with relapsed or refractory aggressive lymphoma.2,3 The benefit of high-dose therapy and ASCT has been superior to conventional salvage chemotherapy in a large randomized study of patients with chemosensitive relapsed NHL.4 Thus, high-dose therapy with ASCT has become a potential curative modality for patients with relapsed aggressive lymphoma. However, not all the patients derive long-term benefit from this treatment and recurrent disease remains the single most common cause of treatment failure after ASCT. New therapeutic approaches that decrease relapse rates after ASCT are therefore needed.
Because NHL is radiosensitive, preparative regimens for ASCT have included chemotherapy augmented by total body irradiation (TBI). Results of phase 1 and 2 studies of fractionated TBI 12.0 Gy, etoposide 60 mg/kg, and cyclophosphamide 100 mg/kg have shown that this regimen is effective in patients with lymphoid malignancies.5,6 The 5-year progression-free survival (PFS) was 52% with a relapse rate of 42% for 134 patients with relapsed NHL who underwent ASCT using this regimen. These results have been confirmed in the Southwest Oncology Group (SWOG) cooperative trial.7 Despite its effectiveness, a relapse rate of 30% to 50% remains considerably high. In addition, most relapses occur at previous sites of disease, suggesting that targeted therapy may decrease relapse.
Radioisotope-labeled monoclonal antibodies combine the targeting properties of monoclonal antibodies with the proven ability of radiation to safely induce cellular damage in target and neighboring cells. In addition, high-energy β particles can kill tumor cells, including those in bulky or poorly vascularized tumors, within range even without direct binding of the antibody, by creating a crossfire effect.8
Two radioisotope-labeled monoclonal antibodies have been approved by the US Food and Drug Administration for treatment of relapsed or refractory NHL: 90Y-labeled ibritumomab tiuxetan (Zevalin) and 131I-labeled tositumomab (Bexxar). In an attempt to deliver targeted radiation to tumor sites, radioimmunotherapy (RIT) has been evaluated in myeloablative trials with and without high-dose chemotherapy. Press et al9,10 pioneered the use of high-dose RIT in conjunction with ASCT in 2 different trials. The first trial used high-dose 131I-tositumomab with autologous bone marrow rescue in 43 patients with B-cell lymphoma in relapse.9 In this study, 19 patients received therapeutic infusion of 234 to 777 mCi (8658-28749 MBq) 131I-labeled antibodies followed by autologous marrow infusion. Sixteen patients achieved a CR, 2 had a partial response, and 1 had a minor response. Nine of 16 complete responders have remained in CR for 3 to 53 months. Toxicities included myelosuppression, nausea, infection, and 2 episodes of cardiopulmonary toxicity. In a second study, Press et al10 evaluated the combination of high-dose 131I-labeled tositumomab, etoposide, and cyclophosphamide in conjunction with ASCT in 38 patients with NHL (26 low-grade, 12 aggressive). Of the 37 evaluable patients, 33 (89%) were alive and 29 (78%) were progression-free after a median follow-up of 1.5 years. Toxicities included grade 4 myelosuppression in all patients, grade 2/3 nausea in 26 (70%), pulmonary infiltrate in 4, and grade 3 veno-occlusive disease (VOD) in 2 patients. These results indicate the feasibility of delivering high-dose RIT in combination with high-dose chemo-therapy in an ASCT setting for NHL.
90Y-ibritumomab tiuxetan is formed by the conjugation of ibritumomab (a murine monoclonal antibody directed against the antigen CD20) to tiuxetan, a metal chelator. Tiuxetan is a second-generation chelator that can bind with high affinity to 90Y for therapy or 111In for imaging purposes. It is approved for treatment of patients with relapsed or refractory low-grade, follicular, or CD20+-transformed B-cell NHL, and follicular NHL, which has failed rituximab.11 In the pivotal phase 3, randomized, controlled trial comparing 90Y-labeled ibritumomab tiuxetan with rituximab, the overall response rates were 80% and 56%, respectively.12 90Y-ibritumomab tiuxetan has been an effective therapy for diffuse large B-cell lymphoma (DLBCL) with a response rate of 58%.13 The 90Y-ibritumomab tiuxetan therapy is well tolerated with the dose-limiting toxicity being myelosuppression.
Given the relatively high risk of relapse with TBI-based conditioning regimens and the safety and feasibility of 90Y-ibritumomab tiuxetan to directly deliver radiation to tumor sites, we conducted a phase 1/2 study using high-dose 90Y-labeled ibritumomab tiuxetan instead of TBI in combination with high-dose etoposide and cyclophosphamide followed by ASCT in patients with poor-risk or recurrent CD20+ B-cell NHL.
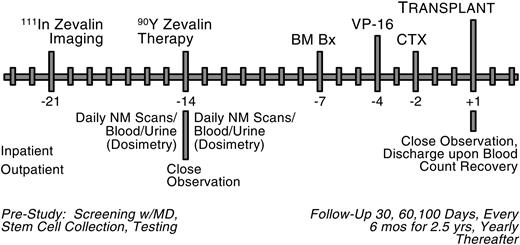
Treatment plan. NM indicates nuclear medicine; BM Bx, bone marrow biopsy; VP-16, etoposide; CTX, cyclophosphamide.
Treatment plan. NM indicates nuclear medicine; BM Bx, bone marrow biopsy; VP-16, etoposide; CTX, cyclophosphamide.
Patients, materials, and methods
Eligibility
Eligible patients were required to be at least 18 years of age and younger than 60 years. Patients were to have histologically confirmed, relapsed, refractory, or poor-risk low- or intermediate-grade CD20+ B-cell NHL including follicular small cleaved, follicular mixed, follicular large cell, diffuse small cleaved, diffuse mixed, diffuse large cell, immunoblastic lymphoma, mantle cell lymphoma, and transformed low-grade disease. Patients were to have a platelet count of at least 150 000/mm3 and 10% or less lymphomatous involvement of bone marrow at the time of stem cell collection. Patients were required to have normal renal function (serum creatinine level ≤ 1.5 mg/dL or a creatinine clearance of ≥ 60 mL/min), adequate pulmonary function (forced expiratory volume at 1 second [FEV1] > 65% of predicted or a diffusing capacity of the lung for CO [DLCO] ≥ 50% of predicted), cardiac ejection fraction of greater than 50% by echocardiogram, and adequate liver function (bilirubin of ≤ 1.5 × normal and aspartate aminotransferase * or alanine transaminase [ALT] ≤ 2 × normal). In addition, patients were to be HIV-, have no active or history of central nervous system (CNS) disease, have Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, or Karnofsky performance status more than 80%. Patients were to be at least 4 weeks from prior radiation or chemotherapy. All patients had autologous peripheral blood stem cells (PBSCs) harvested and cryopreserved before study entry. No in vitro purging was used. Patients were excluded from treatment if they had received prior radioimmunotherapy, had human anti-mouse antibody (HAMA) or antichimeric antibody (HACA) levels, were unable to provide an adequate number of CD34+ cells (≥ 3 × 106 cells/kg), displayed abnormal cytogenetics on bone marrow aspirate, had received a prior bone marrow transplant, experienced prior malignancies, or showed evidence of active hepatitis B or C infection or were positive for hepatitis B surface antigen. All patients gave written informed consent to participate in the protocol, which was approved by the institutional review boards and radiation safety committees of the City of Hope Comprehensive Cancer Center.
Study design
This phase 1/2, open-label study was designed to assess the safety and efficacy of a new preparative regimen consisting of 90Y-labeled ibritumomab tiuxetan and high-dose etoposide and cyclophosphamide followed by autologous stem cell support in patients with relapsed, refractory, and poor-risk CD 20+ NHL. In addition, it was designed to determine the maximum tolerated dose (MTD) of 90Y-ibritumomab tiuxetan that can be given with high-dose etoposide and cyclophosphamide followed by ASCT; however, the dose was capped at 100 mCi (3700 MBq) for practical consideration.
The treatment plan is shown in Figure 1. Prior to study initiation, PBSCs were collected via apheresis after mobilization with chemotherapy and growth factor or growth factor alone. The dosimetry study for high-dose 90Y-ibritumomab tiuxetan is quite complicated and more detailed than that with conventional-dose 90Y-ibritumomab tiuxetan. The patient underwent a dosimetric study with 111In-ibritumomab tiuxetan 1 week (day -21) prior to the administration of the therapeutic dose of 90Y-ibritumomab tiuxetan on day -14. Serial γ-camera images were obtained at approximately 0 (ie, end of infusion), 24, 48, 72-96, 120, and 144 hours after infusion. At each time point, one whole body and 4 spot planar scans were acquired with a dual head γ camera. In addition, 2 single-photon emission computed tomography (SPECT) images were obtained at 24 and 48-72 hours. Nuclear medicine (NM) images were used to estimate the distribution of activity in various organs, especially liver, lungs, kidney, heart, and spleen. Blood samples were be obtained at approximately 0, 2, 4-6, 24, 48, 72-96, 120, and 144 hours after infusion of the antibody to determine antibody clearance and to estimate radiation dose to the bone marrow. Urine samples were collected daily for 6 days for dosimetry study to determine radioisotope clearance. The detailed dosimetry study for high-dose 90Y-ibritumomab tiuxetan will be published in a separate manuscript.
Patients were eligible for treatment if tumor dose was greater than any normal organ, except spleen and bone marrow, that is, liver, lung, and kidney. For patients in CR at time of transplantation, no specific tumor localization was required as long as fluorodeoxyglucose-positron emission tomography (FDG-PET) scan was negative. Tumor dose was calculated from multiple γ-camera images and blood/urine pharmacokinetic profiles. If the patient showed favorable biodistribution, the therapeutic dose was administered on day -14.
90Y-ibritumomab tiuxetan was administered on study day -14 at a dose designed to achieve a maximum absorbed dose of 1000 cGy to any normal organ excluding the spleen and bone marrow. The maximum administered dose was capped at 100 mCi (3700 MBq). Patients were hospitalized overnight for observation after 90Y-ibritumomab tiuxetan administration and for intravenous hydration. Bone marrow biopsy was done on study day -7 to estimate radiation dose and determine when it was safe to give PBSCs. The dose of etoposide, given on study day -4, was initiated at 40 mg/kg in cohort 1 and escalated to 60 mg/kg for cohort 2. Cyclophosphamide was administered on day -2 at a fixed dose of 100 mg/kg ideal body weight. PBSCs were reinfused on study day +1 when the radiation absorbed dose to the stem cells was estimated to be less than 5 cGy. Granulocyte colony-stimulating factor (G-CSF), 5 μg/kg, was given intravenously daily beginning after PBSC infusion.
Complete blood counts and platelet counts, chemistry profiles, and safety evaluations for toxicity were conducted on all treatment days, and additionally on study days 7, 14, 30, 60, 100, and 180 and at years 1 and 2. Serum for HAMA/HACA testing was obtained at study day 60 and 100 and year 1. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (CTC) version 2 with the addition of bone marrow transplantation (BMT) complex/multicomponent events. Toxicities were summarized in terms of type, severity, nadir or maximum values for laboratory measures, time of onset, duration, and reversibility or outcome.
Response evaluation and statistical methods
Computed tomography (CT) evaluations were planned at 1, 3, 6, and 12 months after transplantation, then every 6 months for 2 more years, and then yearly. FDG-PET scanning was recommended if it was abnormal before transplantation or when CT abnormalities were noted. Bone marrow aspiration and biopsy were performed at 6 months after transplantation and yearly thereafter. Bone marrow aspirates underwent cytogenetic analysis, immunophenotyping, and gene rearrangement studies.
Response was determined in comparison with disease status at the time of study entry. Complete response (CR) was defined as complete disappearance of all measurable evidence of disease on physical examination or radiographic evidence of tumor, all disease-related symptoms, and appearance of no new lesions. Partial response (PR) was defined as a 50% or greater reduction in tumor size from baseline in the sum of the products of perpendicular diameters of all measurable lesions with no progression of evaluable disease and no new lesions. Progressive disease was defined as more than 25% increase in the sum of the products of all measurable lesions over smallest sum observed (over baseline if no decrease), or clear worsening of any evaluable disease, or reappearance of any lesion that had disappeared, or appearance of any new lesion. Patients were considered to have stable disease if they did not meet criteria for CR, PR, or progressive disease.
The product-limit method of Kaplan-Meier was used to estimate time-to-event end points such as relapse-free survival (RFS), overall survival (OS), time to relapse, and time to engraftment. Each time-to-event end point was calculated from the date on which the patient went onto the study. Standard statistical methods were used to estimate events such as the incidence of infection and therapy-related myelodysplastic syndrome (MDS), estimated as binomial proportions with exact 95% confidence intervals (CIs). Survival curves were estimated by the method of Kaplan-Meier.
Results
Between May 2000 and November 2003, 41 patients were enrolled and 31 completed the treatment and are included in this report. Ten patients were not able to proceed with treatment due to lack of tumor imaging in 5, unfavorable biodistribution with increased splenic uptake in 1, progressive disease prior to receiving 90Y-ibritumomab tiuxetan in 2, allergic reaction to rituximab immediately prior to imaging dose in 1, and allergic reaction to 90Y-ibritumomab tiuxetan during imaging dose infusion in 1. Six patients were treated in cohort 1 and 25 in cohort 2. The plan for dose escalation of 90Y-ibritumomab tiuxetan in cohort 3 was stopped because the dose of 90Y-ibritumomab tiuxetan was capped at 100 mCi (3700 MBq) and patients in both cohorts 1 and 2 had received this dose.
Patient characteristics are shown in Table 1. There were 17 men and 14 women with a median age of 51 years (range, 25-59.6 years). Lymphoma histology was follicular (FL) grade 1/2 in 8, follicular large cell (FLC) in 4, diffuse large B-cell (DLBCL) in 14, and mantle cell lymphoma (MCL) in 5. Fifty-five percent of patients had bone marrow involvement at diagnosis. The median number of prior chemotherapy regimens was 2 (range, 1-6 regimens). Twenty-nine patients had received rituximab either alone (8), or in combination with salvage chemotherapy (21). Seven patients received the transplant during the first CR/PR including 4 with MCL and 3 with DLBCL with persistent nodal mass with positive FDG-PET scans. Ten received the transplant in the second or subsequent CRs, whereas 9 underwent transplantation during relapse. Five had primary progressive disease but had responded to salvage therapy before transplantation. None of the patients had received prior radiotherapy.
The median dose of 90Y-ibritumomab tiuxetan delivered was 71.6 mCi (2649.2 MBq; range, 36.6-105 mCi [1354.2-3885 MBq]). The 6 patients in cohort 1 were treated with 40 mg/kg etoposide (VP-16) and the 25 patients in cohort 2 were treated with 60 mg/kg VP-16. All patients received cyclophosphamide at a fixed dose of 100 mg/kg.
Hematopoietic recovery
PBSCs were infused as scheduled on day +1 in 26 patients. Five patients had a delay in PBSC infusions for 24 to 48 hours due to high estimated bone marrow radiation dose of more than 5 cGy. The median CD 34+ cells infused was 6.87 × 106/kg (range, 3.1-33.5 × 106/kg). Engraftment failed in one patient, who had received a very high number of PBSCs collected at another institution. For the 30 evaluable patients, the median times to an absolute neutrophil count greater than 0.5 × 109/L and greater than 1.0 × 109/L were both 10 days (range, 8-17 days). The median times to platelet counts higher than 20 × 109/L and 50 × 109/L were 12 days (range, 9-24 days) and 19 days (range 13-178 days), respectively. Patients received a median of 4 (range, 1-19) red blood cell transfusions and 5 (range, 2-37) platelet transfusions. One patient with MCL developed mild pancytopenia at 3 months after transplantation. Bone marrow examination showed hypocellular marrow without lymphoma involvement or any sign of myelodysplasia or abnormal cytogenetics. She remained mildly pancytopenic without needing a transfusion and had a relapse at 12 months with peri-orbital soft tissue mass.
Toxicity
90Y-ibritumomab tiuxetan was well tolerated at this high dose with no adverse events or allergic reactions attributed to the RIT therapeutic regimen. Transplant regimen-related toxicity is summarized in Table 2. Mucositis (81%), neutropenic sepsis (81%), and nausea (84%) occurred in most patients, but these conditions were usually mild to moderate in severity (grade 1-2). One patient had engraftment syndrome at recovery of white blood cell count, which resolved after administration of corticosteroid. Three patients had grade 3 cardiac toxicity including 2 with atrial fibrillation (AF); one was associated with sepsis and the other with history of atrial arrhythmia before transplantation, which developed into AF etoposide-induced hypotension. Both were treated successfully with antiarrhythmic agents. One other patient developed cardiomyopathy and congestive heart failure at 2 months after transplantation. Cardiac function returned to normal 6 months later after appropriate cardiac medications. Four patients had grade 3 pulmonary toxicity due to volume overload (2), congestive heart failure (1), and moderate pleural effusion (1). One patient developed idiopathic interstitial lung disease at 2 years after transplantation. This patient was given rituximab following transplantation because of minimal residual disease noted on bone marrow flow study. Lung biopsy showed bronchiolitis obliterans. His symptoms and radiology abnormality resolved after outpatient treatment with a short course of corticosteroids. Two patients had grade 3 liver toxicities. One patient developed reactivation of hepatitis B infection at 6 months after transplantation. His hepatitis B serologies were negative before transplantation and the abnormal liver function test returned to normal and viral load were negative after treatment with lamivudine. Another patient developed skin rashes, angioedema, with abnormal liver function at day +34 after transplantation. By exclusion, a diagnosis of drug allergy and drug-induced hepatitis was made. Her symptoms resolved and her liver function returned to normal after a short course of corticosteroid therapy. Two patients died. One patient died at day +164 from alcohol-induced liver failure documented on liver biopsy. Another patient died from graft failure at day +44 after transplantation.
All patients had serial testing for HAMA/HACA but none has developed antibodies. There have been no significant changes in cardiac ejection fraction or pulmonary function study at 6 months and at 1 year after transplantation.
The toxicity profiles observed in this study are similar to toxicity observed in patients receiving TBI, etoposide, and cyclophosphamide at our center. The transplantation-related mortality of 3% is in keeping with current experience with other high-dose regimens. All patients had normal marrow cytogenetic studies before transplantation and so far none has developed any clonal disorders on serial follow-up bone marrow biopsies following transplantation. However, the follow-up time is still too short to draw a firm conclusion.
Response evaluation
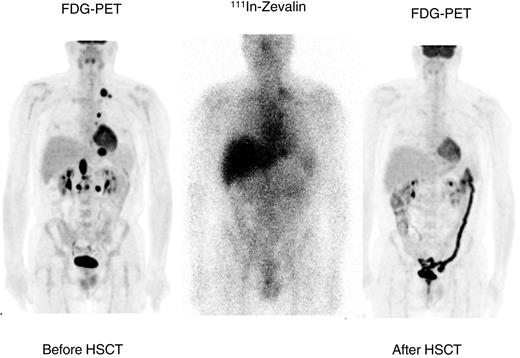
All 13 patients with active disease achieved CR except one who had a persistent positive FDG-PET scan. An example of response seen in a patient with refractory relapsed DLBCL is shown in Figure 2. Five patients received involved-field radiation to sites of prior bulky mass greater than 5 cm following ASCT in keeping with our current practice for posttransplantation radiation therapy. Involved-field radiation was well tolerated except in one patient who developed transient neutropenia and thrombocytopenia during mediastinal radiation that required growth factor support.
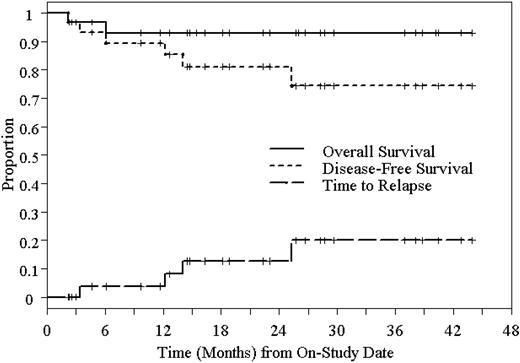
Kaplan-Meier estimated 2-year DFS and OS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL.
Kaplan-Meier estimated 2-year DFS and OS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL.
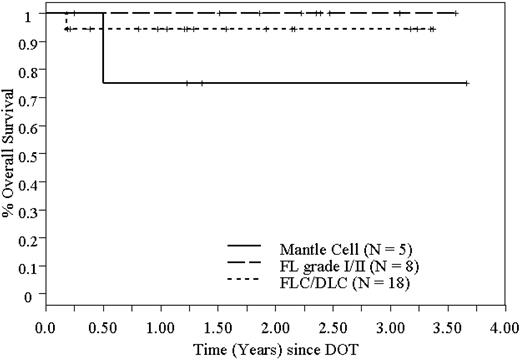
OS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL, based on histology.
OS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL, based on histology.
Relapse
Five patients had relapses at 3, 11, 12, 13, and 24 months after transplantation. All relapses were documented on tissue biopsy. One patient with MCL had a relapse with a peri-orbital soft tissue mass at 12 months. Two relapses occurred in patients with FLC; both had extensive prior treatment of 3 and 6 regimens before transplantation. One patient with primary refractory DLBCL had a relapse at 3 months at a prior site of disease and was treated successfully with salvage radiotherapy and rituximab. Another patient with DLBCL had a relapse in the bone marrow at 11 months even though she never had bone marrow involvement. None of the patients with FL grade 1/2 have had a relapse to date. It should be noted that the biology of relapsed diseases seem to be less aggressive because all patients who had a relapse are still alive after salvage therapy with rituximab alone with or without radiation and without the need for systemic chemotherapy.
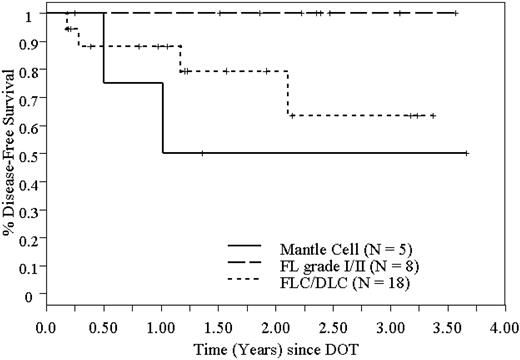
DFS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL, based on histology.
DFS of 31 patients who underwent high-dose 90Y-ibritumomab tiuxetan and ASCT in NHL, based on histology.
Survival
Twenty-four of 31 patients are alive in remission at a median follow-up of 22 months (range, 2-44 months). The Kaplan-Meier estimated 2-year RFS and OS are 78% (95% CI, 61%-96%) and 92% (95% CI, 82%-100%), respectively, and are shown in Figure 3. Because most patients with FLC had areas of DLC in their biopsy specimens, we combined the FLC with DLBCL for the purpose of this analysis. The 2-year RFS and OS are both 100% for patients with FL grade 1 and 2. The 2-year RFS and OS for combined FLC and DLBCL are 74% (95% CI, 47%-100%) and 93% (95% CI, 81%-100%), respectively. For MCL, the 2-year RFS and OS are 50% (95% CI, 1%-99%) and 75% (95% CI, 33%-100%), respectively. Disease-free survival (DFS) and OS based on histology are shown in Figures 4 and 5.
Discussion
This study demonstrates the feasibility of administering high-dose 90Y-ibritumomab tiuxetan with high-dose etoposide and cyclophosphamide followed by ASCT without additional toxicity. The preliminary results are encouraging and the toxicity profiles are very similar to other high-dose regimens. Our results are comparable to the study reported by Press et al10 using 131I-tositumomab in combination with identical doses of etoposide and cyclophosphamide followed by ASCT in 52 patients with relapsed B-cell NHL. In their study, the estimated 2-year OS and PFS were 83% and 68%, respectively. In addition to the difference in radioisotope used, the 2 studies have some different design features to note. First, the MTD of 131I-tositumomab that can be given safely to dose-limiting normal organs, especially the heart and lung, with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide was 2500 cGy. Our study initially planned to escalate dose of 90Y-Ibritumomab tiuxetan to deliver 1000, 1250, 1500.. .2000-2500 cGy to normal organs. Initially, the 90Y-ibritumomab tiuxetan was capped at 100 mCi (3700 MBq) for practical reasons relating to isotope and antibody formulation. The cap was continued when it became evident that we would not be able to increase based on protocol restructure for marrow infusion, which was set at a projected integrated dose of 5 cGy without significantly delaying the stem cell infusion. In fact, 5 patients had slow marrow clearance and had a delay in stem cell infusion for 24 to 48 hours. Because patients in cohorts 1 and 2 received the maximum dose 100 mCi (3700 MBq) 90Yt-ibritumomab tiuxetan, further dose escalation was not carried out. All patients in our study received a 90Y-Ibritumomab tiuxetan dose that delivered 1000 cGy to normal organs. Therefore, we are unable to determine the MTD of 90Y-ibritumomab tiuxetan, which can be given with high-dose etoposide/cyclophosphamide in our study. The lower dose of radiation in our study may explain why we observed less pulmonary toxicity than did Press et al10 (4 patients in the 131I-tositumomab/etoposide/cyclophosphamide study developed delayed interstitial pneumonitis at 2 to 8 months after transplantation). Second, the inclusion criteria are different. The Press et al10 study included only patients in relapse with measurable or evaluable diseases and excluded those in remission. In contrast, our study included both patients in relapse and in remission; 17 patients in our study were in CR at transplantation. Third, patients in Press et al10 study were more heavily pretreated with a median of 3 prior regimens compared to median of 2 prior regimens in our study. Lastly, whereas most patients (73%) in Press et al10 study had FL, 45% of our patients had de novo DLBCL. The experience of 90Y-ibritumomab tiuxetan in relapsed DLBCL is limited; however, our results suggest that it may be an effective therapy for DLBCL and in a high-dose setting. 90Y-ibritumomab tiuxetan may even overcome chemotherapy resistance in patients with relapsed/refractory DLBCL.
90Y-ibritumomab tiuxetan was chosen for this study because it possesses several unique properties favorable for use in the transplantation setting compared to a 131I-labeled radioimmunoconjugate. The pure β emission allows the millicurie dose of 90Y-ibritumomab tiuxetan to be increased without causing significantly increased doses to the hospital personnel caring for the patient, with the high doses being capable of being administered in a standard private room compared to the lead-lined facilities required for the high-dose 131I therapies. Most of patients in our study do not require hospitalization although we normally keep them overnight for observation and hydration. The 2.67-day half-life of the radioisotope allowed for reinfusion of the marrow in a timely manner as we have documented in our study. The 90Y-ibritumomab tiuxetan protocols incorporated rituximab as the unlabeled antibody, an active therapeutic agent that can be combined with other cytotoxic drugs as were planned in the administration of the transplantation regimens.14 The residual rituximab circulating after the conditioning regimen was felt to be potentially advantageous in treating any CD20+ cells in the reinfused product.
The necessity of incorporating a higher dose of RIT with high-dose chemotherapy in the ASCT setting remains to be confirmed. Other investigators have added escalated doses of 131I-tositumomab or 90Y-ibritumomab tiuxetan to high-dose BEAM (carmustine, etoposide, cytarabine, and melphalan) regimen followed by ASCT.15,16 Vose et al conducted a phase 1/2 study evaluating the addition of standard outpatient dose of 131I-tositumomab in 4 doses cohorts (30, 45, 60, and 75 cGy) to the BEAM regimen in 23 patients with chemotherapy-refractory aggressive NHL (FLC = 4, DLC = 14, and MCL = 5).15 The hematologic and nonhematologic toxicities were similar to historical patients receiving BEAM alone, and there were no treatment-related deaths. At a median follow-up of 38 months (range 27-60 months), the 3-year event-free survival and OS were 39% and 55%, respectively. Winter et al added escalating dose of 90Y-ibritumomab tiuxetan to deliver 100, 300, or 500 cGy to normal organs to BEAM in 14 patients with relapsed B-cell NHL.16 The median total dose of 90Y-ibritumomab tiuxetan was 21 mCi (777 MBq) or 0.24 mCi/kg (8.88 MBq/kg). There was no delay in hematopoietic recovery; however, all but 2 patients experienced asymptomatic decreases in diffusion capacity. The 2-year OS and PFS were 75% and 54%, respectively. Encouraging data were reported by Fung et al where standard dose 90Y-ibritumomab tiuxetan (0.4 mCi/kg [14.8 MBq]) was added to BEAM in older patients with poor-risk or relapsed aggressive B-cell NHL.17 The results from these studies suggest that standard dose of RIT can be added to myeloablative regimens and this approach may allow dose intensification without signifi-cant additional toxicity. In addition, it may be easier to deliver this therapy than a higher dose RIT, making it more suitable for older patients or those who are unable to receive TBI.
Patients with FL have an indolent clinical course and are not cured by current treatment approaches. High-dose therapy with ASCT has been shown to improve increase the duration of remission in some patients with relapsed low-grade NHL.18,19 However, because of the long natural history and the continued pattern of relapse after ASCT in some studies, the role of high-dose therapy with ASCT as a potential curative treatment for patients with relapsed low-grade lymphoma has not been clearly established. Because both 131I-tositumomab20 and 90Y-ibritumomab tiuxetan21 have been shown to be effective salvage therapies for relapsed FL and transformed lymphoma, incorporating RIT into the high-dose regimen may potentially improve the outcome of high-dose therapy and ASCT in relapsed and transformed lymphoma. Gopal et al22 conducted a retrospective multivariable comparison of 125 patients with relapsed FL who received either high-dose RIT using 131I-tositumomab (n = 27) or conventional high-dose therapy (n = 98). The patients' characteristics were similar except more patients treated with high-dose RIT had elevated lactic dehydrogenase (LDH) levels before transplantation and were in a high international prognostic risk group. They found that patients treated with high-dose 131I-tositumomab experienced improved OS (P = .02) and PFS (P = .03) when compared to patients treated with conventional high-dose therapy. The estimated 5-year OS and PFS were 67% and 48%, respectively, for high-dose 131I-tositumomab and 53% and 29%, respectively, for conventional high-dose therapy. Our results with high-dose 90Y-ibritumomab tiuxetan, albeit in a small number of patients, are very encouraging. None of the patients with FL grade 1 and 2 has had a relapse and they are all alive in remission. The results from these 2 studies suggest that incorporating RIT into high-dose regimen may lead to improved outcomes of ASCT for relapsed and transformed FL. Further studies are therefore required to determine the curative potential of this approach.
Given the poor prognosis of MCL with median survival of 3 years and no cure with current treatment approaches,23,24 high-dose therapy and ASCT have been performed during first and subsequent remission in patients with MCL. Unlike aggressive NHL, high-dose therapy and ASCT have failed to improve prognosis in relapsed MCL.25 Using ASCT as consolidation treatment during the first CR has improved DFS and remission duration in some studies but relapse continues to occur.26,27 Encouraging results were reported by Gopal et al using high-dose 131I-tositumomab in combination with etoposide and cyclophosphamide and ASCT in 16 patients with relapsed MCL.28 Although most patients were heavily pretreated (median of 3 prior regimens) and 7 had chemotherapy-resistant disease, the 3-year OS and DFS rates were 93% and 61%, respectively. The feasibility of a dose-escalated 131I chimeric anti-CD20 antibody C2B8 (rituximab) with ASCT for relapsed MCL has also been demonstrated in another study.29 Although further study is required, these results suggest again that high-dose RIT may help overcome chemotherapy resistance, in this case in MCL. Given encouraging results with high-dose 131I-tositumomab, we plan to continue to evaluate the role of high-dose 90Y-ibritumomab tiuxetan in the ASCT setting in MCL.
This phase 1/2 study demonstrates that high-dose 90Y-ibritumomab tiuxetan can be safely given in combination with high-dose etoposide and cyclophosphamide in an ASCT setting for NHL without additional transplantation-related toxicity. This regimen is effective in patients with relapsed or poor-risk B-cell NHL. Further studies and longer follow-up are required to determine whether this approach provides longer duration of remission and possible cure and to assess the long-term toxicity of high-dose 90Y-ibritumomab tiuxetan when given in combination with high-dose chemotherapy. The feasibility of incorporating 90Y-ibritumomab tiuxetan in the stem cell transplantation setting should allow this study to be extended to other institutions for a cooperative group trial.
Prepublished online as Blood First Edition Paper, July 7, 2005; DOI 10.1182/blood-2005-03-1310.
Supported by Biogen Idec and in part by United States Public Health Service grants CA30206 and CA33572.
This study was supported in part by Biogen Idec, which manufactures Zevalin (ibritumomab tiuxetan).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Data collection: Jason Chapman, MS, and Joseph Jadav, MS; nurse coordinators/hematology: Emily Krupka, RN, and Jill Land, RN; nurse coordinator/radioimmunotherapy: Phyllis Broene, RN, OCN, and Preeyarat Kloythanomsup, RN; and manuscript preparation, Amy Stillings-Farris.