Abstract
Heparin-induced thrombocytopenia (HIT) is an uncommon but potentially devastating complication of anticoagulation with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). Our objective was to determine and compare the incidences of HIT in surgical and medical patients receiving thromboprophylaxis with either UFH or LMWH. All relevant studies identified in the MEDLINE database (1984-2004), not limited by language, and from reference lists of key articles were evaluated. Randomized and nonrandomized controlled trials comparing prophylaxis with UFH and LMWH and measuring HIT or thrombocytopenia as outcomes were included. Two reviewers independently extracted data on thromboprophylaxis (type, dose, frequency, and duration), definition of thrombocytopenia, HIT assay, and rates of the following outcomes: HIT, thrombocytopenia, and thromboembolic events. HIT was defined as a decrease in platelets to less than 50% or to less than 100 × 109/L and positive laboratory HIT assay. Fifteen studies (7287 patients) were eligible: 2 randomized controlled trials (RCTs) measuring HIT (1014 patients), 3 prospective studies (1464 patients) with nonrandomized comparison groups in which HIT was appropriately measured in both groups, and 10 RCTs (4809 patients) measuring thrombocytopenia but not HIT. Three analyses were performed using a random effects model and favored the use of LMWH: (1) RCTs measuring HIT showed an odds ratio (OR) of 0.10 (95% confidence interval [CI], 0.01-0.2; P = .03); (2) prospective studies measuring HIT showed an OR of 0.10 (95% CI, 0.03-0.33; P < .001); (3) all 15 studies measured thrombocytopenia. The OR was 0.47 (95% CI, 0.22-1.02; P = .06). The inverse variance–weighted average that determined the absolute risk for HIT with LMWH was 0.2%, and with UFH the risk was 2.6%. Most studies were of patients after orthopedic surgery.
Introduction
There is a paucity of data regarding heparin-induced thrombocytopenia (HIT) in venous thromboembolism prophylaxis studies. Despite this, from the perspective of HIT risk, low-molecular weight-heparin (LMWH) should be considered the drug of choice for prophylaxis in patients after orthopedic surgery.
Unfractionated heparin (UFH) and LMWH are commonly used agents for prophylaxis against venous thromboembolism after surgery and in medical patients admitted to the hospital. From the perspective of thromboembolic events, most data suggest the superiority of LMWH prophylaxis, but drug costs remain a barrier to widespread use. Although the use of UFH and LMWH are associated with bleeding, anaphylaxis, and osteoporosis, HIT is generally the most serious adverse effect of these drugs.1-3 A potential advantage of LMWH from clinical and cost perspectives is a lower risk for HIT. HIT can be categorized as the immune-mediated form, or HIT type 2, and as the nonimmunogenic form, or HIT type 1. Given that thrombocytopenia occurs in both forms, it is not sufficient to document decreases in platelet count as the relevant clinical outcome. HIT type 2 is characterized by a major decrease in platelet count and a paradoxical hypercoagulable state that is responsible for the marked increase in morbidity and mortality seen with this condition. Venous and arterial thrombotic events are common consequences.3
HIT type 2 thrombocytopenia is usually defined as a relative decrease in platelet count to less than 50% of baseline or an absolute decrease to less than 100 × 109/L (in some studies, a value lower than 150 × 109/L has been used), typically 5 to 10 days after the initiation of heparin therapy, a pattern indicative of the immunologic etiology of the condition.4 If the onset of thrombocytopenia is earlier, the decreased platelet count may be the result of another cause. If thrombocytopenia develops within the first 24 hours of heparin exposure, HIT is considered in patients who have had recent (100 days or less) heparin exposure, because they may have preformed heparin-PF4 antibodies.4 Clinical suspicion of HIT can also be based on the presence of thrombotic events that result from the paradoxical hypercoagulable state caused by platelet activation and associated thrombin generation. These include increased or recurrent deep vein thrombosis (DVT) or pulmonary embolism (PE). Regardless, the clinical suspicion of heparin-induced thrombocytopenia should be confirmed if results of laboratory tests are positive.5
The frequency of the immune-mediated form, HIT type 2, in prophylaxis studies is unclear, because few studies have addressed HIT type 2 (hereafter referred to as HIT) as either a primary or a secondary outcome. We performed meta-analysis to obtain information on the comparative risks for HIT when UFH and LMWH are used prophylactically in patients at risk for venous thromboembolism.
Methods
We searched the MEDLINE (Ovid) database (1984-2004)38 using and combining the terms “heparin-induced thrombocytopenia,” “low molecular weight heparin,” “prophylaxis,” “randomized controlled trials,” and “prospective studies.” The function Explode was used. The search was not limited to the English language. Additional articles were found by examination of reference lists of retrieved articles.
Study identification and eligibility
To be included in the analyses, studies had to fulfill 3 inclusion criteria. First, they had to be comparisons of prophylactic doses of UFH and LMWH. Second, they had to be assessments of postoperative or medical inpatients receiving thromboprophylaxis. Third, they had to be one of the following study types: RCT recording HIT (defined as decrease in platelets greater than 50% or to less than 100 × 109/L and positive laboratory HIT assay, including enzyme-linked immunosorbent assay [ELISA], 14C serotonin release assay, or adenosine triphosphate [ATP] lumi-aggregometry) as an outcome; nonrandomized prospective study with a comparison group (concurrent or sequential control group) recording HIT (same definition as above); or RCT recording thrombocytopenia (defined as decrease in platelets greater than 50% or to less than 100 × 109/L). Studies could be eligible if they did not use our definition of thrombocytopenia but rather provided the raw data to enable us to extract the appropriate results (eg, Warkentin et al3 ).
Thrombocytopenia monitoring in studies
Eligible studies had to indicate that platelet counts were monitored at least once before and many times after thromboprophylaxis drug exposure, which was usually administered for at least 7 days. Postexposure monitoring of platelets usually started from postoperative day 3 or 4 and would usually continue until at least postoperative day 10. As such, the baseline platelet count in these studies was usually the preoperative platelet count. Most studies did not appear to consider early thrombocytopenia (onset less than 5 days) as suggestive of HIT, though most did not specify whether they used the now accepted strict temporal criterion of thrombocytopenia occurring 5 to 10 days after heparin exposure before considering HIT. Because of these limitations of the available data and because the reported platelet counts could be reasonably assumed to fall within the HIT time range, we decided not to use the strict temporal criterion in our inclusion criteria. When we have evidence that thrombocytopenia rates include early thrombocytopenia, we will report it. Studies that briefly mentioned in their results that no patients experienced thrombocytopenia were not eligible unless they had previously defined the threshold for thrombocytopenia, as outlined here, or at least provided quantitative and temporal data on platelet counts that would have enabled the authors to perform the calculation.
Outcome measures
The primary outcome was HIT incidence with thromboprophylaxis. The secondary outcome was thrombocytopenia incidence with thromboprophylaxis.
Statistical analysis
Mantzel-Haensel odds ratios (ORs) were used to estimate the summary ORs and their 95% confidence intervals (CIs) for the primary and secondary outcomes, comparing LMWH and UFH. A random-effects model was used. Publication bias was assessed by using funnel plots.
Funding source
No funding was received for this meta-analysis.
Results
Three hundred sixty-eight abstracts were reviewed for screening of potentially eligible articles. Two hundred seventy-seven articles were excluded on the basis of the abstract only, mostly because of an inappropriate patient population (therapeutic anticoagulation for venous thromboembolism, acute coronary syndrome, atrial fibrillation, or hemodialysis) or because the study did not compare UFH and LMWH. Reviews or meta-analyses were also excluded (Figure 1).
Ninety-one articles were retrieved and were independently reviewed by at least 2 authors, with completion of a case report form for each article according to our inclusion criteria. Of these, 13 articles were excluded because of an inappropriate patient population (studies evaluating treatment of venous thromboembolism, n = 8; acute coronary syndrome, n = 4; hemodialysis, n = 1); 11 articles were excluded because of the absence of comparison between UFH and LMWH; 32 articles were excluded because no quantitative results were reported on HIT or thrombocytopenia; 12 articles were excluded because of an undefined threshold for thrombocytopenia; 3 RCTs measuring thrombocytopenia were excluded because their definitions of thrombocytopenia were considered inadequate (platelet counts less than 70 × 109/L, less than 80 × 109/L, and less than 50 × 109/L, respectively).6-8 One article9 was excluded because of significant overlap of the reported patients between it and a previous study. One nonrandomized study comparing HIT incidence with LMWH and UFH was excluded because it had been published in abstract form only and appropriate data could not be extracted10 ; 2 articles were meta-analyses; one article was a review.
Flow diagram of study selection. ACS indicates acute coronary syndrome; HD, heart disease.
Flow diagram of study selection. ACS indicates acute coronary syndrome; HD, heart disease.
Fifteen articles were eligible for analysis (Table 1) and were grouped into 3 categories, as follows: 2 RCTs measuring HIT as an outcome3,11 ; 3 nonrandomized prospective studies with a comparison group (concurrent or sequential control group) and measuring HIT as an outcome12-14 ; 10 RCTs not measuring HIT but appropriately monitoring thrombocytopenia as an outcome.15-24
We performed 3 different analyses. The first concerned HIT outcome based on RCTs from category A. The second concerned HIT outcome based on studies from categories A and B. The third concerned thrombocytopenia outcome based on studies from categories A, B, and C.
Primary outcome: HIT incidence
Analysis 1 (totaling 1014 patients) and analysis 2 (totaling 2478 patients) favored the use of LMWH over UFH prophylaxis with ORs of 0.10 (95% CI, 0.01-0.82; P = .03) and 0.10 (95% CI, 0.03-0.33; P < .001), respectively, for HIT incidence (Figures 2 and 3 and Tables 2 and 3). All articles were from postoperative patient populations.
Combining the studies in analysis 2, the inverse variance–weighted average determining the absolute risk for HIT with LMWH was 0.2% (95% CI, 0.1%-0.4%), and with UFH the risk was 2.6% (95% CI, 1.5%-3.8%).
Secondary outcome: thrombocytopenia incidence
Analysis 3 (totaling 7287 patients), risk for thrombocytopenia, was not as strongly in favor of LMWH as the analyses in which HIT was measured as an outcome. There was a trend in favor of LMWH prophylaxis with an OR of 0.47 (95% CI, 0.22-1.02; P = .06), but this did not reach statistical significance (Figure 4 and Table 4).
Analysis 1: HIT outcome (RCTs). Comparison: UFH versus LMWH. There were no LMWH events and 10 UFH events. Test for heterogeneity: χ2 = 0.36; df = 1 (P = .55); I2 = 0%. Test for overall effect: Z = 2.14 (P = .03). ▪ represents the summary OR; vertical line, 95% CI; and ♦, OR and 95% CI.
Analysis 1: HIT outcome (RCTs). Comparison: UFH versus LMWH. There were no LMWH events and 10 UFH events. Test for heterogeneity: χ2 = 0.36; df = 1 (P = .55); I2 = 0%. Test for overall effect: Z = 2.14 (P = .03). ▪ represents the summary OR; vertical line, 95% CI; and ♦, OR and 95% CI.
Discussion
The OR of the risk for HIT in patients receiving postoperative prophylaxis with LMWH or unfractionated heparin strongly favored a lower risk with LMWH. ORs were the same, 0.10, regardless of whether we included only randomized trials or we included all prospective studies with comparator groups. Absolute risk for HIT was only 0.2% with LMWH and was 2.6% with unfractionated heparin. The risk for thrombocytopenia did not significantly differ, underscoring the importance of using a proper definition for HIT and confirming the diagnosis with objective tests. Because most of the studies included were performed in patients after orthopedic surgery, we cannot be sure of the benefit of LMWH in nonorthopedic surgery.
The pathophysiology of HIT may be different in UFH than in LMWH because of the smaller molecule size of LMWH. The size of the heparin molecule at least in part determines its affinity for PF4, as has been shown experimentally.25 Presumably, this makes LMWH less likely to induce HIT than the larger unfractionated heparins. Studies comparing the incidence of HIT antibodies in patients treated with UFH and in those treated with LMWH have confirmed a greater incidence of anti–H-PF4 antibody formation in patients taking UFH than in those taking LMWH, but LMWH may still induce HIT because, within a given preparation of LMWH, the larger molecules are of sufficient size to complex with PF4.26-28 The formation of detectable levels of H-PF4 antibodies in the serum does not necessarily result in the clinically relevant signs of HIT, including thrombocytopenia or the hypercoagulable state; hence, it remains important to know the risk for HIT with LMWH use and with UFH use. Given the large numbers of patients on heparin prophylaxis, even a small incidence of HIT may represent a significantly detrimental effect on patient outcomes. In many centers, UFH is preferred over LMWH because it is less expensive. But LMWH, which is at least as efficacious as UFH, may represent an even less costly alternative if HIT occurs significantly more frequently with UFH and if the cost of treating HIT negates any savings from the less-expensive UFH.
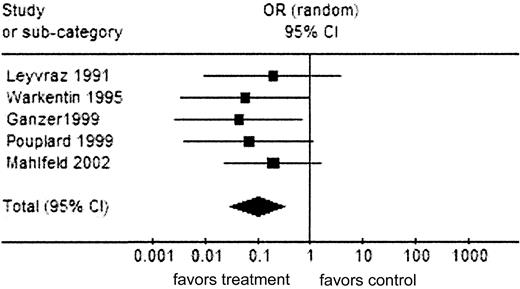
Analysis 2: HIT outcome (RCTs and prospective studies). Comparison: UFH versus LMWH; outcome (RCTs and prospective studies). Total events for treatment-LMWH = 1; for control-UFH = 31. Test for heterogeneity: χ2 = 1.18, df = 4(P = .88), I2 = 0%. Test for overall effect: Z = 3/74 (P < .001). Symbols are as in Figure 2.
Analysis 2: HIT outcome (RCTs and prospective studies). Comparison: UFH versus LMWH; outcome (RCTs and prospective studies). Total events for treatment-LMWH = 1; for control-UFH = 31. Test for heterogeneity: χ2 = 1.18, df = 4(P = .88), I2 = 0%. Test for overall effect: Z = 3/74 (P < .001). Symbols are as in Figure 2.
To make responsible decisions regarding patient care, it is necessary to have reliable estimates of the incidence of HIT in UFH- and LMWH-treated patients. We found no systematic reviews of the risk for HIT with UFH or LMWH in prophylaxis. In several studies, meta-analyses comparing the efficacy and safety of UFH and LMWH had been performed in various settings, including the treatment of patients with venous thromboembolism (VTE)29,30 and with acute coronary syndromes and unstable angina.31 Similarly, cost-effectiveness analyses had been performed in some studies.32 Many of these reviews measured thrombocytopenia as an outcome, but none specifically describe the risk for HIT. One review compared studies recording HIT as an outcome, but the authors did not perform statistical analysis of pooled data.33 We used the conventional definition of HIT, though a recent study suggests that using a 50% or greater decrease in platelet count may be a more sensitive indicator; some cases may be missed if the preoperative platelet count is used as the baseline.34 Therefore, some cases might have been missed in the studies included here. However, when we changed the results in Warkentin et al3 from 8 and 0 to 16 and 2 cases of HIT in the UFH and LMWH groups, respectively, the ORs changed insignificantly: 0.13 (95% CI, 0.03-0.50; P = .003) for the randomized trial analysis and 0.12 (95% CI, 0.04-0.31; P < .001) for the prospective studies.
Unfortunately, few eligible studies evaluated HIT in medical inpatients. Harenberg et al6 studied more than 1500 medical patients but did not use our definition of HIT and did not describe laboratory assays for HIT. They noted no cases of thrombocytopenia lower than 80×109/L in the LMWH group and 4 cases (0.6%) in the UFH group. In the prime study, no cases were described in the 885 patients randomly assigned to LWMH or UFH, but the definition of thrombocytopenia was not described.35 In 2 placebo-controlled randomized trials of LMWH, thrombocytopenia rates were lower than 0.5%, but HIT assays were not reported.36,37 In summary, our study does not enable a reliable estimate of HIT risk in medical patients on prophylaxis with LMWH or UFH, but the risk seems likely to be very low with both drugs.
Analysis 3: thrombocytopenia outcome. Comparison: UFH versus LMWH; outcome: thrombocytopenia (HIT and non-HIT). Total events for LMWH = 152; total events for UFH = 238. Test for heterogeneity: χ2 = 46.11; df = 9 (P < .001); I2 = 80.5%. Test for overall effect: Z = 1.90 (P = .06). Symbols are as in Figure 2.
Analysis 3: thrombocytopenia outcome. Comparison: UFH versus LMWH; outcome: thrombocytopenia (HIT and non-HIT). Total events for LMWH = 152; total events for UFH = 238. Test for heterogeneity: χ2 = 46.11; df = 9 (P < .001); I2 = 80.5%. Test for overall effect: Z = 1.90 (P = .06). Symbols are as in Figure 2.
One obvious limitation of our data is the paucity of randomized trials. We included nonrandomized trials in our second analysis to achieve a more accurate OR (with a narrower CI) and a more accurate estimate of the absolute risk for HIT. Despite the pooling of studies, the estimates of risk are derived from only 2478 patients.
HIT is considerably more common with UFH than with LMWH use. The absolute risk with LMWH is only 0.2%. This information may assist hospitals with formulary decisions if the cost of HIT can be accurately determined. All future clinical trials involving LMWH and UFH should include accurate determinations for HIT to enable more accurate estimates of the risk for this potentially fatal condition.
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2005-04-1546.
P.S.W. is a recipient of a Canada Research Chair.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.