Abstract
The endothelial cell protein C receptor (EPCR) augments protein C activation by the thrombin-thrombomodulin complex. Deletion of the EPCR gene (Procr) in mice leads to embryonic lethality before embryonic day 10 (E10.0). EPCR is detected in the giant trophoblast cells at the feto-maternal boundary from E7.5 and weakly in embryonic aortic endothelial cells from E13.5, suggesting that extraembryonic EPCR expression may be essential for embryonic viability. Using conditional knock-out strategies, we demonstrate that Procr-deficient embryos with EPCR expression on placenta giant trophoblasts can be carried to term and then develop normally. Conversely, EPCR expression in the embryo, without expression in the giant trophoblast cells, does not rescue the mice. In genetically modified mice with low tissue factor activity, Procr deficiency is not lethal to the embryo. As adults, Procr-deficient mice generate more thrombin and activate less protein C in response to procoagulant stimuli. Spontaneous thrombin formation in the deficient animals increases with age. These findings show that extraembryonic EPCR expression is critical for embryo development.
Introduction
The protein C anticoagulant pathway serves as an “on-demand” anticoagulant system that controls thrombin generation by inhibiting factors Va and VIIIa.1 Thrombin not only activates platelets and clots fibrinogen, but also binds to thrombomodulin (TM) on the endothelium, at which time these procoagulant activities of thrombin are blocked, while conversion of protein C to the anticoagulant serine proteinase, activated protein C (APC), is augmented.2 Endothelial cell protein C receptor (EPCR) binds protein C and increases the rate of protein C activation by the thrombin-TM complex in cell culture3 and in vivo.4 EPCR overexpression on the endothelial cell surface increases the rate of protein C activation further.5 Inhibition of EPCR–protein C interaction converts the response to sublethal concentrations of Escherichia coli into a lethal response. These animals exhibit disseminated intravascular coagulation, intense neutrophil influx into the tissues, and elevation of some of the inflammatory cytokines.6
In adults, EPCR is localized primarily on endothelial cells of large blood vessels and is very low or absent from the microvascular endothelium of most tissues.7 EPCR is also abundant on the trophoblast giant cells at the feto-maternal boundary from embryonic day 7.5 (E7.5), suggesting a role in the hemostatic regulation of the maternal blood that irrigates these surfaces. Deletion of the EPCR gene (Procr) in mice leads to embryonic lethality before E10.0. (Gene symbols used in this paper are in accordance with the style guidelines of the National Center for Biotechnology Information, National Institutes of Health. Specifically, the gene encoding EPCR is designated Procr; tissue factor, F3; thrombomodulin, Thbd; Tie2, Tek; and Mox2, Meox2.) At this time, excess fibrin deposition is detected around the trophoblast giant cells (derived from the Procr–/– embryo).8 Immunohistochemical studies in the developing mouse embryo revealed that EPCR is first detectable on aortic endothelium at E13.5.9 Thereafter, EPCR levels increase on endothelial cells in many large vessels. The temporal differences in EPCR expression patterns in the embryo and the trophoblast giant cell coupled with the thrombosis around the trophoblast giant cells suggest that the placental thrombosis probably contributes to embryonic lethality.
One useful approach to understanding the physiologic functions of molecules is to delete the gene. This is especially true if there are reasons to believe that the molecule may have multiple functions. Unfortunately, this informative approach is often circumvented when deletion results in embryonic lethality. EPCR is a case in point. Deletion of the Procr gene results in early embryonic lethality. The structure of EPCR is very similar to the major histocompatibility complex class I/cluster of differentiation antigen 1 (CD 1) family of molecules,10 suggesting potential roles in immunity. In addition, biochemical studies have shown that EPCR substantially enhances the activation of protein C, an anticoagulant, anti-inflammatory, and antiapoptotic protein.11,12
We attempted to rescue Procr–/– embryos using 3 different strategies. First, we generated transgenic mice expressing EPCR under the control of the tyrosine kinase receptor 2 (Tek) promoter5 to examine whether abundant EPCR expression in the embryo, but not on the trophoblast giant cells, would rescue the conventional Procr knock-out mice. Second, the Procr gene was ablated selectively in the embryo13 to examine if extraembryonic expression of EPCR is required for embryonic viability. Third, we tested whether a genetically modified mouse strain with less than 1% tissue factor levels could rescue Procr–/– embryos.14 Our studies show that Procr–/– embryos can be rescued either by selective EPCR expression on giant trophoblast cells or by decreased tissue factor activity. Adult Procr–/– mice are viable, mature essentially normally, and exhibit more thrombin generation than wild-type animals. These observations provide evidence that EPCR expression on the feto-maternal surface is critical for embryo development and that EPCR expression in the developing embryo is not essential.
Materials and methods
Mice
Transgenic mice overexpressing EPCR specifically on endothelium under the control of the Tek promoter/enhancer (Tek-Procr) were generated as described.5 The mice expressing Cre-recombinase under the control of the embryo-specific mesenchyme homeobox 2 (Meox2) promoter (Meox2+/cre)13 were obtained from Jackson Lab (Bar Harbor, ME). The Meox2+/cre mouse was generated as described.13 The Cre-recombinase coding sequence driven by the Meox2 promoter replaced 1 allele of the Meox2 gene. If both alleles of the Meox2 gene are replaced, the mice will show a phenotype similar to the Meox2 gene knock-out mice such as a developmental defect of the limb musculature. The Meox2+/cre mouse is without any detectable phenotype. The mice that lack the endogenous mouse tissue factor gene (mF3–/–) but carry a single allele of a human tissue factor minigene (hF3+) were generated as described.14 Tek-Procr, Meox2+/cre, and mF3 –/–hF3+ mice were backcrossed to C57BL/6J mice for at least 10 generations. Heterozygous EPCR-deficient mice were generated as described8 and backcrossed to C57BL/6J for 7 generations. Genotyping was performed by polymerase chain reaction (PCR) analysis of tail DNA using the oligonucleotide primers described previously.5,8,13,14
Gene targeting and generation of the Procr Lox allele was described previously.8 Briefly, a LoxP flanked neoR cassette was inserted into intron 1 of the murine Procr gene, and another LoxP sequence was inserted into the 5′-untranslated region adjacent to the ATG start codon (Figure 1A). Targeting of the vector into embryonic stem (ES) cells and selection of drug-resistant ES cell clones was performed, and correct ES cell clones were identified by Southern blot hybridization.8 Correctly targeted ES cells with normal karyotype were microinjected into C57BL/6J blastocysts and surgically implanted into uteri of pseudopregnant foster mothers. Sixteen chimeric males were generated and were mated with female Black Swiss mice. From these matings, 5 chimeras demonstrated germ-line transmission. The mice were then backcrossed to C57BL/6J mice for 6 generations. All animal care and experimental procedures complied with the principles of Laboratory and Animal Care established by the National Society for Medical Research and was approved by the Institutional Animal Care and Use Committees of the Oklahoma Medical Research Foundation.
Real-time PCR and immunohistochemistry
Real-time PCR for EPCR mRNA was performed as described.5 For immunohistochemistry, placental/embryonic tissues were fixed in 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) at 4°C for 4 hours and cryoprotected in 0.5 M sucrose/PBS, embedded in optimum cutting temperature compound, and snap-frozen in liquid nitrogen–cooled isopentane. Five-micrometer sections were immunostained with a goat anti–murine EPCR polyclonal antibody. Primary antibodies were detected with rabbit anti–goat immunoglobulin G (IgG) antibody conjugated with fluorescein isothiocyanate. Sections were mounted with a medium containing 4′, 6-diamidino-2-phenylindole dihydrochloride (Vector Lab, Burlingame, CA) and photographed with a Nikon Eclipse E800M microscope (using a Plan Fluoro 20×/0.50 numeric aperture objective) equipped with digital camera DXM1200 and controlled by ACT image acquisition software (all from Nikon, Melville, NY). Embryonic tissue was genotyped as described.8 Other tissues were fixed in 4% PFA/PBS at 4°C for 18 hours and paraffin embedded. Five-micrometer sections were used for detecting EPCR organ distribution and fibrin deposition. A rabbit anti–human fibrinogen polyclonal antibody (DAKO, Carpinteria, CA) was used to detect fibrin/fibrinogen deposition in the tissues as previously described.5
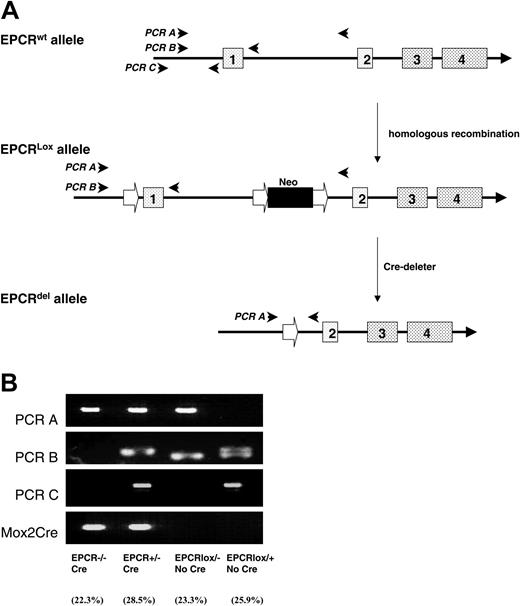
Schematic representation of the generation of Procr Lox mice and PCR genotyping. The ProcrLox allele was obtained by homologous recombination. The Procr-deleted allele was obtained when the region of the Procr gene flanked by LoxP sites was deleted by Cre-recombinase (A). PCR A detects the Procr-deleted allele. The PCR fragment of the wild-type allele is too large to be detected. PCR B detects the EPCR exon 1 wild-type allele. The ProcrLox allele also generates a band that is 36 base pair (bp) smaller due to the replacement of 68 bp in the promoter with the 32-bp LoxP site. PCR C detects the promoter region wild-type allele that does not have the LoxP site inserted. Mox2Cre primers detect the Cre allele. Procr–/–Meox2+/cre pups were born at near Mendelian ratio (43 of 193 pups; 22.3% frequency compared with the expected 25% frequency) (B).
Schematic representation of the generation of Procr Lox mice and PCR genotyping. The ProcrLox allele was obtained by homologous recombination. The Procr-deleted allele was obtained when the region of the Procr gene flanked by LoxP sites was deleted by Cre-recombinase (A). PCR A detects the Procr-deleted allele. The PCR fragment of the wild-type allele is too large to be detected. PCR B detects the EPCR exon 1 wild-type allele. The ProcrLox allele also generates a band that is 36 base pair (bp) smaller due to the replacement of 68 bp in the promoter with the 32-bp LoxP site. PCR C detects the promoter region wild-type allele that does not have the LoxP site inserted. Mox2Cre primers detect the Cre allele. Procr–/–Meox2+/cre pups were born at near Mendelian ratio (43 of 193 pups; 22.3% frequency compared with the expected 25% frequency) (B).
Analytical procedures
The image quantitation method has been described5 with some modifications. Briefly, 12-bit grayscale images (displaying 0 to 4095 gray levels per pixel) were acquired using a CoolSnap HQ cooled CCD camera (Roper Scientific, Tucson, AZ) controlled by MetaMorph 5.0 image analysis software (Universal Imaging, Downingtown, PA). Average fluorescence intensity (AFI) was integrated after threshold exclusion of the background (10 images per sample).
Tissue and plasma EPCR antigen and fibrinogen levels were measured as described.5 Thrombin-antithrombin complex (TAT) was detected in the plasma using a commercial kit (Dade Behring Diagnostics, Deerfield, IL). Circulating APC, protein C, and platelet count were evaluated as described.5 Fibrin Western blot was performed as described.15
Reagent administration
Bovine thrombin and factor Xa with phosphatidylcholine (80%)/phosphatidylserine (20%) vesicles (factor Xa/PCPS) were prepared as described.5 Under intraperitoneal avertin anesthesia, mice were injected through the carotid artery with 15 U/kg per minute bovine thrombin for 2 minutes and killed immediately; blood samples were collected by heart puncture for assays. Two doses of factor Xa/PCPS were used: the low dose was 46.9 pmol/kg factor Xa and 72.2 nmol/kg PCPS, and the high dose was 70.4 pmol/kg factor Xa and 108.3 nmol/kg PCPS. Both doses of factor Xa/PCPS were injected through the tail vein without anesthesia. For measuring hemostatic changes, we used low-dose factor Xa/PCPS, in which all mice survived 10 minutes after the infusion, at which time mice were killed to collect the blood samples. For lung histologic analysis, we used high-dose factor Xa/PCPS. In preliminary experiments, 5 of 6 Procr-deficient mice died before 10 minutes, while all wild-type mice survived at least 10 minutes after high-dose factor Xa/PCPS infusion. The lungs were collected immediately after the EPCR-deficient mice died; the wild-type mice were infused in parallel and killed at the same time as the Procr-deficient mice to collect samples for comparisons. A cannula was introduced into the right atrium, and mice were perfused with 4% PFA/PBS at 50 cm H2O pressure for 3 minutes.
To study the possible effect of low-molecular-weight heparin (LMWH) on embryo survival, pregnant mice were given a daily subcutaneous injection of LMWH (Aventis, Bridgewater, NJ) at 4 or 20 μg/g of body weight starting from E4.5.
Statistics
Results are shown as the mean plus or minus the standard deviation (SD). The Student t test was used to compare values between transgenic mice and wild-type mice. A P value less than .05 was considered statistically significant.
Results
Generation of embryo-specific Procr knock-out mice
Mice heterozygous for the targeted mutation (Procr+/Lox) were generated by blastocyst injection with targeted ES cells and breeding of the resulting germ-line chimeras to Black Swiss females. The mice were then backcrossed to C57BL/6J mice for 6 generations. Crossing Procr+/Lox mice resulted in Procr+/+ and ProcrLox/Lox mice. Due to insertion of the Lox sequence near the initiation codon, EPCR expression levels were decreased in ProcrLox/Lox mice. Compared with its wild-type littermate, the plasma-soluble EPCR level was 16% and liver EPCR levels were only 20%. Except for these lower levels, the pattern of expression was still restricted to the endothelium by immunohistochemistry. Consistent with another report,16 adult ProcrLox/Lox mice with low-level EPCR developed normally and appeared healthy throughout adulthood.
EPCR immunostaining. (A) EPCR staining is readily detected on the giant trophoblasts in (i) Procr+/+ and (ii) ProcrLox embryos at E9.5, whereas no EPCR is observed on the giant trophoblast cells of the (iii) Procr–/–Tek+ embryo. Fluorescein identifies EPCR antigen. (B) Immunohistochemical detection of EPCR in thymus, heart, kidney, and lung in Procr+/+ and ProcrLox mice. Brown horseradish peroxidase reaction product identifies EPCR antigen. EPCR is absent in ProcrLox mice from all large-vessel endothelium. gc indicates trophoblast giant cell; ys, yolk sac; and dc, decidual cell. Original magnification for all panels, × 200.
EPCR immunostaining. (A) EPCR staining is readily detected on the giant trophoblasts in (i) Procr+/+ and (ii) ProcrLox embryos at E9.5, whereas no EPCR is observed on the giant trophoblast cells of the (iii) Procr–/–Tek+ embryo. Fluorescein identifies EPCR antigen. (B) Immunohistochemical detection of EPCR in thymus, heart, kidney, and lung in Procr+/+ and ProcrLox mice. Brown horseradish peroxidase reaction product identifies EPCR antigen. EPCR is absent in ProcrLox mice from all large-vessel endothelium. gc indicates trophoblast giant cell; ys, yolk sac; and dc, decidual cell. Original magnification for all panels, × 200.
The Meox2+/cre mouse has been used previously to delete genes specifically in the developing embryo. For example, rescue of the retinoblastoma gene–deficient embryos was reported.17 To investigate the functions of EPCR in embryo development more completely, we deleted the Procr gene in the embryo specifically using the same strategy. Female Procr+/Lox mice and male Meox2+/cre mice were mated to generate Procr+/LoxMeox2+/cre mice. By breeding female ProcrLox/Lox mice with male Procr+/LoxMeox2+/cre mice, we generated Procr-deficient mice (ProcrLox/LoxMeox2+/cre, abbreviated as ProcrLox). Wild-type control mice, Procr+/+Meox2+/cre (abbreviated as Procr+/+), were generated by breeding littermate female wild-type mice with male Procr+/LoxMeox2+/cre mice. ProcrLox embryos had readily detectable EPCR expression on the giant trophoblast cells during embryogenesis (Figure 2Aii). Notably, ProcrLox pups were born at near the expected Mendelian ratio (Figure 1B). EPCR expression in the lung of ProcrLox mice was undetectable by real-time PCR. Plasma-soluble EPCR and lung tissue EPCR antigen were also below the level of detection by enzyme-linked immunosorbent assay (ELISA, < 125 pg/mL), while in Procr+/+ mice, the plasma-soluble EPCR level was 104 ± 19 ng/mL and lung tissue EPCR level was 602 ± 137 ng/g lung wet weight. Immunohistochemistry indicated EPCR expression was undetectable in any capillaries or small or large blood vessels in ProcrLox mice (Figure 2B).
To eliminate the possibility that the rescue of the ProcrLox pups was due to decreased Mox2 expression caused by the knock-in of the Cre coding sequence, male ProcrLox mice were mated to female conventional knock-out Procr+/– mice described.8 Of 123 pups, none was Procr–/–Meox2+/cre. We conclude that losing one allele of the Meox2 gene does not rescue the ProcrLox pups.
Characterization of Procr-deficient adult animals
Once viable Procr-deficient animals were generated, their hemostatic competence was characterized. ProcrLox mice appeared healthy and no hemorrhage or visible evidence of spontaneous thrombosis (such as necrotic toes or priapism) was observed. Fibrin/fibrinogen staining and fibrin Western blotting were performed in ProcrLox mice and their wild-type controls. There were no differences observed between the unchallenged ProcrLox and wild-type mice (data not shown). Two-month-old ProcrLox mice had slightly increased TAT levels. Fibrinogen levels and the platelet count were both decreased compared with Procr+/+ mice (Table 1). These differences increased and became significant as the mice aged. The circulating protein C antigen level was higher in 2-month-old ProcrLox mice (3.86 ± 0.24 μg/mL vs 3.19 ± 0.30 μg/mL, n = 5 per group, P < .01). Protein C mRNA level in liver was identical between the 2 genotypes. These data support the previous finding that EPCR expression on the endothelium results in a significant vascular compartment of protein C.5 The plasma-soluble TM and tissue TM antigen level was identical between the 2 genotypes, indicating that changes in EPCR expression do not affect TM expression.
Response of ProcrLox and Procr+/+mice to low-dose factor Xa/PCPS challenge
. | 2 months old . | . | 8 months old . | . | Factor Xa/PCPS . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ProcrLox . | Procr+/+ . | ProcrLox . | Procr+/+ . | ProcrLox . | Procr+/+ . | |||
| TAT, apparent μg/L | 2.1 ± 0.4* | 1.4 ± 0.3 | 6.5 ± 2.1† | 1.8 ± 0.3 | 202 ± 29† | 88 ± 19 | |||
| Fibrinogen, % normal C57BL/6J plasma pool | 90.7 ± 3.6 | 99.1 ± 5.1 | 74.2 ± 4.8† | 105.4 ± 5.3 | 26.1 ± 3.8† | 80.7 ± 6.7 | |||
| Platelet count, × 109/L | 731 ± 136 | 801 ± 40 | 756 ± 119* | 946 ± 64 | 353 ± 49† | 576 ± 47 | |||
. | 2 months old . | . | 8 months old . | . | Factor Xa/PCPS . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ProcrLox . | Procr+/+ . | ProcrLox . | Procr+/+ . | ProcrLox . | Procr+/+ . | |||
| TAT, apparent μg/L | 2.1 ± 0.4* | 1.4 ± 0.3 | 6.5 ± 2.1† | 1.8 ± 0.3 | 202 ± 29† | 88 ± 19 | |||
| Fibrinogen, % normal C57BL/6J plasma pool | 90.7 ± 3.6 | 99.1 ± 5.1 | 74.2 ± 4.8† | 105.4 ± 5.3 | 26.1 ± 3.8† | 80.7 ± 6.7 | |||
| Platelet count, × 109/L | 731 ± 136 | 801 ± 40 | 756 ± 119* | 946 ± 64 | 353 ± 49† | 576 ± 47 | |||
TAT level, fibrinogen, and platelet count were determined as described. Values represent the mean ± SD in Procr+/+ and ProcrLox mice at 2 and 8 months of age. These parameters were also measured in 2-month-old animals after infusion with low-dose factor Xa/PCPS. n = 5 for each group.
P < .05.
P < .01.
Upon thrombin infusion, the circulating APC level of ProcrLox mice was only 5.2% of their wild-type controls (4.6 ± 2.5 ng/mL vs 89 ± 23 ng/mL, n = 3, P < .05). This confirmed that EPCR augments protein C activation by the thrombin-TM complex in vivo.4 When EPCR was absent, the coagulation cascade lost an important control element. This hypothesis was verified by low-dose factor Xa/PCPS infusion, after which thrombin formation, fibrinogen, and platelet consumption were augmented in ProcrLox mice (Table 1). At high-dose factor Xa/PCPS, 5 of 6 ProcrLox mice died within 10 minutes, while the wild-type mice all survived. The ProcrLox mice showed more pulmonary emboli than wild-type controls (Figure 3).
EPCR expression in the embryo, but not on the giant trophoblasts, cannot rescue pups missing the endogenous gene
To selectively express EPCR in either the developing embryo or on the trophoblast giant cells, we used the following approaches in this study. Crossing the Meox2+/cre strain with mice carrying the floxed Procr allele generates animals that express EPCR exclusively in extraembryonic tissue (ProcrLox). We show here that a transgenic mouse expressing EPCR driven by the Tek promoter/enhancer overproduces EPCR on endothelial cells5 with little if any EPCR expression in the placental trophoblast giant cells. Therefore, breeding the Tek-Procr strain with Procr-null mice will produce animals that express EPCR exclusively on the endothelium of the embryo proper but not in extraembryonic tissue.
The Tek promoter/enhancer is relatively endothelial-cell specific.18 The Tie2 receptor is expressed on embryonic vasculature from E8.5.19 To evaluate EPCR expression in the Tek-Procr developing embryos, male Tek-Procr mice were bred with female C57BL/6J mice. By immunohistochemistry, EPCR was first detected at E11.5 in Tek-Procr embryos (data not shown), compared with E13.5 in wild-type embryos.9 Because ELISA is more sensitive than immunohistochemistry, it is possible to detect trace levels of EPCR at earlier embryonic stages. At E9.5, the EPCR antigen level in Tek-Procr embryos was 2.0 ± 0.75 ng per fetus (n = 10), about 20-fold higher than that of wild-type fetuses (0.105 ± 0.045 ng, n = 8). The 2 genotypes of embryos were physically the same size at E9.5. We conclude that EPCR driven by the Tek promoter/enhancer is already expressed abundantly in the fetuses at E9.5 and does not cause any obvious abnormalities.
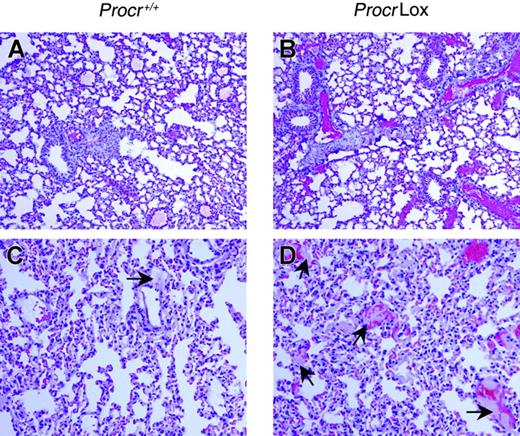
Pulmonary histopathologic analysis of ProcrLox and Procr+/+ mice after high-dose factor Xa/PCPS administration. Immunohistochemical detection of fibrin deposition in the lung of (A) Procr+/+ and (B) ProcrLox mice. Red alkaline phosphatase reaction product identifies fibrin/fibrinogen antigens. ProcrLox mice showed much more intravascular fibrin deposition than Procr+/+ mice. Hematoxylin and eosin (H&E) staining showed more and larger intravascular thrombi in the pulmonary vessels in (D) ProcrLox mice than in (C) Procr+/+ mice. (C-D) Arrows indicate thrombi and red staining indicates red blood cells trapped in the vessels. Slides are representative of 4 mice per group. Fluorescence quantitation shows that the AFI of fibrin/fibrinogen staining is more in ProcrLox mice than wild-type mice (1342 ± 668 vs 388 ± 205, n = 4, P < .05). Original magnification for all panels, × 100.
Pulmonary histopathologic analysis of ProcrLox and Procr+/+ mice after high-dose factor Xa/PCPS administration. Immunohistochemical detection of fibrin deposition in the lung of (A) Procr+/+ and (B) ProcrLox mice. Red alkaline phosphatase reaction product identifies fibrin/fibrinogen antigens. ProcrLox mice showed much more intravascular fibrin deposition than Procr+/+ mice. Hematoxylin and eosin (H&E) staining showed more and larger intravascular thrombi in the pulmonary vessels in (D) ProcrLox mice than in (C) Procr+/+ mice. (C-D) Arrows indicate thrombi and red staining indicates red blood cells trapped in the vessels. Slides are representative of 4 mice per group. Fluorescence quantitation shows that the AFI of fibrin/fibrinogen staining is more in ProcrLox mice than wild-type mice (1342 ± 668 vs 388 ± 205, n = 4, P < .05). Original magnification for all panels, × 100.
It has been observed that Cre-recombinase driven by the Tie2 promoter/enhancer may not completely delete a target gene on giant trophoblast cells,20 indicating that the fragment of the Tie2 promoter does not effectively drive Cre expression on giant trophoblast cells in this time frame. Although the Tie2 receptor itself is strongly expressed on the giant trophoblast at E9.5,21 the Tek promoter alone does not recapitulate the expression pattern of the endogenous Tek gene and can be possibly used to express EPCR in the embryo without significant expression of EPCR in the placental tissue.
To attempt rescue of Procr–/– pups by introducing EPCR expression under control of the Tek promoter/enhancer, transgenic Tek-Procr mice were bred to the Procr+/– mice to produce Procr+/–Tek+ mice, and then the Procr+/–Tek+ mice were crossed. No Procr–/– pups were born (0/256). By timed mating, we found that Procr–/–Tek+ embryos were observed at E9.5 (7/45, 15.6%, expected 18.75%), but not at E12.5 (0/30). Procr–/–Tek+ embryos at E9.5 were smaller than either Procr+/– or Procr+/+ embryos and exhibited signs of the onset of resorption (data not shown). The observations were similar to those of Procr–/– embryos derived from Procr+/– mating.8 Immunohistochemistry at E9.5 revealed that under the control of the Tek promoter/enhancer, although it was readily apparent on the yolk sac, no EPCR staining was found on giant trophoblast cells (Figure 2Aiii).
In theory, the failure to rescue Procr–/– pups by Tek-controlled expression of EPCR in endothelium could have been due to slightly delayed expression of EPCR that therefore failed to rescue the embryo. To address this possibility, we sought to use other means to extend the viability of the developing embryo. Previous studies had shown that anticoagulation from E4.5 prolonged the survival of Procr-null embryos.8 Thus, when the Procr+/–Tek+ mice were crossed and the pregnant mice were administrated with LMWH (4 μg/g) from E4.5 to term, Procr–/–Tek+ embryos should survive long enough for EPCR expression driven by the Tek promoter to commence. Anticoagulant therapy did prolong the survival of Procr–/–Tek+ embryos; of 20 embryos alive at E13.5, 3 were Procr–/–Tek+. Under such an anticoagulant regimen, there were no Procr–/–Tek+ live births from the 36 viable pups. Since EPCR expression in the Tek+ embryo has occurred in this mouse line at or before E9.5, we conclude that failure to express EPCR at early times in the endothelium of the developing mouse was not the cause of embryonic lethality.
Tissue factor deficiency can rescue Procr–/– pups
To test whether thrombosis and/or increased thrombin generation contributed to embryonic lethality, we crossed mice expressing low tissue factor activity (mF3–/–hF3+) with the Procr-deficient mice. mF3–/–hF3+ mice were crossed to Procr+/– mice to yield Procr+/–mF3+/–hF3+ mice. Analysis of live-born pups from Procr+/–mF3+/–mF3+ crossing identified 9 viable Procr–/– animals. All Procr–/– pups lacked mouse tissue factor yet carried the human tissue factor transgene. The yield of viable Procr–/–mF3–/– hF3+ newborns (9/139, 6.47%) conformed to the genotype frequency predicted by Mendelian inheritance (3/64, 4.68%). These observations show that the reduction of tissue factor activity to less than 1% of the normal level rescues Procr–/– embryos. Procr–/–mF3–/– hF3+ mice were viable for at least 8 months. The genotypes Procr–/–mF3+/+ (with hF3+ or hF3–) and mF3–/–hF3– (with or without the EPCR expression) were not found in newborn pups, consistent with intrauterine lethality of embryos completely devoid of either tissue factor14 or EPCR in the environment of normal tissue factor expression.8 A role for coagulation in the embryonic lethality associated with EPCR deficiency is also supported by the observation that LMWH administration to the pregnant females from conventional knock-out Procr+/– crossing could partially rescue the embryos.8 Specifically, 20 μg/g heparin was injected subcutaneously each day from E4.5 to full term. Of 48 pups born, 2 were found to be Procr–/–.
Discussion
Appropriate hemostatic balance is essential for maintenance of pregnancy. Maternal fibrinogen and its conversion to fibrin is required (in controlled quantities) for placental stabilization and the anchoring of placental trophoblasts to the maternal decidua. Mice deficient in fibrinogen22,23 display miscarriage because of intrauterine bleeding around E10.0. However, uncontrolled coagulation initiated by tissue factor at the feto-maternal interface will cause the abortion of embryos as observed in TM deficiency.24 We have also demonstrated that thrombin generation in the microenvironment of the giant trophoblast cells plays an important role in the death of Procr–/– embryos.8 Procr–/– embryos removed from the maternal decidua and cultured in vitro developed beyond E10.5.8 At E9.5, a dramatic increase in the amount of fibrin was detected around the giant trophoblasts. Previous studies on wild-type embryos showed that EPCR expression in embryonically derived cells other than the trophoblast giant cells first becomes detectable in the yolk sac at E10.5; EPCR was only weakly detected in aortic endothelial cells from E13.5.9 These observations indicate that impaired APC generation due to EPCR deficiency at the interface between the embryo and maternal circulation contributes to excess fibrin deposition and the midgestational developmental arrest of these embryos.
To further evaluate the role of EPCR expression on giant trophoblast cells in the maintenance of embryo viability, we took advantage of the Meox2+/Cre ProcrLox/Lox conditional knock-out system to ablate Procr on the embryo but spare the placenta. Previous studies have shown that the Cre-recombinase gene driven by the Meox2 promoter is efficiently expressed and functionally active in all cells of the E6.5 embryo proper, with no expression in the extraembryonic endoderm lineages.13 Thus, Cre is expressed and presumably Procr is deleted earlier than any EPCR expression can be detected on the wild-type fetus.9 By breeding ProcrLox/+Meox2+/cre mice with homozygous ProcrLox/Lox mice, we were able to generate ProcrLox mice. Real-time PCR, immunohistochemistry, and ELISA found no EPCR expression on endothelial cells in these mice.
It has been reported that mice with very low-level EPCR expression can develop normally.16 This raises the question whether the rescue of ProcrLox mice is due to residual undetectable level of EPCR expression in the embryo. As an alternative approach, to test the role of embryonic EPCR in development, we expressed EPCR selectively in the embryo using the Tek promoter/enhancer to drive embryonic EPCR expression in the endogenous Procr-null mice. EPCR expression driven by the Tek promoter/enhancer was much higher in transgenic embryos than that of wild-type embryos at E9.5, yet this did not rescue Procr–/– animals. In these animals, EPCR driven by the Tie2 promoter was undetectable by immunohistochemistry on the giant trophoblast cells at E9.5, while in wild-type placenta, EPCR was abundant on these cells.9 The overexpression of EPCR in the embryo did not itself cause embryonic lethality since the equivalent overexpression on a wild-type background was viable.5 Taken together, when EPCR is not expressed on the giant trophoblast cells, even enhanced expression of EPCR in the embryo does not rescue the embryo. In contrast, when EPCR is expressed on the giant trophoblast cells, Procr-deficient embryos survive. Therefore, it is the loss of extraembryonic function of EPCR that contributes to the lethality of the EPCR-null phenotype.
Adult ProcrLox mice showed less protein C activation during thrombin infusion. This confirmed the previous results using blocking antibody to interfere with the interaction of protein C and EPCR.4 Thrombin formation (reflected by the circulating TAT levels) was higher than in Procr+/+ mice when treated with factor Xa/PCPS, and was accompanied by more fibrinogen and platelet consumption and more pulmonary emboli. The experimental results using adult mice lead to the prediction that EPCR deficiency on the trophoblast would impair protein C activation. The unfettered local thrombin generation initiated by tissue factor could result in excess fibrin deposition and/or more trophoblast apoptosis.8,24 Subsequently, the embryo would be deprived of nutrition and oxygen supply leading to death.
If it is the generation of thrombin around the giant trophoblast that leads to embryonic death, rather than the loss of other possible EPCR functions, reduced tissue factor expression on these cells might rescue these embryos. We crossed Procr+/–mF3+/–hF3+ mice to see whether lower tissue factor expression on the fetus-derived tissue can rescue Procr–/– pups. Procr–/– pups were born when the tissue factor level was lowered to approximately 1% to 2% (mF3–/–hF3+). Local thrombin generation contributes significantly to the death of Procr–/– pups and can be prevented by lowering the tissue factor level on the maternal-fetus interface.
It has been reported that the daily administration of LMWH to Procr+/– interbred pregnant female mice was able to prolong the survival of some Procr-null embryos. LMWH (4 μg/g) could prevent detectable fibrin deposition at the maternal-embryonic interface, but only a subset (∼ 25%) of Procr–/– embryos survived to E15.5.8 Given that Procr-null mice could be born in a low tissue factor mouse background, we tested whether increased doses of LWMH could rescue the developing embryos. The heparin dose was increased to 20 μg/g body weight. Of 48 pups from Procr+/– interbred pairs, 2 Procr–/– pups were born. We concluded that it is possible to rescue Procr–/– pups by inhibiting the hypercoagulable state on the maternal-embryonic interface. One possible caveat with respect to the heparin rescue is that heparin has been shown to enhance protein C activation by factor Xa.25 Thus it is possible that rescue of the deficient animals by heparin was facilitated by APC formation by the factor Xa-heparin complex.
There are many similarities between mice deficient in either of the 2 membrane receptors of the protein C activation complex, EPCR and TM. TM gene (Thbd) deletion embryos die earlier than Procr–/– embryos at E8.5. Death appears to be due, at least in part, to tissue factor–initiated activation of blood coagulation at the feto-maternal interface. This conclusion was based primarily on 2 observations: (1) Thbd–/– mice are viable in a background expressing very low tissue factor24 and (2) reconstitution of TM expression in extraembryonic tissue rescues the Thbd–/– embryos from early lethality.26 Similarly, just as very low EPCR levels are compatible with murine development,16 a Thbd mutant mouse line with severely impaired protein C activation capability,15,27 generated by replacing the wild-type TM gene with a Glu404Pro mutation, resulted in viable mice. In this respect, the TM mutant mice are similar to those with Procr deletion reported here. Both are viable and do not exhibit overt pathologically relevant thrombosis in otherwise healthy animals. However, when challenged by a variety of agents, more severe thrombosis ensues.
It is likely that complete deletion of Thbd or Procr also contributes to abnormal regulation of the inflammatory process. In the case of the TM molecule, the lectinlike domain that is not involved in protein C activation has been shown to reduce leukocyte adhesion and endothelial cell activation,28 and very recently, also to bind and neutralize high mobility group box 1,29 a key late-stage inflammatory mediator contributing to death in severe sepsis. The specific functions of EPCR in regulating inflammation remain to be defined. However, blocking APC binding to EPCR increases interleukin-6 and interleukin-8 elaboration in baboons challenged with E coli,6 attenuates the APC protective effect on ischemia in the brain,11 and inhibits sphingosine 1 phosphate receptor activation.30,31
EPCR expression on the giant trophoblast cells is important in the control of local thrombin formation, and this function is essential for the maintenance of pregnancy. The ability to generate viable Procr-deficient animals not only demonstrates that the deficiency leads to a hypercoagulable state that increases in severity with age, but it also allows further studies on the physiologic functions of EPCR in the adult animals.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-01-0406.
Supported by grant P50 HL54502 (C.T.E.) from the National Institutes of Health. C.T.E. is an investigator of the Howard Hughes Medical Institute. C.T.E. holds the Lloyd Noble Chair in Cardiovascular Research at the Oklahoma Medical Research Foundation.
W.L. and X.Z. performed most of the experiments; J.-.M.G. made the initial constructs used for gene deletion; G.L.F. maintained the mouse husbandry and ES cell preparations; M.B. performed the genotyping; N.L.E. and C.T.E. performed the overall design and interpretation of the experiments; and W.L., X.Z., N.L.E., and C.T.E. wrote the paper and all revisions.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Nigel Mackman for providing the low tissue factor mice. We thank Dr Florea Lupu, Lijun Xia, and Alexander Matveev for technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal