Abstract
At the frontier between innate and adaptive immunity, dendritic cells (DCs) secrete numerous cytokines and express costimulatory molecules that initiate or enhance natural killer (NK) and T-lymphocyte responses. NK cells also regulate DC physiology by killing immature DCs (iDCs), thus limiting inflammation and inappropriate T-lymphocyte tolerization. In a previous study, we have reported that NK cells from acute myeloid leukemia patients (AML-NK cells) have deficient natural cytotoxicity receptor (NCR) expression. Herein, we analyzed the consequences of such a defect regarding the regulatory role of AML-NK cells in DC physiology. We show that NK cells display poor cytolytic capacities against DCs derived from healthy donor monocytes or derived from autologous leukemic blasts. These data point to a novel defect in the regulation of adaptive immune responses initiated by DCs in AML patients. This may lead to specific T-lymphocyte tolerization by spontaneous or ex vivo expanded iDCs expressing leukemia-derived antigens. (Blood. 2005;106: 2186-2188)
Introduction
The development of a specific immune response requires T-lymphocyte interaction with mature dendritic cells (mDCs), since immature DCs (iDCs) do not efficiently present antigens to T lymphocytes.1,2 Since inefficient antigen presentation leads to T-lymphocyte-specific tolerization, excessive amounts of iDCs induce the emergence of tolerogenic T lymphocytes.3-6 Thus, abnormal distribution in DC subpopulations in leukemic patients may contribute to the observed immune deficiency.7 To avoid inappropriate interactions with T-lymphocytes, iDCs may be induced to either mature or, alternatively, to die.8 Control of the balance between mDCs and iDCs is also relevant for immunotherapy protocols since DCs are considered of putative interest in the treatment of cancer.9,10
Recently it has been demonstrated that iDC clearance is at least in part mediated by natural killer (NK) cells. When NK cells overcome the number of iDCs, NK cells are able to kill iDCs.11-13 This killing is permitted by the low human leukocyte antigen (HLA)-class I expression of iDCs and depends mainly on the natural cytotoxicity receptor (NCR) NKp30 triggering molecule.14 We have previously shown that acute myeloid leukemia-natural killer (AML-NK) cells have deficient cytolytic capacities related to low or absent NCR expression.15-17 Herein, we tested the hypothesis that deficient AML-NK cell cytolytic function impairs iDC clearance and thus may contribute to leukemia escape not only from innate but also from adaptive immunity.
Study design
Cells
Peripheral blood samples were obtained from patients or healthy blood donors after informed consent, per the Declaration of Helsinki. Samples were obtained before specific antileukemic therapy and were part of diagnostic procedures. The study protocol was approved by the Institut Paoli-Calmettes institutional review board.
Generation of NK cells
In vitro NK cell expansion was done as previously described.15 Briefly, CD3-CD4-HLA-DR- cells from 7 patients or 3 healthy donors were obtained after negative immunomagnetic selection (Myltenyi Biotec, Paris, France). They were cultured for 3 weeks with 1000 UI/mL recombinant interleukin 2 (IL-2, Proleukin; Chiron, Emeryville, CA), 1.5 ng/mL phytohemagglutinin A (Invitrogen, Cergy-Pontoise, France), and irradiated allogeneic normal peripheral blood mononucleated cells (PBMCs) used as feeder cells in RPMI (Cambrex, Emerainville, France) supplemented with 10% fetal calf serum (Biowest, Paris, France). Cell-surface analysis was done by flow cytometry on a Becton Dickinson FACScalibur cytometer using the Cellquest software (Becton Dickinson, Mountain View, CA). Immunostaining of NK cells was done using fluorescein isothiocyanate (FITC)-conjugated anti-CD3, phycocyanin5 (PC5)-conjugated anti-CD56, and allophycocyanin (APC)-conjugated anti-CD45 (the latter being used for myeloid blast exclusion) monoclonal antibodies (mAbs), all from Immunotech (Marseille, France). NCR (NKp44, NKp30, and NKp46) and other triggering receptor (CD16, 2B4 and NKG2D) expression was measured with phycoerythrin (PE)-conjugated mAb (kindly gifted by Immunotech).
Generation of DCs
To obtain normal iDCs, CD14+ monocytes were isolated by positive immunomagnetic selection (Myltenyi Biotec) from PBMCs of healthy donors and cultured for 5 days in RPMI supplemented with 10% fetal calf serum, 100 ng/mL granulocyte-monocyte colony-stimulating factor (GM-CSF), and 40 ng/mL interleukin 4 (kindly provided by Shering-Plough, Dardilly, France). Leukemia-derived iDCs (LA-DCs) were obtained with the same method as previously described.18 Maturation of monocytederived DCs (Mo-DCs) was obtained by addition of Escherichia coli lipopolysaccharide (LPS) 10 μg/mL (Sigma-Aldrich, Fallavier, France) for 2 additional days of culture.
Cytotoxic assays
The cytotoxic assays were performed by a 4-hour 51chromium-release assay.15 Redirected assays were done using P815 murine mastocytoma cell line (ATCC; LGC Promochem, Molsheim, France) as target cells, while masking experiments were done by using iDCs or mDCs from healthy or AML donors. The concentrations of the various mAbs (all from A. Moretta's laboratory) were 10 μg/mL for the masking experiments (F252 anti-NKp30 immunoglobulin M [IgM] mAb, AG-136 anti-HLAclass I IgM mAb) and 5 μg/mL for the redirected killing experiments (KD1 anti-CD16 IgG1 mAb, AZZ20 anti-NKp30 IgG1 mAb, BAB281 anti-NKp46 IgG1 mAb). All experiments were performed in triplicate in at least 3 independent experiments.
Results and discussion
AML-NK cells were cultured in the presence of exogenous IL-2 for 3 weeks and then assessed for cytolytic activity in a redirected killing assay against murine P815 target cells and against Mo-DCs isolated from healthy donors or from leukemic patients. NK cells obtained from healthy donors and cultured under the same conditions were used as controls in cytotoxicity experiments.
As previously reported, most AML patients (80%) have low NKp30 expression (NKp30dull NK phenotype, Figure 1C), in sharp contrast with the high-level NKp30 expression (NKp30bright NK phenotype, Figure 1A) detected in NK cells from healthy donors. In all instances, for each patient the percentage of NCRdull or NCRbright NK cells is superior to 95% of total NK cells (data not shown). Although not shown, CD16 is a triggering molecule that is expressed at normal levels in AML-NK cells.15 In accordance with these data, the responsiveness of NKp30dull AML-NK cells to anti-NKp30 mAbs in redirected killing assays was significantly lower than that to anti-CD16 mAbs (Figure 1C, column 1). In contrast, NCRbright NK cells from healthy individuals displayed responses to anti-NKp30 mAbs (Figure 1A, column 1) comparable in magnitude with those elicited by anti-CD16 mAbs. Thus the differential responsiveness of AML-NK cells to triggering via NKp30 or CD16 suggested their cytolytic potential was not different from that of controls and that their reduced response to NKp30 mAbs reflected only the low expression of this receptor. Of note, we did not find statistical differences regarding CD56, CD16, 2B4, and NKG2D expression (data not shown).
Few AML patients (20%) are characterized by NK cells expressing an NCRbright phenotype comparable with that of most healthy donors.15 As expected, these NCRbright AML-NK cells displayed efficient cytolytic responses to both anti-NKp30 and anti-CD16 mAbs (Figure 1B, column 1). In all instances, NKp46 and NKp44 redirected killing was deficient in NCRdull AML-NK cells (data not shown).
Recent studies demonstrated that human NK cells display the ability to kill both autologous and allogeneic monocyte-derived iDCs.19 This function depends on the engagement of NKp30 by still-undefined cellular ligands expressed by DCs. Indeed NK cells from controls efficiently killed iDCs in an NKp30-dependent manner as demonstrated by the ability of IgM anti-NKp30 blocking mAbs to inhibit their killing activity (Figure 1A, column 2). On the contrary, killing of iDCs was impaired when NKp30dull AML-NK cells were used as effector cells (Figure 1C, column 2). As expected, NKp30bright AML-NK cells were as efficient as control NK cells from healthy donors (Figure 1B, column 2). In all instances, the iDC killing was inhibited by IgM anti-NKp30 blocking mAb, independently of the levels of cytolytic activity (Figure 1A-C, column 2).
NCRdullAML-NK cell killing of normal monocyte-derived DCs. The expression of NKp30 was analyzed by flow cytometry on NK cells obtained from 3 healthy donors (NCRbright[A]) or 7 AML patients (1 of 2 patients with NCRbright phenotype [B], or 1 of 5 patients with NCRdull phenotype [C]) as described in “Study design.” Black histograms represent the isotype-matched negative controls; and white histograms, the PE-conjugated anti-NKp30 mAb staining. On the corresponding diagrams, the 3 different NK cells (ie, normal NCRbright NK, NCRbright AML-NK, and NCRdull AML-NK) were assessed for cytolytic activity against the P815 murine cell line (column i), immature Mo-DCs (iDC, column ii), and mature Mo-DCs (mDC, column iii). ▪ represent the redirected killing with the IgG1 anti-CD16 mAb; •, the redirected killing with the IgG1 anti-NKp30 mAb; and ♦, the redirected killing with an irrelevant IgG1 mAb. ▵ represent NK cells against iDCs, and the crosses represent the natural killing of NK cells against iDCs blocked with an IgM anti-NKp30 mAb. Grey circles represent the natural killing of NK cells against mDCs, and ○ represent the blocking of HLA class I engagement by IgM anti-HLA class I mAb. These data are from 1 experiment of 5 representative and independent performed. Error bars indicate ± standard deviation (SD).
NCRdullAML-NK cell killing of normal monocyte-derived DCs. The expression of NKp30 was analyzed by flow cytometry on NK cells obtained from 3 healthy donors (NCRbright[A]) or 7 AML patients (1 of 2 patients with NCRbright phenotype [B], or 1 of 5 patients with NCRdull phenotype [C]) as described in “Study design.” Black histograms represent the isotype-matched negative controls; and white histograms, the PE-conjugated anti-NKp30 mAb staining. On the corresponding diagrams, the 3 different NK cells (ie, normal NCRbright NK, NCRbright AML-NK, and NCRdull AML-NK) were assessed for cytolytic activity against the P815 murine cell line (column i), immature Mo-DCs (iDC, column ii), and mature Mo-DCs (mDC, column iii). ▪ represent the redirected killing with the IgG1 anti-CD16 mAb; •, the redirected killing with the IgG1 anti-NKp30 mAb; and ♦, the redirected killing with an irrelevant IgG1 mAb. ▵ represent NK cells against iDCs, and the crosses represent the natural killing of NK cells against iDCs blocked with an IgM anti-NKp30 mAb. Grey circles represent the natural killing of NK cells against mDCs, and ○ represent the blocking of HLA class I engagement by IgM anti-HLA class I mAb. These data are from 1 experiment of 5 representative and independent performed. Error bars indicate ± standard deviation (SD).
The same populations of NK cells were also assessed for cytolytic activity against mature Mo-DCs. These target cells, unlike iDCs, express high levels of HLA class I molecules that protect them from NK-mediated killing.As expected, mature Mo-DCs were resistant to lysis by both AML-NK and control NK cells (Figure 1, right panels). However, killing by NCRbright NK cells (Figure 1A-B) was reversed by blocking anti-HLAclass I mAb. Under the same conditions, NKp30dull AML-NK cells were still unable to kill mature Mo-DCs, indicating that their poor cytolytic activity was not due to inhibitory receptor (killer cell inhibitory receptor [KIR], CD94/NKG2A) interaction with HLA class I molecules.
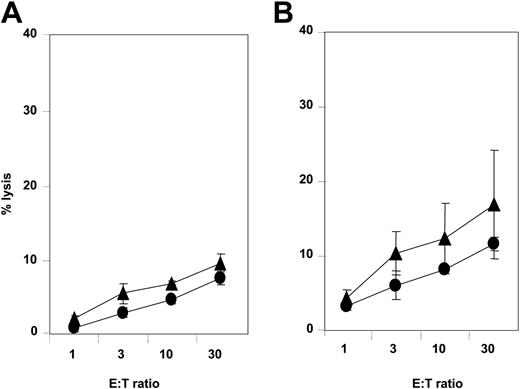
NCRdullAML-NK cell killing of leukemia-derived DCs. NCRdull AML-NK cells were obtained and amplified as described in “Study design” and assessed for spontaneous cytolytic activity against autologous (A) or allogeneic (B) iDCs (▴) and mDCs (•). These data are from 1 experiment of 3 representative and independent performed. Error bars indicate ± standard deviation (SD). E/T indicates effector-target ratio.
NCRdullAML-NK cell killing of leukemia-derived DCs. NCRdull AML-NK cells were obtained and amplified as described in “Study design” and assessed for spontaneous cytolytic activity against autologous (A) or allogeneic (B) iDCs (▴) and mDCs (•). These data are from 1 experiment of 3 representative and independent performed. Error bars indicate ± standard deviation (SD). E/T indicates effector-target ratio.
Since CD14+ AML blasts differentiate, at least ex vivo, in DC-like cells, novel immunotherapeutic approaches using LA-DCs could represent a way to enhance specific immune responses against leukemia.18,20 This prompted us to assess the ability of NKp30dull AML-NK cells to kill LA-DCs. Whereas NKp30bright NK cells efficiently killed immature LA-DCs (data not shown), NKp30dull AML-NK cells displayed weak cytotoxicity against both autologous (Figure 2A) and allogeneic (Figure 2B) immature LA-DCs.
Our data indicate that the majority of AML patients have NK cells characterized by a defect in the ability to kill either normal immature Mo-DCs or immature LA-DCs. Since NCRbright NK cells are able to kill both normal iDCs (Figure 1) and LA-DCs (data not shown), this defect is likely to be caused by the apparent defect in NCR expression and not by the absence of ligands. In both cases, this dysregulation of iDC clearance might result in induction of abnormal interactions between iDCs and T lymphocytes and induce tolerization against iDC-presented tumor antigens. As a consequence, we hypothesize that patients with the common NCRdull phenotype may not benefit from LA-iDC vaccination against leukemia due to inefficient regulation of immature LA-DC maturation.4,5 Nevertheless, this approach should be useful in NCRbright patients. This implies that NCR expression must be analyzed at diagnosis for each patient in order to determine individually the best strategy for leukemia immunotherapy. This immunotherapy has still to be ideated, but, since the NK cells we tested were obtained after a 3-week in vitro expansion with high-dose IL-2, it is unlikely this IL-2 stimulation approach could overcome NCR deficiency at least regarding iDC killing. Nonetheless, we cannot exclude that the defect in NK cell function (or only the decreased expression of NKp30) could be complemented by NK cells in other compartments, such as lymph node or spleen. Such a hypothesis is under investigation in our laboratory. In conclusion, we have described here a novel mechanism of tumor escape indirectly related to impaired NK-mediated selection of maturing DCs. This dysfunction may alter both the anti-infectious and antitumor immune responses and strongly suggests that the restoration of normal AML-NK cell functions should be a goal for future laboratory and clinical investigations.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2005-03-1270.
Supported by Groupement Entreprise Français Lutte Cancer, Fédération Nationale des Centres de Lutte Contre le Cancer, Fondation pour la Recherche Médicale, and the Ligue Contre le Cancer.
An InsideBlood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Just-Landi and F. Mallet for excellent technical assistance. We also thank Julie Veran and Naira BenMami for cell culture contribution.

![Figure 1. NCRdull AML-NK cell killing of normal monocyte-derived DCs. The expression of NKp30 was analyzed by flow cytometry on NK cells obtained from 3 healthy donors (NCRbright[A]) or 7 AML patients (1 of 2 patients with NCRbright phenotype [B], or 1 of 5 patients with NCRdull phenotype [C]) as described in “Study design.” Black histograms represent the isotype-matched negative controls; and white histograms, the PE-conjugated anti-NKp30 mAb staining. On the corresponding diagrams, the 3 different NK cells (ie, normal NCRbright NK, NCRbright AML-NK, and NCRdull AML-NK) were assessed for cytolytic activity against the P815 murine cell line (column i), immature Mo-DCs (iDC, column ii), and mature Mo-DCs (mDC, column iii). ▪ represent the redirected killing with the IgG1 anti-CD16 mAb; •, the redirected killing with the IgG1 anti-NKp30 mAb; and ♦, the redirected killing with an irrelevant IgG1 mAb. ▵ represent NK cells against iDCs, and the crosses represent the natural killing of NK cells against iDCs blocked with an IgM anti-NKp30 mAb. Grey circles represent the natural killing of NK cells against mDCs, and ○ represent the blocking of HLA class I engagement by IgM anti-HLA class I mAb. These data are from 1 experiment of 5 representative and independent performed. Error bars indicate ± standard deviation (SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-03-1270/6/m_zh80180583850001.jpeg?Expires=1769106356&Signature=djnq~dIEu1g5LlITschNW~TuZT8qLxMxIst9ObGfMImQIa6sL-MsbQz7gD-i0V-3G46IB4M5CA1KUY78AQ4yFIVF9419OV5f13psyINj5nEqOVdznJi9-IRO74A2a~YDPfwTwyVHKWPj3hxK5fe-Ou1dqMo8JzewZXjFTv59wQaWIwVFv3NNyW8OQ3qmPF5jZByzm9rJNRkTUucQBs~Q9YAH86IV~P-p6~T15sHtnowTtuPghh0x6tMP4J5ohZzRNLFrqxwFXlsURmzRenHCE1-Y-XEgJmYUZtNOxrUho2tzws7n8-X8c7CcFtMcgVAgbLxC50XaqxIiidPGh3t1ZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)