Abstract
The clinical history of patients with heart failure (HF) is complicated by arterial thromboembolism. Platelet activation is reported in this population, but the underlying mechanism has not been clarified. Forty-two patients with HF scored according to New York Heart Association (NYHA) classification had higher levels of collagen-induced platelet aggregation, platelet tumor necrosis factor-α (TNF-α) receptor expression, and serum thromboxane B2 and higher circulating levels of TNF-α than 20 healthy subjects. Coincubation of platelets from HF patients with an inhibitor of TNF-α receptors significantly reduced collagen-induced platelet aggregation. In vitro study demonstrated that TNF-α amplified the platelet response to collagen; this effect was inhibited by TNF-α receptor antagonist and inhibitors of arachidonic acid metabolism. This study showed that TNF-α behaves as a trigger of platelet activation through stimulation of the arachidonic acid pathway.
Introduction
Chronic congestive heart failure (HF) is an important cause of morbidity and mortality in the Western population.1 HF predisposes to thromboembolism, particularly ischemic stroke, in a relatively high percentage of patients.1 The association between HF and thromboembolic stroke is higher in patients with severe HF than in patients with mild to moderate HF,2 but the mechanisms favoring thromboembolism have not been fully clarified.3
The proinflammatory cytokine tumor necrosis factor-α (TNF-α) is up-regulated in HF patients and seems to be involved in the pathophysiology of this disease.4,5 Because previous in vitro studies have demonstrated that TNF-α may promote platelet aggregation,6,7 we investigated whether circulating levels of TNF-α could have a role in eliciting platelet aggregation in patients with HF.
Study design
Patients
We analyzed 20 health subjects (HSs) and 42 patients with diagnoses of HF who were enrolled from the outpatient clinic of our facilities. The study population's characteristics are reported in Table 1. Inclusion and exclusion criteria and diagnostic methods have been previously reported.8 HF was scored according to the New York Heart Association (NYHA) classification. All patients gave informed consent to participate in the study. The study protocol was approved by the institutional ethics committee of the Department of Experimental Medicine and Pathology of the University of Rome “La Sapienza.”
Platelet activation
Agonist-induced platelet aggregation (PA) (Born method) was performed in washed platelets as described in Leo et al9 and Gresele et al10 and were expressed as the light transmission (LT) percentage difference between platelet-rich plasma and platelet-poor plasma 3 minutes after addition of the agonist. Collagen-induced platelet thromboxane B2 (TxB2) was measured as previously described.11 Serum TxB2 was measured as previously described.12 Plasma TNF-α levels were determined using TNF-α enzyme-linked immunosorbent assay (ELISA) kits.8
Flow cytometry analysis of TNF-α receptors
TNF-α receptor 1 (TNFR1) and TNFR2 expression on platelet membrane was analyzed using the specific fluorescein-isothiocyanate (FITC)-labeled monoclonal antibodies (mAbs) anti-TNFR1 and anti-TNFR2 (R&D Systems, Minneapolis, MN). All assays included samples to which an irrelevant isotype-matched antibody was added.
Twenty microliters mAb was added to 200 μL platelet suspension (2 × 108/mL) previously fixed with (2%) paraformaldehyde (0.1% bovine serum albumin [BSA]) and were incubated for 60 minutes at 4°C. The unbound mAb was removed by the addition of 0.1% BSA phosphate-buffered saline (PBS) and centrifugation at 5000g for 3 minutes (twice). Fluorescence intensity was analyzed on an Epics XL-MCL Cytometer (Coulter Electronics, Hialeah, FL) equipped with an argon laser at 488 nM. For every histogram, 50 000 platelets were counted to evaluate the percentage of positive platelets. Antibody reactivity was reported as mean fluorescence intensity.
In vitro study
PA and TxB2 formation were measured, as previously described,9 in platelets taken from aspirin-free patients and were incubated at 37°C for 15 minutes with TNF-α (20-40 pg/mL) and a subthreshold concentration of collagen11 (0.5 μg/mL), adenosine diphosphate (ADP) (0.4 μM), or thrombin (0.01 U/mL) and were or were not added to 14 μM arachidonoyl trifluoromethyl ketone (AACOCF3), an inhibitor of phospholipase A2 (PLA2) enzyme,9 100 μM aspirin, an inhibitor of cyclooxygenase enzyme,9 or 1 μM WP9QY, an inhibitor of TNF-α receptors.13 For these experiments, AACOCF3, aspirin, and WP9QY were incubated for 10 minutes at 37°C before agonists were added.
Statistical analysis
Data are reported as mean plus or minus SD. Variables were analyzed using the Student t test for unpaired data.
When comparing more than 2 groups, 2-way analysis of variance (ANOVA) test and the Kruskall-Wallis test as a post hoc test were used. If a significant difference was found, the Mann-Whitney U test (2-tailed) was used to determine the differences between each pair of groups. Correlation analysis was performed using Pearson correlation coefficient. Significance was accepted at P less than .05.
Results and discussion
Clinical characteristics and drug therapy of patients with HF according to NYHA classification are reported in Table 1.
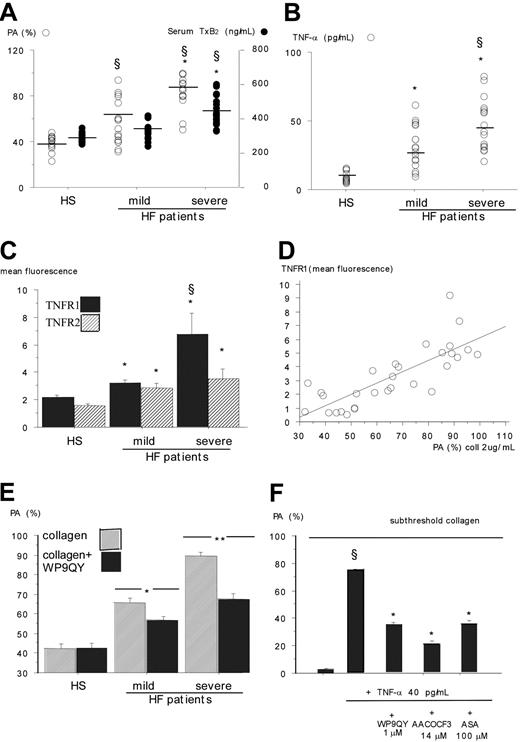
Patients with HF had higher platelet aggregation (PA) (LT, 72% vs 40%; P < .001) and serum TxB2 (377 ng/mL vs 284 ng/mL; P < .001) concentrations than HSs; after excluding 4 aspirin-treated patients, such increases were still evident and were associated with HF severity (Figure 1A). These findings were not observed using other agonists, such as ADP or thrombin, and were not influenced by HF etiology (data not shown).
TNF-α was significantly higher in patients than in HSs (39.6 ± 12 pg/mL vs 8.5 ± 3 pg/mL; P < .001), progressively increased from mild to severe (Figure 1B), and significantly correlated with the production of PA (r = 0.72; P < .001) and serum TxB2 (r = 0.53; P < .001), suggesting a possible cause-and-effect relationship between the increase of this cytokine and platelet function.
Recent studies demonstrate that, on activation, platelets express TNFR and that engagement of TNF-α with its receptors elicits platelet activation.14 Therefore, flow cytometry analysis was performed in a subset of 20 patients with HF and in 10 HSs matched for sex and age (Figure 1C). Compared with controls, patients with HF had higher expression of TNFR1, depending on HF severity; TNFR2 was also increased in patients compared with controls, but the difference was less marked. A significant correlation was observed between platelet TNFR1 and PA (Figure 1D), suggesting that the up-regulation of TNFR1 is implicated in the activation of platelets.
Platelet aggregation, platelet TNF-α receptor expression, and plasma TNF-α in HSs and HF patients.(A) Collagen-induced platelet aggregation (PA) and serumTxB2 production in patients with mild and severe HF (*P < .001 severe vs mild HF) and in HSs (§P < .001). (B) TNF-α plasma levels in patients with mild and severe HF (*P < .001 vs HSs; §P < .001 vs mild HF). (C) TNFR1 and TNFR2 expression on platelet surfaces in patients with mild HF (n = 10; 9 men, 1 woman; age, 58 ± 10 years) and severe HF (n = 10; 9 men, 1 woman; age, 58 ± 6 years) and in HSs (n = 10; 9 men, 1 woman; age, 58 ± 7 years) (*P < .001 vs HSs; §P < .001 vs mild HF). (D) Correlation between platelet TNFR1 and PA (r = 0.79; P < .001) in 20 patients with HF and 10 HSs. (E) Collagen-induced PA in aspirin-free patients with mild (n = 10) or severe (n = 10) HF and in HSs (n = 10) in the presence or absence of the TNFR inhibitor WP9QY (*P < .01; **P < .001) (F) Effect of WP9QY or AACOCF3 or acetylsalicylic acid (ASA) on TNF-α-induced activation of collagen (0.5 μg/mL)-primed platelets taken from 3 patients with mild HF and 2 patients with severe HF (§P < .001 vs TNF-α-free platelets; *P < .001 vs platelets added with collagen and TNF-α). Data (n = 5) are expressed as mean ± SD.
Platelet aggregation, platelet TNF-α receptor expression, and plasma TNF-α in HSs and HF patients.(A) Collagen-induced platelet aggregation (PA) and serumTxB2 production in patients with mild and severe HF (*P < .001 severe vs mild HF) and in HSs (§P < .001). (B) TNF-α plasma levels in patients with mild and severe HF (*P < .001 vs HSs; §P < .001 vs mild HF). (C) TNFR1 and TNFR2 expression on platelet surfaces in patients with mild HF (n = 10; 9 men, 1 woman; age, 58 ± 10 years) and severe HF (n = 10; 9 men, 1 woman; age, 58 ± 6 years) and in HSs (n = 10; 9 men, 1 woman; age, 58 ± 7 years) (*P < .001 vs HSs; §P < .001 vs mild HF). (D) Correlation between platelet TNFR1 and PA (r = 0.79; P < .001) in 20 patients with HF and 10 HSs. (E) Collagen-induced PA in aspirin-free patients with mild (n = 10) or severe (n = 10) HF and in HSs (n = 10) in the presence or absence of the TNFR inhibitor WP9QY (*P < .01; **P < .001) (F) Effect of WP9QY or AACOCF3 or acetylsalicylic acid (ASA) on TNF-α-induced activation of collagen (0.5 μg/mL)-primed platelets taken from 3 patients with mild HF and 2 patients with severe HF (§P < .001 vs TNF-α-free platelets; *P < .001 vs platelets added with collagen and TNF-α). Data (n = 5) are expressed as mean ± SD.
To explore this issue, PA was measured in platelets added or not added to WP9QY. Although the TNFR inhibitor did not affect the aggregation of platelets from HSs, it significantly inhibited PA in patients with HF; those with severe HF had higher percentages of inhibition (Figure 1E). Taken together, these findings suggest that in patients with HF, platelet hyperactivation could be related to the increased levels of TNF-α, that, on interaction with its receptors, enhanced platelet responsiveness to the agonist.
To explore whether TNF-α behaved in vitro as a platelet agonist, it was added at concentrations up to 40 pg/mL to platelets taken from patients with HF, but no change of PA could be observed (data not shown). This finding is apparently at variance with previous studies showing that TNF-α is a platelet agonist6,7 ; however, the order of magnitude of TNF-α used in those studies was much higher (1-50 ng/mL) than what we used (20-40 pg/mL), indicating, that at physiologic concentrations, TNF-α is not an aggregating agent. Conversely, TNF-α elicited PA and TxB2 formation in collagen-primed platelets (Figure 1F).
On the basis of the previous study suggesting that TNF-α elicits the activation of PLA2 on interaction with its receptor, TNRF1,15 we hypothesized that stimulation of the arachidonic acid pathway could have a key role in TNF-α-mediated platelet activation. This hypothesis was supported by evidence that an inhibitor of PLA2 and aspirin significantly inhibited TNF-α-induced PA (Figure 1F). Amplification of the platelet response to collagen depended on TNF-α interaction with its receptors because adding TNFR antagonist significantly inhibited PA (Figure 1F). The specificity of this effect was corroborated by the lack of influence of TNFR antagonist in platelets stimulated with collagen alone (not shown).
TNF-α (40 pg/mL) also amplified the response to collagen in platelets taken from HSs (PA untreated platelets, 3% ± 1% LT; PA subthreshold collagen-treated platelets, 43% ± 4% LT). Adding platelets with TNFR or arachidonic acid pathway inhibitors significantly inhibited PA (not shown).
Amplification of platelet response to TNF-α was not observed with thrombin or ADP, likely because of the minor role of the arachidonic acid pathway in platelet activation induced by these agonists16 (not shown).
In conclusion, this study provides evidence of a progressive increase in platelet aggregation and TxB2 formation from mild to severe HF. Enhanced circulating levels of TNF-α may account for platelet hyperfunction because TNF-α is able to activate platelets through stimulation of the arachidonic acid pathway. These findings provide new insight into the mechanism that could favor thromboembolism in patients with HF.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-03-1247.
Supported in part by a public grant (Ministero dell'Instruzione, dell'Università e della Ricerca [MIUR] 2000) to Francesco Violi.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.