Abstract
A number of recent reports have documented that cells possessing hematopoietic-reconstitution ability can be identified and isolated from a variety of solid organs in the adult animal. In all studies to date, however, purified organ-derived stem cells demonstrate a diminished repopulating capacity relative to that of purified bone marrow–derived hematopoietic stem cells (BM HSCs). It has therefore been unclear whether organ-derived HSCs possess functional properties distinct from those of BM HSCs, or simply have not been purified to a comparable extent. Here we report the identification of a rare subset of cells in adult murine liver that possess potent blood-repopulating potential, approaching that of BM HSCs. The cells, isolated on the basis of dye-efflux activity and CD45 expression (termed CD45+ liver side population [SP] tip cells), exhibit a surface phenotype similar to that of freshly isolated BM HSCs derived from normal adult animals, but are phenotypically distinct in that they do not express the stem-cell marker c-kit. Single-cell transplantation studies indicate that CD45+ liver SP tip cells can be generated from BM HSCs, suggesting a relationship between stem-cell populations in the liver and bone marrow compartments. Overall, these studies have important implications for understanding extramedullary hematopoiesis, and may be relevant to current strategies aimed at inducing tolerance to transplanted organs.
Introduction
Hematopoiesis in adults is believed to originate primarily from hematopoietic stem cells (HSCs) that reside within the bone marrow.1 Rare circulating forms of these stem cells are also detectable in the peripheral blood where they are speculated to traffic between the bone marrow and other organ compartments.2-4 In occasional disease states, bone marrow failure may lead to extramedullary hematopoiesis or the production of blood lineages from solid organs, such as the liver, that are normally considered nonhematopoietic.5-9
Because definitive hematopoiesis in the embryo occurs in the fetal liver prior to the migration of HSCs to bone marrow,10 there has been continued interest in determining whether stem-cell populations possessing hematopoietic potential can be isolated from adult liver. In one early study, Taniguchi et al used the classic markers of bone marrow HSCs (stem-cell antigen-1 [Sca-1+], c-kit+, and lineage [Lin–]) to isolate cells of this surface phenotype from adult murine liver and found that these cells displayed blood-repopulating activity when injected without competitor marrow into irradiated recipients.11 The HSC activity in that study, however, was far weaker than that typically found in HSCs purified from bone marrow. More recently, investigators have attempted to isolate and identify liver-derived stem cells through the use of a fluorescence-activated cell sorter (FACS)–based method, which relies on dual wavelength analysis of Hoechst 33342–stained cells, that we had previously developed for the isolation of bone marrow–derived stem cells (termed side-population or SP cells).12,13 In one such study in which dye-efflux methodology was applied to human fetal liver tissue, CD34+ SP cells were found to be enriched 10-fold in transplantable HSC activity.14 In addition, murine adult liver SP cells have been reported to possess in vitro hematopoietic activity,15,16 and the in vivo ability to repopulate c-kit–deficient recipient mice, albeit after transplantation of thousands of cells.17 Analogous studies of other organ-derived SP populations have also demonstrated the ability of the cells to provide for hematopoietic reconstitution, again at levels significantly lower that that observed with bone marrow SP cells.18 Overall, based on the relatively weak in vivo reconstitution activity reported for all purified organ-derived stem-cell populations, it has not been possible to determine whether organ-derived stem cells in general possess functional properties distinct from those of bone marrow–derived cells or if the methods used to date for the purification of those cells do not result in efficient purification of the relevant cells.
Here we provide evidence that stem cells exist in adult murine liver that possess potent hematopoietic-reconstitution activity comparable with that of highly purified bone marrow–derived stem cells. The cells appear to be phenotypically distinct from normal bone marrow SP cells, yet may be bone-marrow derived.
Materials and methods
Mice
Single-cell suspensions for FACS analysis and RNA extraction were prepared from 8- to 10-week-old female C57BL/6j mice. Transplantation studies used 8-week-old male donor C57BL/6-Ly5.1 (CD45.1; B6.SJL-Ptprca Pep3b/BoyJ) or C57BL/6-GFP mice that constitutively and ubiquitously express the enhanced green fluorescent protein (GFP) reporter gene under regulatory control of the chicken beta-actin promoter with cytomegalovirus enhancer (C57BL/6j-Tg(ACTB-EGFP)1Osb/J). Recipient mice were congenic 8- to 10-week-old female C57BL/6-Ly5.2 (CD45.2). All mice were obtained from Jackson Labs (Bar Harbor, ME) and maintained in a pathogen-free animal facility. Animal studies were approved by the Standing Committee on Animals of Harvard Medical School.
Preparation of single-cell suspensions and Hoechst 33342 staining
Adult mice were killed by CO2 inhalation followed by cervical dislocation. Livers were perfused free of blood by transection of the portal vein followed by irrigation of the liver with approximately 15 mL ice-cold sterile phosphate-buffered saline (PBS) via the right ventricle until the liver was visibly clear. To obtain single-cell suspensions, harvested livers were placed in plastic culture dishes, finely minced with razor blades, and enzymatically digested for 1 hour at 37°C in a solution of 0.1% Collagenase A in 2.4 U/mL Dispase II (Roche, Indianapolis, IN) with 2.5 mM CaCl2. The resulting digest was then filtered through a 70-μm Falcon cell strainer (BD Biosciences, San Jose, CA) to remove debris and washed with HBSS+ (calcium- and magnesium-free Hanks balanced salt solution supplemented with 2% heat-inactivated fetal bovine serum [FBS], 1% penicillin/streptomycin, and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]; Invitrogen, Carlsbad, CA). After centrifugation, cell pellets were resuspended in HBSS+ for Hoechst staining. Bone marrow single-cell suspensions were prepared by crushing femurs, tibiae, and iliac crests with a pestle.19 Peripheral blood for transplantation was obtained from the right ventricle by aspiration into a heparinized syringe via a 28-gauge needle. Hoechst staining of all cell preparations was performed by incubating 4.5 million cells/mL in HBSS+ with 8.8 μg/mL Hoechst 33342 dye (Molecular Probes, Eugene, OR) for 90 minutes at 37°C based on a modification (see Mostoslavsky et al20 for details) of our original method.12 Where indicated, an aliquot of cells was incubated with 50 μM verapamil (Sigma-Aldrich, St Louis, MO) during Hoechst staining. As we have found variations from manufacturers' labeled dye concentrations in different batches of Hoechst dye,19 all experiments were performed using dye whose concentration was first established by ultraviolet (UV) spectroscopy. After Hoechst staining, cell suspensions were washed in HBSS+, and red blood cells were depleted by centrifugation over Ficoll-Paque Plus (830g for 20 minutes; Amersham Biosciences, Piscataway, NJ). The buffy-coat layer was collected and washed once with HBSS+.
Antibody staining
Hoechst-stained cells were resuspended in ice-cold HBSS+ at 2 × 107 cells/mL and then stained for 30 minutes on ice with Fc Block (2 μL/106 cells; BD Pharmingen, San Diego, CA) and one or more of the following monoclonal antibodies: phycoerythrin (PE)– or FITC (fluorescein isothiocyanate)–conjugated anti-CD45, PE-conjugated anti–Sca-1, allophycocyanin (APC)–conjugated anti–c-kit, PE-conjugated anti-CD34 (RAM34), FITC-conjugated anti-CD31, FITC-conjugated anti-CD29, PE-conjugated anti-CD49f, or biotinylated lineage (Lin) markers (anti-TER119, -CD3, -B220, -CD11b, and –Gr-1). Biotinylated lineage markers were detected after second-step staining with APC-conjugated streptavidin. All antibodies and second-step reagents were purchased from BD Pharmingen. Positive FACS gates for each antibody marker were set according to the fluorescence intensity of simultaneously prepared negative controls stained with nonspecific fluorescence-conjugated antibodies of identical isotype. Immediately prior to cell sorting, cells were resuspended in HBSS+ containing propidium iodide (PI) (2 μg/mL; Molecular Probes) and filtered through a 40-μm Falcon cell strainer (BD Biosciences).
Flow cytometry
Cell sorting was performed on a MoFlo triple laser instrument (DakoCytomation, Fort Collins, CO) using Summit 3.1 software (Dako Cytomation). Analysis of raw data was completed with FlowJo software (Treestar, Ashland, OR). The laser emissions were 488, 350, and 647 nm. Fluorescence was detected with the following bandpass filters: 530/40 for FITC and GFP, 580/30 for PE, 670/30 for PI, 405/30 for Hoechst blue, 570/20 for Hoechst red, and 670/20 for APC (Omega Optical, Brattleboro, VT). Hoechst blue and red fluorescence were detected in linear-scale acquisition. First, a live cell gate was created excluding cell fragments (low forward scatter) or events that contained high PI fluorescence. Cells within this live cell gate were displayed on a Hoechst-red–Hoechst-blue histogram, and side population (SP) cells were identified after collecting at least 1 × 105 events (gating algorithm illustrated in Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Competitive long-term blood repopulation
CD45.2 recipient mice were lethally irradiated with a total of 14 Gy, delivered as 2 doses of 7 Gy given 3 hours apart on the day before transplantation. Mice were maintained on standard chow and water supplemented with 2 mg/mL neomycin sulfate antibiotic (Sigma-Aldrich) for 14 days. Donor cells obtained from CD45.1 or GFP+ mice were mixed with 2 × 105 unfractionated CD45.2 competitor bone marrow cells and intravenously injected retro-orbitally into CD45.2 recipient mice under isoflurane anesthesia. Peripheral blood was obtained from the retro-orbital plexus every 4 weeks, and red blood cells were depleted by lysis (Red Blood Cell Lysing Buffer; Sigma-Aldrich). White blood cells were then stained using either fluorescence-conjugated anti–CD45.1-PE and –CD45.2-FITC antibodies (for recipients of CD45.1 transplants) or pan-anti–CD45-PE (for recipients of GFP+ transplants; BD Pharmingen). Levels of blood chimerism were calculated as the proportion of CD45-labeled white blood cells that expressed the donor CD45.1 or GFP marker gene.
Single hematopoietic stem-cell transplantation
Single bone marrow SP cells from a donor GFP+ transgenic mouse were sorted into separate wells of a 96-well plate that contained 100 μL HBSS+ and 3 × 105 Sca-1–negative competitor marrow cells from a wild-type mouse. The entire contents of each well were then injected into the retro-orbital venous plexus of each recipient. A separate syringe was used for each well to avoid cross-contamination. An additional 96-well plate sorted with single cells by this method was examined by microscope to confirm that 100% of wells contained only one sorted cell. Eighteen mice that received single-cell transplants were then followed to select 2 recipients with more than 40% GFP+ blood chimerism more than 3 months after transplantation.
Reverse-transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from 8500 cells sorted from each bone marrow or liver population (Absolutely RNA Nanoprep Kit; Stratagene, La Jolla, CA). DNase treatment of all RNA extracts was included according to the manufacturer's instructions. cDNA was prepared by reverse transcription under standard conditions using random hexamer priming (First-Strand cDNA Synthesis Kit; Amersham Biosciences). PCR amplification of c-kit and glyceraldehyde phosphate dehydrogenase (GAPDH) cDNA was performed using FastStart Taq DNA Polymerase (Roche) and the following primer pairs: c-kit forward, 5′-GCACTTGAGTGCTACACTCTTGCACCT-3′; c-kit reverse, 5′-TCTTCAGAACTGTCAACAGTTGGACAACA-3′; GAPDH forward, 5′-TTCCAGTATGACTCCACTCACG-3′; and GAPDH reverse, 5′-GTTCACACCCATCACAAACATG-3′. Conventional PCR conditions using one primer pair per reaction tube were as follows: 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute, 40 cycles. Multiplex PCR to amplify both genes in the same reaction tube was performed under identical conditions with both primer pairs added to the same PCR reaction tube.
Statistical analysis
Average blood chimerism was compared between groups by 2-tailed unpaired Student t test. A difference was considered statistically significant if P was less than .05.
Results
Detection of SP cells in adult murine liver
Hoechst 33342 dye staining of cell suspensions prepared from adult mouse livers revealed an easily distinguishable side population (SP) of Hoechst-effluxing cells comprising 0.1% to 0.2% of analyzed events (Figure 1A). Hoechst efflux could be inhibited in more than 90% of this population by the calcium channel blocker, verapamil. Ficoll gradient centrifugation resulted in a significant depletion of cell fragments (Figure 1B), and this accomplished a 10- to 15-fold enrichment of SP cells in Hoechst-stained single-cell suspensions. In comparison, Ficoll gradient centrifugation of Hoechst-stained bone marrow cells resulted in a 5- to 10-fold enrichment of SP cells so that bone marrow SP cells comprised 0.1% of marrow preparations (Figure 1C), rather than the 0.01% to 0.02% of cells contained in an SP gate of non-Ficolled marrow (data not shown).19,21
Characterization of the liver SP surface phenotype
A more detailed examination of the liver side population revealed 2 apparent subpopulations of liver SP cells: a cluster of cells toward the SP “tip” that exhibited lower Hoechst fluorescence (termed tip cells) and a population of SP cells located higher in the SP gate (termed shoulder cells) (Figure 1D-E). Analysis of tip cells, shoulder cells, and non-SP cells for the expression of the panhematopoietic surface marker, CD45, (Figure 1F-H) indicated that while 30% of the total Ficolled liver cell suspensions were CD45+, 60% to 80% of SP shoulder cells were CD45+. In contrast, SP tip cells were almost uniformly CD45 deficient, as only 3% of this population expressed CD45.
Tip, shoulder, and non-SP cell populations were then further analyzed for surface expression of stem-cell antigen-1 (Sca-1), c-kit, markers of differentiated hematopoietic lineages (Lin), and beta-1 and alpha-6 integrins (CD29 and CD49f, respectively), antigens that have been reported to be present on fetal liver progenitors22,23 (Table 1). While non-SP and SP shoulder cells were heterogeneous with regard to CD45, Sca-1, c-kit, CD29, and CD49f staining, more than 95% of SP tip cells exhibited a surface staining pattern that was CD45–/c-kit–/Lin–/CD29+/CD49f+, a surface phenotype that is identical to that described for multipotent fetal liver progenitors.22,23 Of interest, liver SP tip cells did not express c-kit, a marker previously shown to be expressed by freshly isolated bone marrow–derived HSCs (BM HSCs) derived from normal adult animals.24-29
Hematopoietic-reconstitution activity of liver SP cells
To identify and quantify the HSC content in the adult liver, different cell preparations were evaluated in an in vivo competitive repopulation assay. First, unfractionated cell suspensions obtained from the adult livers of CD45.1 mice were mixed with 2 × 105 unfractionated competitor bone marrow cells from CD45.2 mice and injected into lethally irradiated CD45.2 recipients to quantify the total hematopoietic-reconstitution activity in liver. In agreement with previously published work,11 large doses of unfractionated liver cell suspensions were shown to possess detectable long-term blood-repopulating cells 3 months after transplantation. However, while 5% of peripheral blood was derived from injections of 5 × 105 unfractionated liver or 0.5 × 105 Ficolled liver preparations, no detectable blood chimerism could be observed after transplantation of fewer cells (5 × 104 unfractionated liver or 0.5 × 104 Ficolled liver cells; Table S1).
Next, more selective subsets of liver-derived cells were transplanted in an effort to identify subpopulations of liver cells possessing enriched reconstitution activity. As the capacity to efficiently efflux Hoechst dye appears to be a property of HSCs found in the bone marrow as well as in cell suspensions obtained from muscle12,18,30 and human fetal liver,14 we initially sought to determine whether HSCs could be purified from adult murine liver based solely on dye-efflux methodology. Liver SP cells (1 × 104) transplanted in competition with unfractionated bone marrow provided 15% long-term blood chimerism, while 25 × 104 non-SP liver cells were incapable of engrafting above 1% (n = 4 per group; Table S1). These results suggested that, as in bone marrow, Hoechst-efflux capacity identifies nearly the entirety of the hematopoietic-reconstituting potential of liver.
Adult liver and bone marrow SP cells and identification of the liver CD45+ SP tip. FACS analysis of Hoechst-stained single-cell suspensions identifies a side population (SP) of cells in murine adult liver (A), Ficolled adult liver (B), and Ficolled bone marrow (C). Closer inspection of the dot plot of liver SP shown in panel B reveals a cluster of SP cells with highly efficient dye-efflux properties (termed SP tip cells) that is distinct from cells found higher in the SP gate (SP shoulder cells; gates from B are redrawn in D to show cell frequencies of tip and shoulder subpopulations). Hoechst dye efflux in SP cells is inhibited by verapamil (E). Frequencies of cells expressing the panhematopoietic surface marker, CD45, in liver SP tip, shoulder, and non-SP cells are shown in panels F, G, and H, respectively. Rare CD45+ cells, representing just 3% of SP tip cells, are identified in panel F. Numbers in all graphs represent percentage of events contained in the illustrated gate.
Adult liver and bone marrow SP cells and identification of the liver CD45+ SP tip. FACS analysis of Hoechst-stained single-cell suspensions identifies a side population (SP) of cells in murine adult liver (A), Ficolled adult liver (B), and Ficolled bone marrow (C). Closer inspection of the dot plot of liver SP shown in panel B reveals a cluster of SP cells with highly efficient dye-efflux properties (termed SP tip cells) that is distinct from cells found higher in the SP gate (SP shoulder cells; gates from B are redrawn in D to show cell frequencies of tip and shoulder subpopulations). Hoechst dye efflux in SP cells is inhibited by verapamil (E). Frequencies of cells expressing the panhematopoietic surface marker, CD45, in liver SP tip, shoulder, and non-SP cells are shown in panels F, G, and H, respectively. Rare CD45+ cells, representing just 3% of SP tip cells, are identified in panel F. Numbers in all graphs represent percentage of events contained in the illustrated gate.
CD45+ SP tip cells display potent hematopoietic-reconstitution activity
Due to the heterogeneity of the liver SP (Table 1), we next assessed the relative reconstitution activity of liver SP cells further fractionated on the basis of relative dye-efflux activity (ie, tip, shoulder) and CD45 expression. All of the reconstitution activity of liver SP cells was found to reside in the CD45+ fraction, as mice that received 5000 CD45+ liver SP cells averaged 18% long-term peripheral blood chimerism, whereas no mice that received 5000 CD45– liver SP cells showed any detectable peripheral blood chimerism (n = 4 per group; Figure S1).
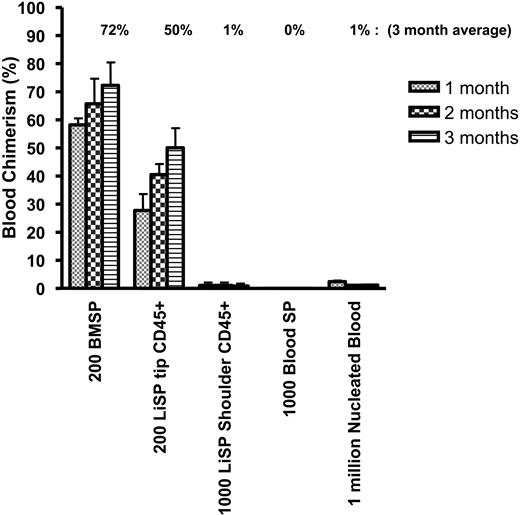
As indicated previously, the vast majority (97%-98%) of CD45+ liver SP cells reside in the SP shoulder, while the cell cluster with the highest Hoechst-efflux potential (SP tip cells) contains only rare (3% of SP tip; 0.03% of Ficolled liver) CD45+ cells. It was therefore of interest to next determine the relative reconstitution potential of CD45+ SP cells derived from the tip or shoulder of the SP gate. As shown in Figure 2, despite their rarity, CD45+ liver SP tip cells exhibited potent hematopoietic-reconstitution activity and accounted for the vast majority of the hematopoietic-reconstitution activity of the overall CD45+ SP population (and the reconstitution of total liver cells). As few as 200 CD45+ SP tip cells in competition with 2 × 105 marrow cells reproducibly provided 50% average peripheral blood chimerism long term. In comparison, an identical number of bone marrow SP cells competitively transplanted in the same experiment provided 72% average peripheral blood chimerism.(Figure 2). To further quantify the reconstitution activity of the CD45+ SP tip cells, a second series of studies examined recipients that received transplants of 200, 100, 50, or 10 cells in competition with 2 × 105 bone marrow competitor cells (Figure 3A). Robust long-term blood reconstitution could be detected after competitive transplantation of 100 cells (18.9% average), and low but detectable levels of peripheral blood chimerism could be found in one animal after transplantation of as few as 10 cells (0.2% in 1 of 4 recipients; Figure 3B). Analysis of animals 7 months after reconstitution with CD45+ SP tip cells confirmed the multipotent nature of the cells, as robust contributions to the lymphoid (CD3+, B220+), myeloid (CD11b+, Gr-1+), and erythroid (Ter-119+) lineages were observed in animals that received transplants of 200 cells (Figure 4).
Given the scarcity of the HSCs that we identified in adult liver tissue, we addressed the possibility that circulating HSCs within the peripheral blood compartment could be contaminating the liver cell suspensions and might thereby account for the blood-repopulating potential observed. However, this was shown not to be the case, since 1 million nucleated peripheral blood cells contributed only marginally to hematopoietic reconstitution long term (1% average chimerism) and 1000 peripheral blood SP cells possessed no significant competitive hematopoietic-reconstituting potential (< 0.1% blood chimerism in all animals; Figure 2). Moreover, CD45+ SP cells purified from livers that were not perfused free of blood had no more reconstituting potential than cells purified from saline-perfused livers (Figure 2).
Kinetics of competitive blood repopulation after transplantation of SP cells purified from adult bone marrow, liver, or peripheral blood. Purified cell populations from CD45.1 mice were transplanted in competition with 2 × 105 unfractionated bone marrow cells into lethally irradiated CD45.2 mice. Average peripheral blood chimerism arising from the donor test cells was followed monthly. Two hundred liver SP (LiSP) tip CD45+ cells demonstrated potent hematopoietic-repopulation activity that approached bone marrow (BM) SP (P = .08). In contrast, nucleated peripheral blood cells, blood SP cells, and LiSP shoulder CD45+ cells possess limited, if any, competitive repopulation potential compared with bone marrow SP (P < .05) (n = 4 per group per experiment repeated twice; average chimerism at 3 months is enumerated above each bar; bars represent mean chimerism and error bars show SEM).
Kinetics of competitive blood repopulation after transplantation of SP cells purified from adult bone marrow, liver, or peripheral blood. Purified cell populations from CD45.1 mice were transplanted in competition with 2 × 105 unfractionated bone marrow cells into lethally irradiated CD45.2 mice. Average peripheral blood chimerism arising from the donor test cells was followed monthly. Two hundred liver SP (LiSP) tip CD45+ cells demonstrated potent hematopoietic-repopulation activity that approached bone marrow (BM) SP (P = .08). In contrast, nucleated peripheral blood cells, blood SP cells, and LiSP shoulder CD45+ cells possess limited, if any, competitive repopulation potential compared with bone marrow SP (P < .05) (n = 4 per group per experiment repeated twice; average chimerism at 3 months is enumerated above each bar; bars represent mean chimerism and error bars show SEM).
Peripheral blood chimerism after competitive transplantation of limiting dilutions of CD45+ liver SP tip cells. (A) Average 3-month blood chimerism arising from competitive transplantation of 200, 100, 50, or 10 purified CD45+ liver SP tip cells (LiSP) (n = 4 per group; error bars represent SEM). (B) Peripheral blood FACS analysis from a recipient in each group. CD45.1+/CD45.2– events illustrate long-term blood reconstitution from varying doses of purified liver-derived cells. CD45.2+/CD45.1– events arise from 2 × 105 transplanted unfractionated competitor bone marrow cells or residual recipient cells that survived lethal irradiation. Numbers in dot plots represent percentage of cells within each gate.
Peripheral blood chimerism after competitive transplantation of limiting dilutions of CD45+ liver SP tip cells. (A) Average 3-month blood chimerism arising from competitive transplantation of 200, 100, 50, or 10 purified CD45+ liver SP tip cells (LiSP) (n = 4 per group; error bars represent SEM). (B) Peripheral blood FACS analysis from a recipient in each group. CD45.1+/CD45.2– events illustrate long-term blood reconstitution from varying doses of purified liver-derived cells. CD45.2+/CD45.1– events arise from 2 × 105 transplanted unfractionated competitor bone marrow cells or residual recipient cells that survived lethal irradiation. Numbers in dot plots represent percentage of cells within each gate.
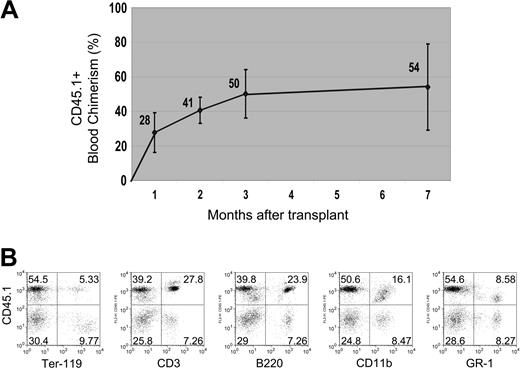
Multilineage blood differentiation 7 months after transplantation. (A) Peripheral blood chimerism, measured by FACS, is shown arising from 200 transplanted liver CD45+ SP tip cells (from a CD45.1 donor) over a 7-month period (n = 4 mice per group per experiment, repeated twice; error bars reflect standard deviation of the mean). (B) Representative FACS analysis of peripheral blood at 7 months shows robust engraftment of differentiated lymphoid (CD3+ or B220+), myeloid (CD11b+ or GR-1+), and erythroid (Ter-119+) hematopoietic lineages derived from the transplanted donor (CD45.1+) cells. Numbers in dot plots represent percentage of cells within each quadrant.
Multilineage blood differentiation 7 months after transplantation. (A) Peripheral blood chimerism, measured by FACS, is shown arising from 200 transplanted liver CD45+ SP tip cells (from a CD45.1 donor) over a 7-month period (n = 4 mice per group per experiment, repeated twice; error bars reflect standard deviation of the mean). (B) Representative FACS analysis of peripheral blood at 7 months shows robust engraftment of differentiated lymphoid (CD3+ or B220+), myeloid (CD11b+ or GR-1+), and erythroid (Ter-119+) hematopoietic lineages derived from the transplanted donor (CD45.1+) cells. Numbers in dot plots represent percentage of cells within each quadrant.
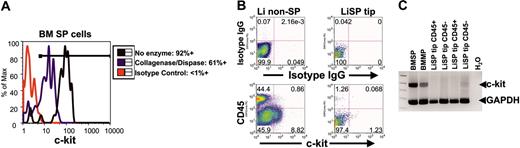
CD45+ SP tip cells possess phenotypic characteristics distinct from bone marrow HSCs
As indicated earlier, immunophenotyping studies of SP tip cells showed that the cells do not express c-kit, a marker found to be expressed on freshly isolated BM HSCs derived from normal adult animals.24-29 Given the potential significance of this result, it was important to rule out the possibility that the enzymatic methods (collagenase and dispase digestion) used to generate single-cell suspensions from total liver prevented the ability to detect c-kit expression by FACS analysis. To begin to address this issue, bone marrow samples were subjected to the same digestion protocol used for the generation of single-cell suspensions of liver-derived cells, and were subsequently analyzed for c-kit expression by FACS. As shown in Figure 5A, while c-kit surface immunostaining was diminished relative to untreated cells, the expression of c-kit on bone marrow SP cells was still observed in approximately 60% of the cells. We also directly determined whether enzymatic digestion of bone marrow cells in any way compromised their biologic function in vivo through competitive repopulation assays, and found that SP cells that had been exposed to collagenase, dispase, or both enzymes showed no difference in long-term blood repopulation compared with controls that were not exposed to enzymes (data not shown).
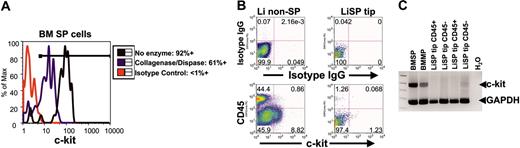
CD45+ SP tip cells from liver (Li) are phenotypically distinct from bone marrow (BM) SP cells. (A) Effects of enzyme digestion (collagenase and dispase) on c-kit immunostaining in BM SP cells. Despite diminished intensity of c-kit surface immunostaining after enzyme digestion, 61% of BM SP cells still exhibit c-kit immunofluorescence above background. (B) After identical enzyme digestion, c-kit immunostaining is readily detected above background in CD45-Li non-SP cells and minimally detected in few CD45-LiSP cells. In contrast, no CD45+ SP tip cells from liver exhibit c-kit surface staining. IgG indicates immunoglobulin G. (C) Multiplex RT-PCR assay for c-kit and GAPDH gene expression shows no detectable c-kit mRNA in multiple sorted samples of CD45+ LiSP tip cells. In contrast, c-kit expression is easily detected in samples prepared from identical numbers of sorted BM SP and BM main population (MP). Expression of the housekeeping gene GAPDH is present in all samples. Numbers in dot plots represent percentage of cells contained in each quadrant.
CD45+ SP tip cells from liver (Li) are phenotypically distinct from bone marrow (BM) SP cells. (A) Effects of enzyme digestion (collagenase and dispase) on c-kit immunostaining in BM SP cells. Despite diminished intensity of c-kit surface immunostaining after enzyme digestion, 61% of BM SP cells still exhibit c-kit immunofluorescence above background. (B) After identical enzyme digestion, c-kit immunostaining is readily detected above background in CD45-Li non-SP cells and minimally detected in few CD45-LiSP cells. In contrast, no CD45+ SP tip cells from liver exhibit c-kit surface staining. IgG indicates immunoglobulin G. (C) Multiplex RT-PCR assay for c-kit and GAPDH gene expression shows no detectable c-kit mRNA in multiple sorted samples of CD45+ LiSP tip cells. In contrast, c-kit expression is easily detected in samples prepared from identical numbers of sorted BM SP and BM main population (MP). Expression of the housekeeping gene GAPDH is present in all samples. Numbers in dot plots represent percentage of cells contained in each quadrant.
Analysis of total liver cell suspensions similarly indicated that c-kit surface staining was dim, but readily detectable, despite enzyme digestion (Figure 5B). In contrast to marrow preparations, however, the vast majority of surface expression appeared to be in non-SP CD45– liver cells (8%-40%), with rare surface staining in CD45+ non-SP cells (1%) and very rare staining in CD45– SP cells (1% of CD45– liver SP tip cells). Of importance, CD45+ liver SP tip cells showed no detectable surface c-kit immunostaining. To provide independent confirmation of the lack of c-kit expression in these cells, RNA was prepared from identical numbers of CD45+ liver SP tip cells and bone marrow SP cells and analyzed for the expression of c-kit RNA via conventional as well as multiplex RT-PCR. As shown in Figure 5C, while c-kit mRNA could be detected in bone marrow SP and MP cells, and was barely detectable in CD45– liver SP tip cells, no RNA signal was detected in RNA samples from CD45+ liver SP tip cells. These results strongly suggest that CD45+ liver SP tip cells possess a surface phenotype distinct from that of bone marrow SP cells.
CD45+ SP tip cells can be generated from bone marrow SP cells after bone marrow transplantation
As the fetal liver is the principal site of hematopoiesis in the mid- and late-gestation embryo,31 it is possible that the HSC activity detected in adult liver represents a remnant of fetal activity derived during development. Alternatively, HSCs in the adult liver may originate from rare marrow-derived circulating HSCs that are detectable in the peripheral blood of adult mice.2 To partially address this question, we asked whether in the experimental setting of bone marrow transplantation, liver CD45+ SP tip cells could “derive” from transplanted bone marrow SP cells. In a first study, animals that received transplants of either 2 × 106 total marrow cells or 200 purified bone marrow SP cells from GFP+ donor mice were analyzed 5 months after transplantation for the presence of donor-derived (GFP+) CD45+ liver SP tip cells. As shown in Figure 6, 33% to 66% of CD45+ liver SP tip cells present in recipients engrafted with either total bone marrow or purified bone marrow SP cells were GFP+ (n = 3 per group; Figure 6A-B). To more firmly establish whether CD45+ liver SP tip cells were clonally derived from bone marrow SP cells in these studies, animals received transplants of single GFP+ bone marrow SP cells, and subsequently were analyzed for the presence of GFP+ CD45+ liver SP tip cells. After 4 and 12 months, 10% and 64%, respectively, of CD45+ liver SP tip cells were GFP+ (Figure 6C). In addition, the majority of bone marrow and blood cells in these recipients of single cells were GFP+. Notably, the vast majority of engraftment in the SP or non-SP of liver occurred in CD45+ cells (Figure 6A-D). Less than 1% of CD45– liver SP cells were GFP+ up to 1 year after transplantation; however, very rare GFP+ events that did not appear to immunostain with anti-CD45 antibodies could be detected.
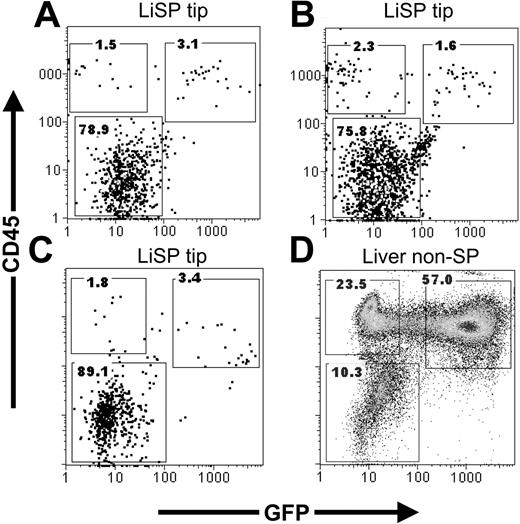
Liver CD45+ SP tip cells can be generated from bone marrow (BM) SP or single stem cells after transplantation. FACS analysis of liver (Li) SP tip cells in lethally irradiated recipients that were transplanted with (A) 2 × 106 unfractionated GFP+ marrow, (B) 200 purified bone marrow (BM) SP, or (C) single BM SP cells. CD45+ Li SP tip cells derived, in part, from donor (GFP+) marrow cells in each recipient. (D) CD45+ Li non-SP cells also derived from the transplanted single BM SP cell. Numbers in dot plots represent percentage of cells contained in each gate.
Liver CD45+ SP tip cells can be generated from bone marrow (BM) SP or single stem cells after transplantation. FACS analysis of liver (Li) SP tip cells in lethally irradiated recipients that were transplanted with (A) 2 × 106 unfractionated GFP+ marrow, (B) 200 purified bone marrow (BM) SP, or (C) single BM SP cells. CD45+ Li SP tip cells derived, in part, from donor (GFP+) marrow cells in each recipient. (D) CD45+ Li non-SP cells also derived from the transplanted single BM SP cell. Numbers in dot plots represent percentage of cells contained in each gate.
Discussion
Although transplantable blood-repopulating cells within the livers of adult rodents and humans have been well documented by others,11,14,17 previous reports have begged the question of whether cells with potential similar to bone marrow HSCs might be detected in liver tissue if these cells could be isolated with high-enough purity. We identified HSCs within the adult liver that lack c-kit expression yet possess robust long-term blood-repopulating potential. We found that these cells reside within the very rare CD45+ fraction of the tip region of the liver SP population and do not appear to be contaminating peripheral blood cells. Through competitive repopulation studies, we show for the first time that adult solid-organ–derived HSCs can approach BM HSCs in blood-repopulating capacity.
Our findings contrast with those of Taniguchi et al who first isolated c-kit–expressing cells from adult livers that gave rise to low levels of long-term blood chimerism (6%) after transplantation.11 Our results also differ from previous reports that have detected limited HSC activity within Hoechst-effluxing liver or muscle populations where (in contrast to BM HSCs) thousands of purified cells were required for blood repopulation.17,18,30,32 Our method, which relies on the purification of the highest Hoechst-effluxing cells (SP tip cells) in combination with CD45 surface expression, provides a 1500-fold enrichment in cells with blood-repopulating potential compared with Ficolled total liver preparations.
Although the histologic location of HSCs in the liver is not yet known, these cells do not appear to reside within contaminating circulating blood. HSCs could not be flushed out of liver tissue, and preparations of liver cells contained competitive blood-repopulating potential well above that which could be accounted for by peripheral blood. Still, we cannot exclude the possibility that liver HSCs could reside within an endovascular compartment of liver, perhaps firmly adherent to vessel walls.
That CD45+ liver SP tip cells do not express c-kit distinguishes this population of cells from normal BM-derived HSCs. While CD45+ liver SP tip cells may represent an HSC population distinct from BM HSCs, it is also possible that the cells are indeed related to BM HSCs and that c-kit expression is altered as a consequence of migration of BM HSCs into the liver. This latter possibility is supported by the bone marrow transplant experiments involving GFP-marked BM SP cells. While previous studies have shown that c-kit expression is associated with most, if not all, conventional HSC activity present in unmanipulated bone marrow,24-29 c-kit–negative HSCs with normal reconstitution activity can be isolated from animals treated with 5-fluorouracil (5-FU).24,33,34 A novel c-kit–negative stem-cell population has also been isolated from normal unmanipulated marrow that possesses the capacity for only a delayed reconstitution (10-12 months after transplantation) of lethally irradiated recipients.35 These and other studies33 have raised the possibility that c-kit–negative cells may represent “dormant” populations of HSCs.
It is clear that further studies to identify and understand differences in the genetic programs of liver HSCs and BM HSCs are warranted. For example, liver-derived CD45+ SP tip cells are heterogeneous with regard to Sca-1 surface staining, and it is not yet known whether Sca-1–negative cells of this population are capable of equivalent blood repopulation. This would contrast with the known Sca-1+ phenotype of marrow HSCs. Indeed, work by Uchida et al supports this possibility as both Sca-1+ and Sca-1– liver SP cells were capable of long-term blood repopulation after transplantation of 1 × 105 or more cells without competitor marrow.17
As has been found in previous studies of liver- and muscle-derived HSCs, we found blood-repopulating cells within the liver to be exclusively CD45+ or hematopoietic in lineage.17,36 Whether progenitors of liver parenchymal cells, such as hepatocytes, cholangiocytes, and oval cells, are contained within the liver SP population is not yet clear, although initial experiments that raise this possibility have been reported.15,37 Further transplantation studies using models of liver injury will be required to fully evaluate the liver-reconstituting potential of liver SP cells. In particular, the 95% of liver SP tip cells that lack hematopoietic potential but share the CD45–/Ter-119–/CD29+/CD49f+/c-kit– surface phenotype of multipotent fetal liver parenchymal progenitors warrant further functional investigation.
Our studies suggest that adult liver HSCs are not necessarily just remnants left over from the fetal liver. On the contrary, we found that adult CD45+ liver SP tip cells as well as blood and marrow cells were able to derive clonally from a single transplanted adult bone marrow stem cell. Our findings may imply a circulating network of HSCs that traffic between marrow, blood, and solid organ. Still, it is not known whether HSCs from adult liver are dormant and require trafficking to a bone marrow niche in order to participate in hematopoiesis. Reports of active centers of hematopoiesis in human liver grafts and extramedullary hematopoiesis from liver in cases of severe marrow failure8,9,38 raise the alternate possibility that HSCs of the adult liver may participate in hematopoiesis in situ.
The presence of potent HSCs within an organ that is typically considered nonhematopoietic has broad implications for the field of organ transplantation. It is well known that human organ recipients develop improved graft tolerance to transplanted livers over time.7 Furthermore, in experimental rodent and pig models, orthotopic liver transplantation has also been reported to induce donor-specific tolerance.39,40 Hematopoietic chimerism between recipient immune cells and those arising from the donor graft is one of several mechanisms postulated to contribute to tolerance of the transplanted organ. A better understanding of the potent HSCs present in harvested livers may lead to the development of novel therapies that encourage hematopoietic chimerism as a means to reduce organ rejection after liver transplantation.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2005-03-1017.
Supported by National Institutes of Health (NIH) grants 5PO-HL54785 (R.C.M.) and 1 KO8 HL071640-01 (D.N.K.).
D.N.K. and A.J.F. performed research; D.N.K. and R.C.M. designed research, analyzed data, and wrote the paper.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.