The expression of cancer germline antigens (CGAgs) is normally restricted to the testis but is also present in many types of malignant cells including plasma cells from patients with myeloma. Because T-cell immune responses to CGAg have been identified in patients with solid tumors, this may offer a novel target for immunotherapy in patients with myeloma. We have used 12 peptide epitopes from a range of CGAgs to screen for CGAg-specific T cells in blood from patients with multiple myeloma at various stages of their disease. T cells from 15 of 37 patients responded to one or more CGAg peptides and the magnitude of the CGAg-specific CD8+ T-cell response ranged between 0.0004% and 0.1% of the total CD8+ T-cell pool. Serial analyses showed that these immune responses were detectable in individual patients at multiple time points during the course of their disease. In patients undergoing treatment or in disease relapse, the magnitude of the CGAg-specific T-cell response was positively correlated with the level of paraprotein. Functional T cells specific for CGAgs are therefore present in a proportion of patients with multiple myeloma and offer the possibility of a novel approach for immunotherapy in this disease.
Introduction
Multiple myeloma is a hematologic malignancy characterized by an accumulation of monoclonal malignant plasma cells in the bone marrow.1-4 Autologous peripheral blood stem cell (PBSC)-supported high-dose melphalan is now considered standard therapy for younger patients with myeloma but does not offer the prospect of a cure. Although allogeneic stem cell transplantation is associated with significant morbidity and mortality, a graft-versus-myeloma effect has been clearly demonstrated.1-4 Tumor-specific immunotherapy offers considerable potential in the management of patients with malignant disease but effective T-cell therapy will be dependent on the identification of protein antigens that are expressed selectively on tumor cells.
Efforts to target multiple myeloma by immunotherapy have focused largely on the use of immunoglobulin idiotype as a specific-specific antigen. Several studies have shown that idiotype-induced cytotoxic T lymphocytes (CTLs) can kill myeloma cells but direct evidence of T-cell recognition of peptides derived from immunoglobulin idiotype is awaited.5-8
Other emerging targets for the cellular immunotherapy of myeloma include the glycosylated type 1 transmembrane glycoprotein (MUC1) and the Wilms tumor antigen (WT-1).9,10 However, expression of these proteins is not exclusive to tumor cells and effective T-cell immunity might therefore be associated with healthy tissue damage.11
Cancer germline antigens (CGAgs) offer considerable promise for tumor immunotherapy because their normal physiologic expression is restricted to the premeiotic spermatogonia cells of the testis, an immunologically privileged tissue.12 Interestingly, T-cell tolerance to peptides derived from CGAg proteins appears to be incomplete because functional T-cell responses have been found to be generated as a consequence of CGAg protein expression in tumor cells.13 Expression of CGAg proteins can be detected in cells from several different types of malignancy including melanoma and a range of epithelial tumors and sarcomas.14 Forty-four cancer germline gene families have been identified, 19 of which encode proteins associated with cancer-related immune responses in humans.14
Most studies that have investigated the use of CGAgs as targets for immunotherapy have been conducted in patients with malignant melanoma. The majority of clinical trials in patients with metastatic melanoma have been based on the use of vaccines containing peptides derived from CGAg proteins.15-20 Encouragingly, tumor regressions have been observed in 5% to 20% of patients and no vaccine toxicity has been reported.15
Recently, expression of several members of the CGAg family, including melanoma-associated gene (MAGE), LAGE, sperm protein 17 (Sp17), and NY-ESO, has been described in the malignant plasma cells from patients with multiple myeloma as well as in transformed plasma cell lines.21-26 CGAg expression in multiple myeloma is more common in advanced disease such as patients at stages II or III of the Durie-Salmon classification scheme. The potential importance of CGAg-specific T-cell immunity in such patients with multiple myeloma is yet to be determined.
In the current study we have investigated the presence of CD8+ T-cell immunity to CGAgs in 37 patients with multiple myeloma at various stages of their disease. To do this, we used the cytokine secretion assay based on expression of interferon γ (IFN-γ) following exposure to peptide epitopes derived from CGAg proteins.27
We found that 15 of 37 patients responded to one or more of the CGAg peptides and that the magnitude of this T-cell response was dependent on the degree of tumor burden. CGAg-specific T cells could be expanded in short-term cultures, exhibited staining with HLA-peptide tetramer, and demonstrated functional responses in enzyme-linked immunospot (ELISPOT). Furthermore, immunohistochemical staining of bone marrow trephines from patients exhibiting a CGAg-specific response confirmed the presence of CGAg protein expression in all cases from which appropriate clinical material was available. These results indicate that functional T-cell immune responses to CGAg proteins are present in many patients with multiple myeloma and raise the prospect of a novel target for immunotherapy in this disease.
Materials and methods
Patient samples
Patients were recruited from outpatient clinics at the University Hospital Birmingham and Heartlands Hospital and Solihull National Health Service (NHS) Trust. Informed written consent was obtained from all patients and local ethical permission (South Birmingham Local Regional Ethics Committee) was obtained prior to the study. Peripheral blood samples (20 mL) were prospectively taken from patients with multiple myeloma at various stages of disease including presentation and during the plateau phase (Table 1). Where possible, samples were taken at monthly intervals and mononuclear cells were isolated by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). Freshly isolated cells were used in all assays. Control subjects were obtained from age-matched healthy volunteers without a history of malignant disease.
The Birmingham Blood Transfusion Service (BTS) performed HLA typing.
Detection of CGAg-reactive T cells using the cytokine secretion assay
Twelve peptides from a range of cancer testis antigens (LAGE-1, NY-ESO, MAGE-1, MAGE-2, MAGE-3, and MAGE-4; Alta Bioscience, Birmingham, United Kingdom) were chosen because they have been previously defined as epitopes for CD8+ T cells and are restricted by a variety of HLA alleles (HLA-A*0201, A*0101, A*0301, A*2401, B*0702; Table 2).28 The IFN-γ cytokine secretion assay (CSA) was used to detect functional T-cell responses, and magnetic selection with anti-phycoerythrin (PE) microbeads was used to increase the sensitivity of detection of responding T cells.29 The CSA was performed according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, freshly isolated PBMCs were seeded at a cell density of 107/mL in culture media containing RPMI 1640 (Invitrogen, Paisley, United Kingdom) supplemented with 10% human serum (H+D Supplies Buckinghamshire, United Kingdom) and l-glutamine (Invitrogen). The cells were then left unstimulated overnight at 37°C 5% CO2. The next day peptides were added to the cultures for 3 to 6 hours at a final concentration of 10 μg/mL. Dimethyl sulfoxide (DMSO) and staphylococcal enterotoxin B (SEB; 1 μg/mL; Sigma, Poole, United Kingdom) were used as negative and positive controls, respectively. After stimulation the cells were labeled with IFN-γ catch reagent for 5 minutes on ice and then incubated for 45 minutes at 37°C under continuous rotation. The cells were labeled with IFN-γ detection antibody (PE-labeled) for 10 minutes, washed, and then magnetically labeled using anti-PE magnetic beads. Enrichment of IFN-γ secretion cells was performed by magnetic separation using the auto-magnetically activated cell sorting (MACS) separation program, Posseld (Miltenyi Biotech, Bergisch Gladbeck, Germany) The positively selected cells were counterstained with anti-CD8-PC5-conjugated monoclonal antibody. Propidium iodide (PI; 1 μg/mL; Sigma) was added prior to analysis to allow exclusion of dead cells. Analysis was performed with a Coulter EPICS XL flow cytometer (Beckman Coulter, High Wycombe, United Kingdom). The frequency of cytokine-secreting antigen-specific T cells was calculated as a percentage of the total CD8+ T-cell pool on the basis of the number of cells detected in the positively selected fraction compared to the total number of CD8+ T cells within PBMCs (Figure 1).29 Where the patient's HLA genotype was unknown, PBMCs were screened initially against peptide pools and the response to single peptides was elicited on subsequent analysis and confirmed by HLA type.
Production of HLA-peptide tetramers
HLA-peptide tetramers were generated using standard protocols.30 Briefly, HLA-A*0201 and B*0702 molecules were refolded in vitro with β2-microglobulin and peptides MAGE-A2-157-166, LAGE-1-Alt ORF or MAGE-A1-289-298. Refolded HLA-peptide monomer complexes were enzymatically biotinylated and then purified by gel filtration and ion exchange. Monomer complexes were then multimerized using streptavidin-PE at a molar ratio of 4:1 (molecular process). For staining, cells were washed, resuspended in MACS buffer, and incubated for 15 minutes at 37°C with tetramer. Cells were washed twice prior to staining with ant-CD8-PC5 (Beckman Coulter) monoclonal antibody for 20 minutes at 4°C followed by flow cytometric analysis.
Generation of CGAg-specific T-cell lines and clones
For expansion of CGAg-specific T cells, 2 × 106 PBMC/mL were cultured in 96-, 48-, or 24-well plates in RPMI 1640, 10% human serum, and interleukin 7 (IL-7; 25 ng/mL; PeproTech, London, United Kingdom). Recombinant IL-2 (100 U/mL; Chiron, Emeryville, CA) was added on day 5 and cultures were restimulated at days 14, 21, and 27 with irradiated (40 Gy) peptide-loaded T2 cells. Tetramer staining was used to identify T-cell expansion in peptide-stimulated cultures. If tetramer-positive cells had expanded in culture they were enriched using anti-PE beads (as described) and isolated using 2 MS Columns (Miltenyi Biotec). The enriched cells were then cloned by limiting dilution analysis (LDA) over irradiated (40 Gy) allogeneic PBMCs and partially matched lymphoblast cell line (LCL) in RPMI 1640 supplemented with 10% human serum, IL-2 (100 U/mL), IL-7 (25 ng/mL), and phytohemagglutinin (PHA; 5 μg/mL; Sigma).
Cultured ELISPOT analysis
PBMCs were isolated from fresh blood and washed twice with RPMI 1640. Then, 30% of PBMCs were cryopreserved for use as autologous antigen-presenting cells in the ELISPOT assay and the rest of the cells were used to set up a peptide-specific T-cell line as described (see “Generation of CGAg-specific T-cell lines and clones”).
Cells were harvested at day 14, washed twice, and resuspended in filtered media (RPMI 1640 + 10% human serum) seeding in triplicate at 105/well in a multiscreen 96-well plate (Millipore, Watford, United Kingdom) precoated with an IFN-γ capture antibody (Mabtech, Stockholm, Sweden). Cells were incubated either alone, with autologous PBMCs loaded with DMSO, or with PBMCs loaded with specific peptide. ELISPOT plates were incubated for 18 to 24 hours at 37°C using an AID ELISPOT plate reader (AID-Diagnostika, Strassberg, Germany).31 The antigen-specific response was calculated by subtracting the background response (T cells plus autologous loaded with DMSO) from the value obtained from wells containing peptide. Statistical significance was assessed according to standard methodology in which the mean number of spots in the peptide test must be 20 spots greater than the mean plus or minus 2 SD of spots on the negative control wells.32
ELISA for quantitation of IFN-γ cytokine production
Tetramer-positive clones were stimulated for 16 hours on T2 cells loaded with CGAg peptide at a final concentration of 10 μg/mL. Supernatants were harvested and analyzed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (PharMingen/Becton Dickinson, Oxford, United Kingdom).
Immunohistochemical analysis
Tissue localization of NY-ESO and MAGE was performed by immunohistochemistry. Slides were subjected to heat-incurred epitope removal in 0.001 M EDTA (ethylenediaminetetraacetic acid), pH 8, for 15 minutes in an 800-W microwave oven and endogenous peroxidase activity was quenched with 0.6% hydrogen peroxide in methanol. Tissue sections were then incubated in primary antibodies to NY-ESO-1 proteins (N-12; Santa Cruz Biotechnology, Santa Cruz, CA) and MAGE A proteins (FL-309; Santa Cruz Biotechnology) at a concentration of 1:25 for 60 minutes. N-12 is a goat polyclonal antibody and epitope mapping shows specificity for an internal region of human NY-ESO-1. FL-309 is a rabbit polyclonal with epitope specificity corresponding to amino acids 1-309 of all human MAGE family members. Antibody binding was detected using a standard avidin/biotin complex method according to the manufacturer's instructions (Vector Quick Kit; Vector Laboratories, Peterborough, United Kingdom). 3-3-Diaminobenzidine (DAB) was used as a chromogen for visualization. For the dual staining the same method was used as described with NY-ESO/MAGE being stained first followed by CD138 (syndecan-1). Silver/Gray SG (Vector) was used as a chromogen. Testis with intact spermatogenesis served as a control tissue. Appropriate negative controls omitting the primary reagent were included for each case.
Detection of tumor burden in patients with multiple myeloma
The tumor burden of patients with multiple myeloma is assessed routinely in the clinic by detecting fluctuations in levels of monoclonal immunoglobulin paraprotein or free light chain (λ or κ). Paraprotein concentration is indicated by nephelometric measurement of the total level of the relevant immunoglobulin class detected in the serum (g/L). Excess free light chain is detected in the urine of patients. Urinary excretion of free light chain is assayed for the relevant type (λ or κ) and is expressed as grams per gram of urinary creatinine.
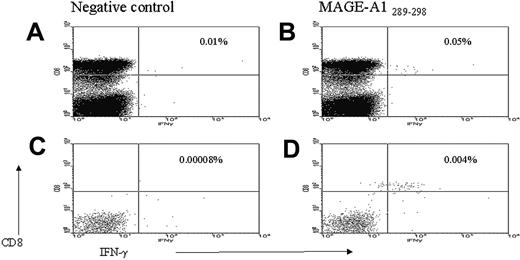
Enumeration of frequency of CD8+ T cells responding to CGAg peptides by FACS analysis. PBMCs were stimulated with CGAg peptides for 3 to 6 hours prior to labeling with antibodies to PE-labeled anti-IFN-γ monoclonal antibody and anti-PE magnetic beads. Enrichment of IFN-γ-secreting cells was performed by magnetic separation. PBMCs stimulated with DMSO alone prior to (A) and following (C) magnetic selection. IFN-γ production in response to MAGE-A1-289-298 peptide prior to (B) and following (D) magnetic selection. The illustrated value refers to the percentage of CD8+ T cells responding to either DMSO alone (A,C) or MAGE-A1-289-298 peptide (B,D). The CGAg-specific T-cell response in the positively selected fraction was calculated as a percentage of the total CD8+ T-cell pool (see “Materials and methods”). In this example 4.5 × 106 PBMCs were stimulated with peptide of which flow cytometric analysis revealed 24% to be CD3+CD8+. From these 1 080 000 CD8+ T cells, 47 cells were isolated in the positively selected fraction, indicating that the percentage of the CGAg-specific T-cell response in PBMCs was at least 0.004% (47/1 080 000 × 100) of the CD8+ T-cell pool.
Enumeration of frequency of CD8+ T cells responding to CGAg peptides by FACS analysis. PBMCs were stimulated with CGAg peptides for 3 to 6 hours prior to labeling with antibodies to PE-labeled anti-IFN-γ monoclonal antibody and anti-PE magnetic beads. Enrichment of IFN-γ-secreting cells was performed by magnetic separation. PBMCs stimulated with DMSO alone prior to (A) and following (C) magnetic selection. IFN-γ production in response to MAGE-A1-289-298 peptide prior to (B) and following (D) magnetic selection. The illustrated value refers to the percentage of CD8+ T cells responding to either DMSO alone (A,C) or MAGE-A1-289-298 peptide (B,D). The CGAg-specific T-cell response in the positively selected fraction was calculated as a percentage of the total CD8+ T-cell pool (see “Materials and methods”). In this example 4.5 × 106 PBMCs were stimulated with peptide of which flow cytometric analysis revealed 24% to be CD3+CD8+. From these 1 080 000 CD8+ T cells, 47 cells were isolated in the positively selected fraction, indicating that the percentage of the CGAg-specific T-cell response in PBMCs was at least 0.004% (47/1 080 000 × 100) of the CD8+ T-cell pool.
Statistical analysis
Mann-Whitney U test was used to test the level of significance between myeloma patients and age-matched controls using Prism 4 for windows 1992-2003 GraphPad software (GraphPad, San Diego, CA).
Results
CGAg-specific CD8+ T cells are detectable in patients with multiple myeloma
We initially used the IFN-γ CSA to detect functional T-cell responses to 12 CGAg peptides in 37 patients with multiple myeloma. These peptides were selected based on their previous identification as immunogenic peptides from a range of CGAgs restricted by several HLA alleles (Table 2). In 15 of the 37 patients, we could detect a CD8+ T-cell response to one or more of the CGAg peptides and in all 15 cases the HLA type of the patient correlated with the restriction element of the immunogenic peptide. Twelve of these 15 patients had stage II or III disease according to the Durie and Salmon classification scheme, whereas 3 had stage I multiple myeloma. The median age of all patients screened was 65 years and this did not differ between the group of patients exhibiting a T-cell response and the group of patients with no response.
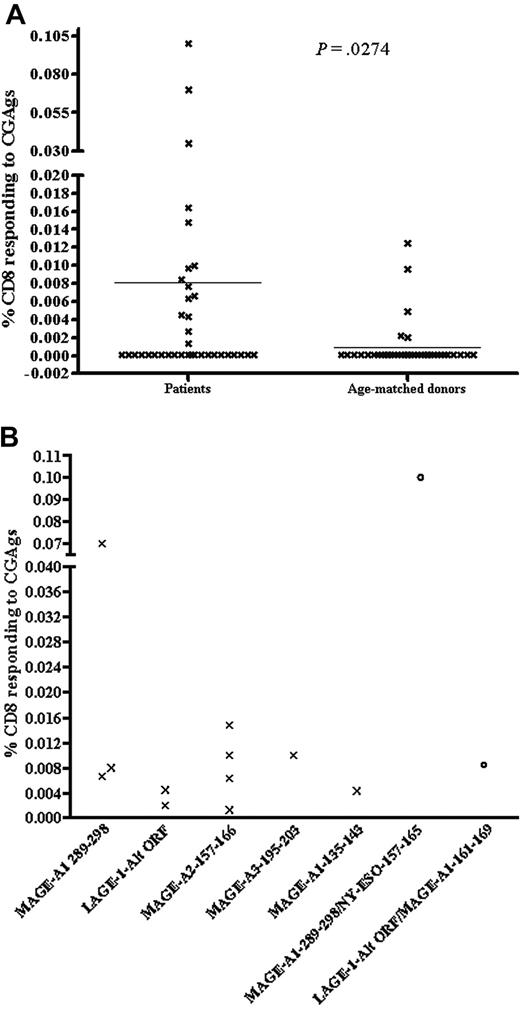
To increase the sensitivity of the IFN-γ CSA down to a level of 0.01% to 0.0001%, the frequency of cytokine-secreting antigen-specific T cells was calculated from the positively enriched sample following magnetic selection.27 An illustration of how responses were enumerated is displayed in Figure 1. The mean percentage of CD8+ T-cell response to CGAg of all patients compared to age-matched donors was 0.008% and 0.0009%, respectively (Figure 2A; P = .027). Of the 15 patients exhibiting a T-cell response, we found that the frequency of the CGAg-specific CD8+ T-cell response ranged between 0.0004% and 0.1% of the total CD8+ T-cell pool with a mean value of 0.02%. It should be considered, however, that because up to 50% of positive cells can be lost during this selection process, this value is likely to represent an underestimate of the true frequency.29 Thirty-seven age-matched healthy donors were also screened for the presence of CGAg-specific T cells using the same technique and in 5 of these 37 donors we detected a response ranging between 0.0125% and 0.002% of the CD8+ T-cell pool (Figure 2A). The mean of T-cell responses detected in the 5 healthy donors who responded was 0.006% and was therefore significantly lower in frequency compared with those responses (0.02%) detected in the patients with multiple myeloma. This indicates that the CGAg-specific T-cell responses we detected in patients with multiple myeloma were most likely generated as a direct consequence of the disease.
Due to the lack of donors with comparable HLA types (HLA-A*0201, B*0702), it was difficult to assess for patterns of immunodominance of a given peptide over another. The T cells reacted against a range of peptides representing epitopes from the MAGE-A1, MAGE-A2, MAGE-A3, and LAGE-1 proteins. Five of the 12 peptides were restricted to HLA-A*0201 and, interestingly, responses were detected only to 2 of these, MAGE-A2157-166 and LAGE-1-Alt-ORF. Six patients were HLA*A0201 and 4 responded to peptide MAGE-A2157-166, whereas 2 responded to peptide LAGE-1-Alt-ORF. In addition, 2 patients who were HLA-A*0201, B*0702 responded to MAGE-A1-289-298 peptide restricted to HLA*B0702. Additionally, one patient with HLA type of HLA-A*0101, A*2401, B*0702 responded to MAGE-A3-195-203 peptide restricted to HLA-A*2401. Also a notably strong response of 0.07% of CD8+ T cells specific for MAGE-A1-289-298 was detected for patient 4 (Figure 2B) and one patient (patient 13) gave a significantly high response of 0.1% to a peptide pool of MAGE-A1-289-298/NY-ESO-1-157-165. However, serial analysis was not permitted due to death of this patient and therefore the individual peptide specificity of this high response could not be determined.
CD8+ T-cell responses to CGAgs. (A) Maximal levels of CGAg-specific T cells in individual patients (n = 37) and age-matched controls (n = 37). The y-axis illustrates the percentage of CD8+ T cells responding to CGAgs calculated from the enriched sample (see “Materials and methods”). The horizontal line indicates the mean percentage of CD8+ T cells in response to CGAg peptide of all 37 patients and aged-matched controls (patients, 0.008% versus age-matched controls, 0.0009%). Undetected responses were given a value of 0.0001% to calculate statistics. The Mann-Whitney U test was used to test level of significance between myeloma patients and age-matched controls (P = .027). (B) Maximum CD8+ T-cell response to individual CGAg peptides in myeloma patients. The graph shows the maximal IFN-γ response against individual CGAg peptides from 11 different donors in whom single peptide-specific responses were elicited (X) and an additional 2 donors who showed responses against a pool of 2 peptides (○). In the latter 2 donors subsequent analysis of individual peptide-specific responses was not possible due to the early death of the patients. Individual CGAg peptides are shown on the x-axis, and the maximal CGAg-specific T-cell response is shown on the y-axis as a proportion of the CD8+ T-cell pool.
CD8+ T-cell responses to CGAgs. (A) Maximal levels of CGAg-specific T cells in individual patients (n = 37) and age-matched controls (n = 37). The y-axis illustrates the percentage of CD8+ T cells responding to CGAgs calculated from the enriched sample (see “Materials and methods”). The horizontal line indicates the mean percentage of CD8+ T cells in response to CGAg peptide of all 37 patients and aged-matched controls (patients, 0.008% versus age-matched controls, 0.0009%). Undetected responses were given a value of 0.0001% to calculate statistics. The Mann-Whitney U test was used to test level of significance between myeloma patients and age-matched controls (P = .027). (B) Maximum CD8+ T-cell response to individual CGAg peptides in myeloma patients. The graph shows the maximal IFN-γ response against individual CGAg peptides from 11 different donors in whom single peptide-specific responses were elicited (X) and an additional 2 donors who showed responses against a pool of 2 peptides (○). In the latter 2 donors subsequent analysis of individual peptide-specific responses was not possible due to the early death of the patients. Individual CGAg peptides are shown on the x-axis, and the maximal CGAg-specific T-cell response is shown on the y-axis as a proportion of the CD8+ T-cell pool.
CGAg-specific T cells are functional after short-term culture
To confirm the functional capacity of T cells detected by cytokine secretion, cultured ELISPOT assays were performed on patients 7, 21, 22, and 23 after short-term expansion with peptide. PBMCs from each patient were stimulated with peptide and at day 14 were tested for their recognition of cognate peptide in an IFN-γ ELISPOT assay (Figure 3). In addition, T-cell cultures were stained with tetramer where available. In all 4 cases, T-cell cultures showed peptide-specific recognition, confirming that the CGAg-specific T cells are functional on recognition of antigen. Moreover, the frequency of response ranged between 0.035% and 0.05% of T cells, which was directly comparable with the frequency of T cells staining with tetramer.
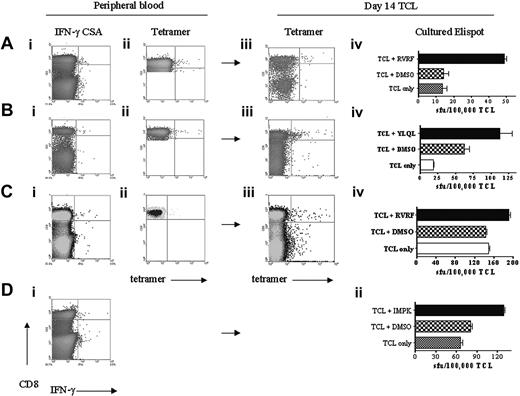
Functional analysis of short-term T-cell cultures. PBMCs were isolated and subjected to CSA and staining with HLA-peptide tetramer. ELISPOT analysis on freshly isolated PBMCs was unreliable due to the low frequency of responding cells (data not shown) and so T cells were cultured with peptide for 14 days prior to an ELISPOT assay. Staining with HLA-peptide tetramer was performed at the same time. (A) Patient 21. (Ai) CSA of PBMCs illustrating 0.03% CD8 specific to CGAg peptide MAGE-A1289-298. (Aii) MAGE-A1-289-298 tetramer stain of PBMCs also stained 0.03% of the CD8 T-cell pool. (Aiii) MAGE-A1-289-298 tetramer stain of T-cell line after 14 days of culture showed 0.09% of CD8 T cell-binding tetramer. (Aiv) IFN-γ ELISPOT analysis at day 14 T-cell line (TCL) revealed that 0.04% of T cells responded to MAGE-A1-289-298 peptide. Similar analysis for peripheral blood in patient 22 revealed 0.04% of CD8+ T cells specific to MAGE-A2-157-166 peptide(Bi) and 0.04% of CD8 staining with MAGE-A2-157-166 tetramer (Bii). At day 14, 0.26% of CD8 stained with tetramer (Biii) and 0.05% of T cells responded to MAGE-A2-157-166 peptide in ELISPOT assay (Biv). (C) Similar analysis for peripheral blood in patient 7 illustrated 0.02% of CD8+ T cells specific to MAGE-A1-289-298 (Ci) and 0.03% of CD8 staining with MAGE-A1-289-298 tetramer (Cii). At day 14, 0.05% of CD8 stained with tetramer (Ciii) and 0.04% of T cells responded to MAGE-A1-289-298 peptide in ELISPOT assay (Civ). (D) A tetramer for the peptide MAGE-A3-195-203 restricted to HLA-A*2401 was not available; however, analysis of peripheral blood from patient 23 revealed 0.05% of CD8+ T cells specific to MAGE-A3-195-203 peptide by IFN-γ CSA. At day 14, 0.05% of T cells responded to the peptide in ELISPOT assay (Dii). (Note: To maximize the number of events analyzed by FACS, PBMCs were gated on CD8 only when tetramer staining was performed on peripheral blood.)
Functional analysis of short-term T-cell cultures. PBMCs were isolated and subjected to CSA and staining with HLA-peptide tetramer. ELISPOT analysis on freshly isolated PBMCs was unreliable due to the low frequency of responding cells (data not shown) and so T cells were cultured with peptide for 14 days prior to an ELISPOT assay. Staining with HLA-peptide tetramer was performed at the same time. (A) Patient 21. (Ai) CSA of PBMCs illustrating 0.03% CD8 specific to CGAg peptide MAGE-A1289-298. (Aii) MAGE-A1-289-298 tetramer stain of PBMCs also stained 0.03% of the CD8 T-cell pool. (Aiii) MAGE-A1-289-298 tetramer stain of T-cell line after 14 days of culture showed 0.09% of CD8 T cell-binding tetramer. (Aiv) IFN-γ ELISPOT analysis at day 14 T-cell line (TCL) revealed that 0.04% of T cells responded to MAGE-A1-289-298 peptide. Similar analysis for peripheral blood in patient 22 revealed 0.04% of CD8+ T cells specific to MAGE-A2-157-166 peptide(Bi) and 0.04% of CD8 staining with MAGE-A2-157-166 tetramer (Bii). At day 14, 0.26% of CD8 stained with tetramer (Biii) and 0.05% of T cells responded to MAGE-A2-157-166 peptide in ELISPOT assay (Biv). (C) Similar analysis for peripheral blood in patient 7 illustrated 0.02% of CD8+ T cells specific to MAGE-A1-289-298 (Ci) and 0.03% of CD8 staining with MAGE-A1-289-298 tetramer (Cii). At day 14, 0.05% of CD8 stained with tetramer (Ciii) and 0.04% of T cells responded to MAGE-A1-289-298 peptide in ELISPOT assay (Civ). (D) A tetramer for the peptide MAGE-A3-195-203 restricted to HLA-A*2401 was not available; however, analysis of peripheral blood from patient 23 revealed 0.05% of CD8+ T cells specific to MAGE-A3-195-203 peptide by IFN-γ CSA. At day 14, 0.05% of T cells responded to the peptide in ELISPOT assay (Dii). (Note: To maximize the number of events analyzed by FACS, PBMCs were gated on CD8 only when tetramer staining was performed on peripheral blood.)
Interestingly, in the majority of cases, proliferation of the CGAg peptide-specific T cells was limited although similar culture conditions allowed expansion of virus-specific T cells from the same patients (data not shown). The poor proliferation potential of CGAg-specific T cells may therefore reflect an inherent property of these cells, which could relate to the conditions of their priming at the site of tumor and may at least partly explain the inability of these cells to control tumor growth.33-35
LDA of CGAg-specific T cells
To further characterize the functional capacity of the CGAg-specific T-cell responses, T-cell lines were cloned by LDA. Expansion of CGAg-specific T-cell clones was infrequent, but a T-cell clone against the MAGE-A2-157-166 peptide presented by HLA-A*0201 was established from patient 22 (clone 1). This clone stained positively with MAGE-A2-157-166/HLA-A*0201 tetramer (Figure 4A) and exhibited a high level (1789 pg/mL) of IFN-γ production in response to HLA-A*0201 target cells loaded with MAGE-A2-157-166 peptide (Figure 4B). Unfortunately, a cytotoxicity assay on tumor cells could not be performed due to the short lifespan of the clone in culture. Additionally, a T-cell line established from patient 11 comprised 59% CGAg-specific CTLs when stained with tetramer MAGE-A2-157-166 (Figure 4C) although it exhibited weak IFN-γ production in response to HLA-A*0201 target cells coated with peptide (data not shown).
Characterization of a MAGE-A2-157-166-specific T cell. (A) FACS analysis of a MAGE-A2-157-166-specific T-cell clone generated from patient 22 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis. (B) IFN-γ production by MAGE-A2-157-166-specific T-cell clone following stimulation with peptide-loaded target cells (effector-target [E/T] ratio = 0.1:1). T2 target cells were loaded with peptide MAGE-A2-157-166 and incubated with clone 1 from patient 22. IFN-γ production was observed following incubation with peptide-loaded targets (▪) but not unloaded targets (▦). (C) FACS analysis of a MAGE-A2157-166-specific T-cell clone generated from patient 11 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis.
Characterization of a MAGE-A2-157-166-specific T cell. (A) FACS analysis of a MAGE-A2-157-166-specific T-cell clone generated from patient 22 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis. (B) IFN-γ production by MAGE-A2-157-166-specific T-cell clone following stimulation with peptide-loaded target cells (effector-target [E/T] ratio = 0.1:1). T2 target cells were loaded with peptide MAGE-A2-157-166 and incubated with clone 1 from patient 22. IFN-γ production was observed following incubation with peptide-loaded targets (▪) but not unloaded targets (▦). (C) FACS analysis of a MAGE-A2157-166-specific T-cell clone generated from patient 11 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis.
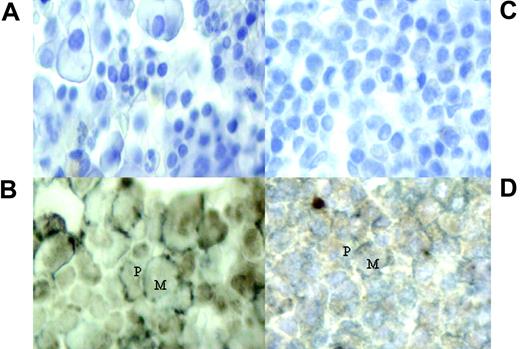
Dual immunohistochemistry staining of bone marrow trephines with primary antibodies to CD138 and MAGE/NY-ESO. (A,C) Negative stains of bone marrow from patients 4 and 22, respectively. (B,D) Cytoplasmic and nuclear expression of MAGE and NY-ESO/LAGE-1 within the CD138+ cells of patients 4 and 22, respectively. Expression of CGAgs shown as brown stain (M) and CD138 plasma cells are stained with a silver/gray surface stain (P). Images were captured with a Nikon Eclipse E400 microscope with a 40×/0.65 NA objective (Tokyo, Japan) and a Nikon E995 camera.
Dual immunohistochemistry staining of bone marrow trephines with primary antibodies to CD138 and MAGE/NY-ESO. (A,C) Negative stains of bone marrow from patients 4 and 22, respectively. (B,D) Cytoplasmic and nuclear expression of MAGE and NY-ESO/LAGE-1 within the CD138+ cells of patients 4 and 22, respectively. Expression of CGAgs shown as brown stain (M) and CD138 plasma cells are stained with a silver/gray surface stain (P). Images were captured with a Nikon Eclipse E400 microscope with a 40×/0.65 NA objective (Tokyo, Japan) and a Nikon E995 camera.
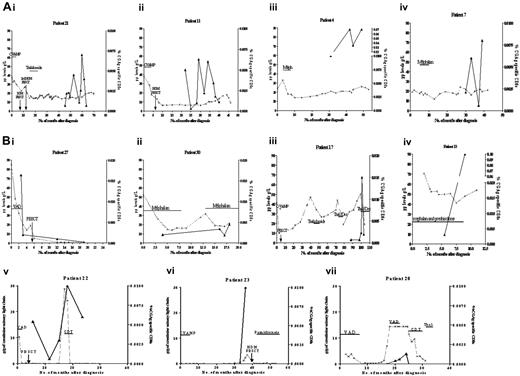
Serial analysis of CGAg-specific CD8+ T-cell responses in 11 patients with multiple myeloma. The x-axis indicates the number of months from diagnosis, the dashed line represents paraprotein level, and the black line represents CD8+ T-cell response to CGAg peptide from the point of analysis. (A) Four patients (21, 11, 4, and 7) were at a plateau stage of disease. (B) Seven patients (13, 22, 20, 30, 17, and 27) illustrated changing tumor burden. Treatment regimens are shown. Thal indicates thalidomide; CVAMP, cyclophosphamide, vincristine, Adriamycin, methylprednisolone, and prednisolone; PBSCT, peripheral blood stem cell transplant; Melph, melphalan; Dex, dexamethasone; VAD, vincristine, doxorubicin, dexamethasone; CDT; cyclophosphamide, dexamethasone, thalidomide.
Serial analysis of CGAg-specific CD8+ T-cell responses in 11 patients with multiple myeloma. The x-axis indicates the number of months from diagnosis, the dashed line represents paraprotein level, and the black line represents CD8+ T-cell response to CGAg peptide from the point of analysis. (A) Four patients (21, 11, 4, and 7) were at a plateau stage of disease. (B) Seven patients (13, 22, 20, 30, 17, and 27) illustrated changing tumor burden. Treatment regimens are shown. Thal indicates thalidomide; CVAMP, cyclophosphamide, vincristine, Adriamycin, methylprednisolone, and prednisolone; PBSCT, peripheral blood stem cell transplant; Melph, melphalan; Dex, dexamethasone; VAD, vincristine, doxorubicin, dexamethasone; CDT; cyclophosphamide, dexamethasone, thalidomide.
CGAg proteins are expressed in the bone marrow of patients with CGAg-specific immunity
We next attempted to confirm the presence of CGAg protein expression in tumor cells from patients demonstrating T-cell immunity. Bone marrow trephine samples taken at the time of disease diagnosis were available for 7 of 15 patients (nos. 4, 7, 17, 27, 30, 36, and 37) who exhibited CGAg-specific immune responses. We used a dual staining technique to assess the presence of CGAg protein expression in plasma cells. Antibodies specific for the MAGE and NY-ESO proteins were used to stain CGAg proteins, whereas an antibody to syndecan-1 (CD138) was used as a marker for plasma cells. All 7 samples examined showed evidence of expression of MAGE and NY-ESO within the CD138 plasma cells (Figure 5).
The magnitude of CGAg-specific immune responses correlates with tumor burden
To investigate the magnitude of the response in relation to the activity of the paraproteinemia, we performed serial analyses of CGAg-specific immune responses in 11 of the 15 patients who exhibited a detectable T-cell response (Figure 6A-B). In the remaining 4 cases only single time points were available for analysis. Four of the 10 patients (nos. 21, 11, 4, and 7) had stable disease (Figure 6A) and 7 patients (nos. 13, 17, 20, 22, 23, 27 and 30) demonstrated a fluctuating tumor burden (Figure 6B). For patients with stable disease, CGAg-specific immune responses were studied at several time points during the plateau phase of their disease (Figure 6A). CGAg-specific T-cell responses were detected consistently in all 4 patients although the magnitude of these responses demonstrated fluctuation up to 5-fold during periods up to 20 months (Figure 6A). The reasons for this apparent fluctuation are not clear although similar fluctuations in response to persistent antigen such as latent viruses have been reported.36
Patients in whom there were changes in tumor burden often demonstrated CGAg-specific immune responses that correlated with the level of paraprotein. Patient 27 was studied during treatment with VAD (vincristine, Adriamycin [doxorubicin], dexamethasone) chemotherapy, which elicited a marked decrease in tumor burden (Figure 6B). A strong CGAg-specific CD8+ T-cell immune response was detected at disease presentation, which represented 0.02% of the CD8+ T-cell pool. Concurrent with the subsequent administration of chemotherapy, the level of the immune response decreased over the corresponding period of 2 months to a low level of 0.002% of the total CD8+ T cells (Figure 6B). Six patients had relapsing disease (Figure 6B) and in each case there was a trend toward an increase in CGAg-specific immune response being associated with an increase in tumor burden. Interestingly, for patient 17, there was a 9-fold increase in CGAg-specific immune response following administration of dexamethasone-thalidomide, which may possibly reflect the immunomodulatory activity of this combination of agents.
The magnitude of the T-cell response to CGAg peptides has been shown as a percentage of the CD8+ T-cell pool but was also calculated as the absolute number of cells and showed a similar relationship with paraprotein level (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
Discussion
Tumor-specific immunotherapy offers considerable promise in the management of patients with malignant disease, but effective T-cell therapy will depend on the identification of protein antigens that are expressed selectively on tumor cells. CGAgs offer considerable promise in this regard in that their physiologic expression within the testis prevents their effective presentation to the immune system; hence, T-cell tolerance does not appear to be established. A range of CGAg proteins has been identified in tumor cells and the mechanisms that lead to expression of CGAg proteins are only partially resolved. Epigenetic regulation seems critical and demethylation of CGAg promoter sequences has been observed in malignant cells.14 Several recent studies have demonstrated a wide range of CGAg expression in plasma cell lines and primary tumor cells from patients with myeloma and protein expression is most frequently detected in patients with more advanced disease.21-26 At present, the evidence concerning the expression of CGAgs in benign monoclonal gammopathy is conflicting.24,26
CGAg-specific T cells were first identified in patients with melanoma and have subsequently been isolated from patients with a range of tumors. However, it is clear that the frequency of T cells specific for CGAg in such patients is typically low and certainly of lower magnitude than the immune response against the differentiation antigens, Melan-A or tyrosinase.15-20 This has precluded attempts to define the frequency of CGAg-specific T cells in the peripheral blood of such patients although a recent report has identified NY-ESO-specific T cells representing 0.2% and 0.6% of the CD8 population in 2 patients with myeloma.25 In our study, we combined the techniques of cytokine secretion and magnetic selection to accurately determine the number of CGAg-specific T cells in peripheral blood with a sensitivity of 0.0001% of the CD8+ T-cell pool.29 We found that the mean CGAg-specific CD8+ T-cell frequency in responding multiple myeloma patients was 0.02% (range, 0.0004%-0.1%). This frequency is comparable to previous reports that documented that the magnitude of CGAg-specific CD8+ T cells in melanoma patients is between 10-3 and 10-4 of CD8 T cells with the use of HLA-peptide tetramer-guided cell sorting.18-20 Germeau et al19 have recently determined the frequency of the MAGE-3168-176-specific CD8+ T-cell response in patients with melanoma who undergo immunization with recombinant canarypox containing MAGE-3.A1 peptide. The sensitivity of detection of peptide-specific immunity in this study was 8 × 10-7
CD8 T cells, which is comparable to our own approach. Interestingly the author observed that peptide immunization was associated with an increase in the number of tumor-specific CD8+ T cells with specificity against other proteins from the MAGE family. Such work depends on the ability to derive cell lines from autologous tumor, which is rarely possible with myeloma but holds out the prospect that boosting CGAg-specific immunity against a single antigen may increase protective immunity against heterogeneous tumor-associated proteins. Because myeloma is a tumor of B cells, it may have the potential to present antigen directly to T cells although the presentation capacity of malignant plasma cells is believed to be limited.37 Antigen presentation of CGAg protein may therefore result from cross-priming through dendritic cells rather than directly from tumor cells.
CGAg-specific T cells could be expanded in short cultures but more prolonged expansion was difficult. This may reflect an inherent property of CGAg-specific CTLs because virus-specific T cells isolated from the same patients were able to expand as well as those isolated from control individuals.33-35 The conditions that allow expansion of CGAg-specific CTLs in vitro are yet to be defined but may require the use of autologous dendritic cells or high-affinity peptide analogues.25
Interestingly, we detected CGAg-specific T cells in 5 of the 37 age-matched controls. However, the overall frequency was lower than that seen in the cohort of patients (mean, 0.0009% versus 0.008%; P = .027). Several possibilities could explain the presence of the CGAg response we observed in age-matched controls. First, it is possible that breakdown of immune tolerance to CGAg proteins or release of CGAg protein from the testis may occur, perhaps as a result of inflammation or as a consequence of age-associated immunosenescence. Second, an aberration in CD4+CD25+ regulatory T cells may affect the CGAg T-cell response: CD4+CD25+ regulatory T cells play an important role in the suppression of CGAg-specific immune responses and depletion of regulatory T cells facilitates isolation of CGAg-specific T cells.38-40 Finally, it is possible that we detected a CGAg T-cell response in healthy donors due to the presence of subclinical or premalignant transformed cells. Indeed, benign paraproteinemia is common in elderly donors and limited expression of CGAg protein by immunohistochemistry has been reported in these cells. It is also likely that detection of T-cell immunity is a more sensitive method by which to detect CGAg expression than immunohistochemistry.24,26 Further studies are required to determine the clinical significance of CGAg-specific immune responses in healthy donors.
The potential role of a CGAg-specific immune response in the control of tumor cell growth is unclear and is still the subject of debate within tumor immunology. CGAg-specific T cells from cancer patients have been shown to be capable of cytotoxicity on tumor targets, including plasma cells, and there is thus considerable hope that these responses could have a direct effect on tumor cell growth.21-26 In contrast, such immunity may reflect a response to cross-presentation of necrotic tumor and have little functional value. In our cohort of patients with myeloma, there was a relationship between the magnitude of the CGAg-specific T-cell response and the size of the tumor burden, which was more pronounced in the 7 patients who demonstrated a changing paraprotein burden. The magnitude of the CGAg-specific responses increased in all 6 patients with relapsing disease yet decreased in one patient (no. 27) who responded to treatment from diagnosis. Importantly, in one patient (no. 22) with responding disease, the increase in immune response was detected prior to a rise in paraprotein level. These observations, therefore, indicate that close monitoring of the CGAg-reactive immune response may be a useful predictor of disease activity.
In conclusion, our data provide evidence that CGAg-specific CD8+ T cells are present in a substantial proportion of patients with multiple myeloma and, although further studies are necessary, CGAg-derived peptides may well provide suitable candidates for immunotherapy based on adoptive cellular therapy or vaccination to increase the number or functional activity of the CGAg-specific immune response in vivo for the treatment of myeloma.
Prepublished online as Blood First Edition Paper, September 6, 2005; DOI 10.1182/blood-2005-02-0563.
Supported by the Leukaemia Research Fund.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Julie Arrazi for collection of blood samples from patient cohorts.

![Figure 4. Characterization of a MAGE-A2-157-166-specific T cell. (A) FACS analysis of a MAGE-A2-157-166-specific T-cell clone generated from patient 22 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis. (B) IFN-γ production by MAGE-A2-157-166-specific T-cell clone following stimulation with peptide-loaded target cells (effector-target [E/T] ratio = 0.1:1). T2 target cells were loaded with peptide MAGE-A2-157-166 and incubated with clone 1 from patient 22. IFN-γ production was observed following incubation with peptide-loaded targets (▪) but not unloaded targets (▦). (C) FACS analysis of a MAGE-A2157-166-specific T-cell clone generated from patient 11 following staining with HLA-peptide tetramer HLA-A2/MAGE-A2-157-166. Staining with tetramer is represented on the x-axis, and CD8+ staining is shown on the y-axis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/13/10.1182_blood-2005-02-0563/2/m_zh80240587850004.jpeg?Expires=1770265353&Signature=UeLVa5Hbqo3T3YEU3JVqiN6dy63TOQhIQMVLI3-fT-2Z5BPIyRsHCYAx8OyHJk3AgXsEhBQ11sm1HFjhc6QpG1psx3yXRzIaGLJBi9DpXylgxDhOCRuSzGKZ-gvAe0XhoOuJs8kkU-v73Ys~wbM1x5hnUC-2eG1lBMmgS4nPMZINT5jlUvRRC5HB6AQhE3DJBIJTj4GbrmCI2cik7wUK-sRSgsV2fLPx78Nz3NH72B2aamHIxOxC-Jp7Y3DGn0J3T1fZjbAWqQQq1S7bMCEofvUO9Lb0ItkHZL1G3aGvDgsuUxwrRlKyhya49KHaO~dvYVQwBRC5ThsGYPRTqPewuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)