In immunotherapy, dendritic cells (DCs) can be used as powerful antigen-presenting cells to enhance or suppress antigen-specific immunity upon in vivo transfer in mice or humans. However, to generate sufficient numbers of DCs, most protocols include an ex vivo culture step, wherein the cells are exposed to heterologous serum and/or antigenic stimuli. In mouse models of virus infection and virus-induced autoimmunity, we tested how heterologous serum affects the immunomodulatory capacity of immature DCs generated in the presence of IL-10 by comparing fetal bovine serum (FBS)- or normal mouse serum (NMS)-supplemented DC cultures. We show that FBS-exposed DCs induce a systemic immune deviation characterized by reduction of virus-specific T cells, delayed viral clearance, and enhanced systemic production of interleukin 4 (IL-4), IL-5, and IL-10 to FBS-derived antigens, including bovine serum albumin (BSA). By contrast, DCs generated in NMS-supplemented cultures modulated immunity and autoimmunity in an antigen-specific fashion. These cells did not induce systemic IL-4, IL-5, or IL-10 production and inhibited generation of virus-specific T cells or autoimmunity only if pulsed with a viral antigen. These data underscore the importance of using autologous serum-derived immature DCs in preclinical animal studies to accurately assess their immunomodulatory potential in future human therapeutic settings, where application of FBS is not feasible.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells important for induction of both tolerance and immunity,1-3 and animal studies have sought to use these features to induce either antigen-specific immunity, antigen-specific tolerance, or antitumor immunity.4,5 Methods have been developed to generate large numbers of DCs in vitro from spleen or bone marrow precursors in mice or from monocytes in humans.6-9 Most of these protocols involve the use of growth factors such as granulocyte macrophage-colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), which can specifically promote DC generation. Other and less well-characterized factors are provided by, for example, heterologous fetal bovine serum (FBS), which has been used extensively.
By nature, DCs sample their environment by antigen uptake,10,11 and antigens are subsequently presented on major histocompatibility complex class I (MHC-I) and MHC-II molecules. Thus, in our opinion it is quite likely that DCs cultured in medium containing exogenous proteins will process and present not only an experimental antigen (eg, an antigenic peptide), but also antigens derived from heterologous proteins (eg, bovine serum albumin [BSA] from FBS). In addition, DCs rapidly respond to a variety of maturation and modulatory stimuli and it is not clear if and how a culture step in heterologous serum might affect subsequent DC function upon in vivo transfer.
Nevertheless, DCs propagated or cultured in FBS-containing medium in vitro have been used in a number of different in vivo protocols, including attempts to immunize against model antigens,12 induction of antitumor immunity,13 induction of and protection against autoimmunity,14-17 and protection against allograft rejection.18 However, in some studies, protection by DC therapy was antigen independent and could be due to a skewing of the immune response rather than induction of antigen-specific tolerance.
In immunotherapy, immature DCs are thought to be capable of inducing antigen-specific tolerance,3,19,20 and IL-10 is an immunoinhibitory cytokine that prevents DC maturation, thereby keeping the cells in an immature state.21 Use of immature DCs or DCs grown in vitro in the presence of IL-10 is of high clinical interest, since such cells can induce antigen-specific tolerance if they have been pulsed with a cognate antigen prior to in vivo injection.22-24 Antigen-specific tolerance is the ultimate clinical goal to prevent or treat autoimmune diseases and enhance graft acceptance.
Based on these considerations, we reasoned that in protocols where immature DCs are used to induce tolerance, exposure to heterologous proteins (eg, FBS) could result in systemic immune deviation or modulation of immunity rather than targeted induction of antigen-specific tolerance or unresponsiveness. This would make extrapolation of such observations to human settings, where ultimately no serum or autologous serum is used, quite problematic.25,26 Such potential problems could be avoided if immature DCs could be generated with normal mouse serum (NMS) instead of FBS.
We therefore tested how bone marrow-derived DCs grown in the presence of IL-10 in either FBS- or NMS-supplemented culture medium would influence immune responses in 3 models: healthy normal mice receiving unpulsed syngeneic DCs, mice infected with lymphocytic choriomeningitis virus (LCMV), and the RIP (rat insulin promoter)-LCMV model of virus-induced type 1 diabetes (T1D), where disease is initiated by viral infection.27-29
Our data show that FBS-exposed DCs induce systemic and antigen-independent immune deviation due to T-cell responses to FBS antigens, whereas NMS-derived DCs could modulate immunity only if pulsed with a relevant antigen in vitro. Based on these data, we conclude that immunomodulatory DCs cultured in the presence of heterologous serum strongly interfere with the systemic generation of immune responses and mask antigen-specific effects, which is due, at least in part, to responses to FBS antigens themselves. We would therefore suggest that heterologous serum should be replaced by autologous serum in preclinical proof-of-concept studies involving the in vivo use of in vitro-generated DCs.
Materials and methods
Mice and virus stocks
BALB/c, C57BL/6, MHC-II knockout (KO) mice (ABBN12M), and OT-I mice were from Taconic M&B (Ry, Denmark) and Jackson Labs (Bar Harbor, ME). Generation and characterization of RIP-LCMV transgenic mice that develop T1D after LCMV infection have been described.27,28 RIP-NP (H-2d) transgenic mice expressing the viral nucleoprotein from LCMV strain Armstrong in the β cells of their islets29 were bred at La Jolla Institute for Allergy and Immunology (LIAI). All mice were housed under specific pathogen-free conditions. Virus stock consisted of LCMV-Armstrong (clone 53b) and was plaque-purified 3 times on Vero cells and prepared by a single passage on BHK-21 cells. Mice were infected with a single dose of 105 plaque-forming units (pfu) administered intraperitoneally. Analysis of virus titer was done by plaque assay as described.30 All animal studies have been approved by either the Danish Animal Experimentation Inspectorate or the review committee at LIAI.
Generation of DCs and adoptive transfer
DCs were generated largely as described.6,31,32 Briefly, bone marrow cells were harvested from the femur and tibiae and washed in ice-cold Hanks balanced salt solution (HBSS) following lysis of red blood cells. T cells were depleted using baby rabbit complement (Harlan Sera Labs, Leicestershire, United Kingdom) and hybridoma antibodies against mouse CD4 (RL172.4), CD8 (31M), and Thy1 (AT83). B cells were not depleted as these disappeared during culture (C.H. and H.M., unpublished data, January 2005). Remaining cells were washed extensively and plated in RPMI-1640 (Invitrogen, Carlsbad, CA) with either 10%, 5%, or 1.5% FBS (Invitrogen or Hyclone, Logan, UT) or 1.5% normal mouse serum (Taconic M&B) with 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 50 μM 2-mercaptoethanol at 4 × 106 cells/well to 5 × 106 cells/well in a 6-well plate (Nunc, Roskilde, Denmark). Three different batches of FBS from 2 different suppliers were tested, and DCs grown in all 3 batches displayed similar responses in vivo. Fresh medium was added every other day. Bone marrow-derived DCs (BMDCs) developed in the presence of GM-CSF (Pharmingen, San Diego, CA; 20 ng/mL days 0-4, or 10 ng/mL days 5-8) and 20 ng/mL IL-10 or IL-4 (Pharmingen) when indicated (days 0-8). Cells were pulsed with 10 μg/mL NP-118 peptide (RPQASGVYM), 10% FBS (for NMS-DCs and splenic DCs), and activated by 10 ng/mL interferon γ (IFN-γ) and/or 1 μg/mL lipopolysaccharide (LPS) on day 7 as indicated and harvested on day 8. Splenic DCs were isolated using CD11c-microbeads and AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) and were MHC-IIhi, CD11chi. For adoptive transfer, cells were washed extensively in phosphate-buffered saline (PBS) and 1 × 106 cells were injected intraperitoneally.
Blood glucose measurements
Blood glucose concentration was determined on tail vein blood using the OneTouch Ultra system (Lifescan, Milpitas, CA). Mice with measurements greater than 300 mg/dL were considered diabetic.
Mixed leukocyte reaction
BALB/c DCs were harvested on day 8, washed, counted, and irradiated and placed in U-bottom, 96-well plates. Allogeneic T cells (1 × 105) were enriched from C57BL/6 mouse spleen using T-cell enrichment columns (R&D Systems, Minneapolis, MN; 85%-90% CD3+ cells) and added to the wells. After 96 hours, cells were pulsed with 0.5 μCi (0.0185 MBq) 3H-thymidine and the plates were incubated for another 18 hours. Finally, T-cell proliferation was evaluated by measuring the incorporation of radioactivity by scintillation counting as described.31
In vitro stimulation, ELISA, and flow cytometry
Spleens were harvested from uninfected or LCMV-infected mice at the indicated times. Single-cell suspensions were obtained by disrupting tissue through a 100-μm cell strainer (Falcon tubes; Becton Dickinson, San Jose, CA) and lysis of erythrocytes. Complete FBS or NMS medium was used to culture 1 × 106 cells/well (see “Generation of DCs and adoptive tranfer”) with 1 μg/mL BSA (Sigma-Aldrich, St Louis, MO), 2.5 μg/mL conA, 10 μg/mL platebound anti-CD3, and 2 μg/mL soluble anti-CD28 in 1.5% NMS. Where indicated, samples were depleted of CD4+, CD8+, or CD19+ cells before culture, using microbeads and the AutoMACS system (Miltenyi Biotec). Quantification of cytokine levels in cell culture supernatants after in vitro stimulation was performed after 72 hours in a sandwich enzyme-linked immunosorbent assay (ELISA), using capture and detection antibody pairs (OptEIA) from BD Pharmingen. For intracellular staining, spleen cells were restimulated for 4 hours with 10 μg/mL NP-118 peptide in the presence of 1 μg/mL brefeldin A (Sigma-Aldrich). Cells were stained for surface expression of CD4 and CD8, fixed, permeabilized, and stained for intracellular IFN-γ. Samples were acquired using a FACSCalibur (Becton Dickinson, San Jose, CA). For stimulation of OT-I cells, mesenteric lymph node cells (5 × 105) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cocultured with 5 × 103 NMS-IL-10 DCs or NMS-IL-4 DCs matured with LPS or IFN-γ and prepulsed for 24 hours with 10 μg/mL of the Kb-restricted OVA257-264 peptide SIINFEKL in 96 U wells. Where indicated, 50 ng/mL mIL-2 was added directly to the cocultures. Samples were stained for expression of CD8 combined with either Annexin V and 7-AAD (Pharmingen) or the Kb-OVA257-264 tetramer, which was generated as previously described.33,34
Immunohistochemistry
Tissues were immersed in Tissue-Tek OCT (Bayer, Leverkusen, Germany) and quick frozen on dry ice. Using a cryo-microtome and sialin-coated Superfrost Plus slides (Fisher Scientific, Hampton, NH), 6-μm tissue sections were cut. Sections were fixed with 95% ethanol at -20°C, and after washing in PBS, an avidin/biotin-blocking step was included (Vector Laboratories, Burlingame, CA). Primary and biotinylated secondary antibodies (Vector Laboratories) were incubated with the sections for 60 minutes each, and color reaction was obtained by sequential incubation with avidin-peroxidase conjugate (Vector Laboratories) and diaminobenzidinehydrogen peroxide. Primary antibodies used were rat anti-mouse CD8a (Ly2) and rat anti-mouse CD4 (L3T4; Pharmingen), and they were diluted in PBS with 10% FBS.
Flow cytometric analysis
DCs were harvested, washed in PBS, and stained with the following antibodies: CD11b-phycoerythrin (PE) (M1/70), CD11c-allophycocyanin (APC) (HL3), CD40-PE (3/23), CD86-fluorescein isothiocyanate (FITC) (GL1), H2Kb-FITC (AF6-88.5), and I-A/E-PE (M5/114.15.2), all from Pharmingen. Cells were analyzed on a FACS-Calibur (BD Pharmingen). A total of 10 000-20 000 events were acquired and live cells were gated based on forward/side scatter properties. Data were analyzed with CellQuest software (BD Pharmingen).
Statistics
Statistical analysis was carried out using the Student t test (equal or unequal variance was evaluated using the F test) and the Fisher exact test.
Results
Phenotype of DCs generated in FBS- and NMS-supplemented cultures
Our goal was to generate immature DCs capable of preventing the expansion of virus-specific T cells after injection into naive mice. Consequently, immature DCs were generated from bone marrow precursors both in the presence and in the absence of the immunomodulatory cytokine IL-10.21,24,31
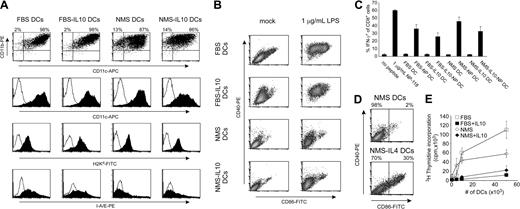
We compared DCs generated in the presence of FBS versus NMS, yielding a total of 4 different DC subsets. Flow cytometric analyses demonstrated that all 4 subsets of DCs were CD11bhiCD11chi (Figure 1A, top panels) and MHC-I+. FBS-generated DCs were MHC-IIint-hi whereas NMS-generated DCs were MHC-IIlo (Figure 1A, bottom panels). To further investigate the maturation level of the DCs, all 4 subsets were matured with LPS for 24 hours and expression of CD40 and CD86 was analyzed. Both FBS-generated subsets (with or without IL-10) could be matured in the presence of LPS, resulting in upregulation of CD40 and CD86. In contrast, both NMS-generated subsets up-regulated CD40 only moderately in response to LPS and the CD86 expression remained very low after maturation (Figure 1B). This was not due to an intrinsic failure of NMS-derived DCs to mature, since as shown in Figure 1D, a considerable subset of DCs generated in NMS supplemented with the DC maturation factor IL-4 expressed high levels of both CD40 and CD86.
Phenotypic characterization of DC subsets. (A,B) DCs were propagated from bone marrow precursors under the conditions indicated with or without IL-10 in FBS- or NMS-supplemented cultures. On day 8, cells were harvested and analyzed for surface markers either unstimulated (A) or stimulated with 1 μg/mL LPS for the last 24 hours (B). Histograms shown are representative of 3 to 7 observations. (C) DCs were pulsed or not with 10 μg/mL NP-118 peptide for the last 24 hours of culture and used as APCs for splenocytes from LCMV-infected syngeneic mice and analyzed by intracellular cytokine staining (ICCS) as described. As positive control, peptide was added directly to splenocytes or splenocytes were left unstimulated. (D) Phenotype of NMS DCs grown with or without IL-4. (E) DCs were used as stimulator cells in an allogeneic mixed lymphocyte reaction (MLR) using 105 C57BL/6-derived T cells as responders. T-cell activation was measured by 3H-thymidine incorporation after 5 days.
Phenotypic characterization of DC subsets. (A,B) DCs were propagated from bone marrow precursors under the conditions indicated with or without IL-10 in FBS- or NMS-supplemented cultures. On day 8, cells were harvested and analyzed for surface markers either unstimulated (A) or stimulated with 1 μg/mL LPS for the last 24 hours (B). Histograms shown are representative of 3 to 7 observations. (C) DCs were pulsed or not with 10 μg/mL NP-118 peptide for the last 24 hours of culture and used as APCs for splenocytes from LCMV-infected syngeneic mice and analyzed by intracellular cytokine staining (ICCS) as described. As positive control, peptide was added directly to splenocytes or splenocytes were left unstimulated. (D) Phenotype of NMS DCs grown with or without IL-4. (E) DCs were used as stimulator cells in an allogeneic mixed lymphocyte reaction (MLR) using 105 C57BL/6-derived T cells as responders. T-cell activation was measured by 3H-thymidine incorporation after 5 days.
To show that all DC subsets could present antigen in the context of MHC-I in vitro, we pulsed DCs overnight with the dominant H-2d-restricted epitope of the LCMV nucleoprotein, the NP118 peptide, and used them as antigen-presenting cells for antigen-experienced splenocytes from memory LCMV-infected mice (35 days after virus inoculation) in an in vitro recall assay. This confirmed that all DC subsets could present the NP118 peptide to T cells to a similar degree as measured by IFN-γ production (Figure 1C). Finally, to test the ability of the DC subsets to activate naive T cells, we performed a mixed leukocyte reaction using allogeneic T cells as responders. This experiment demonstrated that whereas FBS DCs were more potent than NMS DCs, both DC subsets grown in the presence of IL-10 were less efficient than DCs grown without IL-10 in activating naive, allogeneic T cells (Figure 1E).
In conclusion, DCs were generated under all 4 culture conditions and, as expected, the presence of IL-10 during DC differentiation resulted in a fairly immature (or at least not fully mature) DC phenotype.
T-cell stimulation in vivo by DC subsets
We and others10,17,35 have noted that syngeneic, unpulsed FBS-derived DCs, upon adoptive transfer into naive mice, can influence immunity, irrespective of in vitro antigen pulsing.
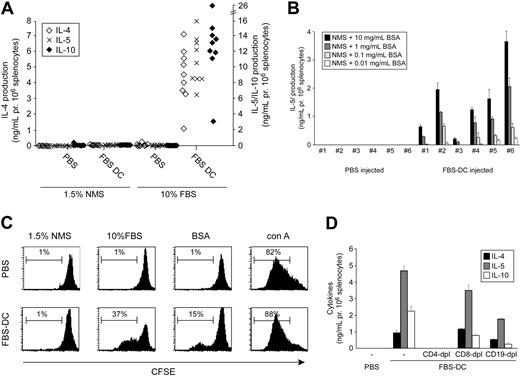
We tested if this could be due to priming to FBS antigens by immunizing naive mice 2 times with syngeneic, unpulsed DCs. Splenocytes were subsequently analyzed in vitro for cytokine production. Strikingly, as demonstrated in Figure 2A, splenocytes from animals treated with FBS DCs secreted high amounts of the Th2 cytokines IL-4, IL-5, and IL-10 when cultured in FBS-containing medium in vitro. This cytokine production correlated with proliferation of CD4+ and CD8+ T cells (as evidenced by CFSE dilution; Figure 2C) and was dependent on the presence of FBS in vitro since no cytokines or proliferation were observed when splenocytes were cultured in NMS medium (Figure 2A). Furthermore, part of the response was due to priming against BSA present in serum, since splenocytes from FBS DC-treated mice proliferated and produced IL-4, IL-5, and IL-10 in response to BSA in a dose-dependent manner (Figure 2B-C, only IL-5 is shown). Moreover, the production of cytokines to FBS antigens was systemic and not dependent on the BALB/c background, since cells isolated from both mesenteric and pancreatic lymph nodes responded to FBS in a manner similar to that of splenocytes and since similar results were obtained in C57BL/6 and NOD mice (Figure 3). We tested whether induction of an FBS-specific type 2 immune response by FBS DCs was due to the lack of IL-12 production by using LPS- or LPS+IFN-γ-activated DCs. These treatments induce low and high IL-12p70 production in DCs, respectively. However, even LPS+IFN-γ-activated FBS DCs induce a type 2 response to FBS, although this was less potent in some animals (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
T-cell activation in vivo by FBS DCs. (A) BALB/c mice were treated with 106 syngeneic FBS DCs or PBS on days -14 and -7. Splenocytes were harvested on day 0 and 106 cells were cultured in medium containing 10% FBS or 1.5% NMS for 72 hours and cytokine production was analyzed. Each dot represents an individual mouse. (B) As in panel A, except that cells were cultured in 1.5% NMS medium with the indicated concentrations of BSA and cytokine production was measured. (C) As in panel A, but cells were labeled with CFSE before culture and analyzed by flow cytometry. Cells are gated on live CD4+ and CD8+ cells. (D) As in panel A, except that cells from FBS DC-treated animals were depleted of the indicated subsets before culture. Figures are representative of 2 to 4 independent experiments.
T-cell activation in vivo by FBS DCs. (A) BALB/c mice were treated with 106 syngeneic FBS DCs or PBS on days -14 and -7. Splenocytes were harvested on day 0 and 106 cells were cultured in medium containing 10% FBS or 1.5% NMS for 72 hours and cytokine production was analyzed. Each dot represents an individual mouse. (B) As in panel A, except that cells were cultured in 1.5% NMS medium with the indicated concentrations of BSA and cytokine production was measured. (C) As in panel A, but cells were labeled with CFSE before culture and analyzed by flow cytometry. Cells are gated on live CD4+ and CD8+ cells. (D) As in panel A, except that cells from FBS DC-treated animals were depleted of the indicated subsets before culture. Figures are representative of 2 to 4 independent experiments.
In an attempt to identify the source of cytokine production, we depleted splenocytes from FBS DC-treated mice of CD4+, CD8+, and CD19+ cells. As shown in Figure 2D, only depletion of CD4+ cells was able to completely remove cytokine production in vitro. This, together with the demonstration that cytokines could be induced by anti-CD3/anti-CD28 or conA stimulation (Figure 3), suggests that the source of cytokine production was indeed CD4+ T cells.
In a similar assay, we then tested the other DC subsets. In contrast to the results above, splenocytes from animals treated with NMS or NMS-IL-10 DCs did not produce IL-4 or IL-10, whereas FBS-IL-10 DCs had an effect similar to that of FBS DCs (Figure 4, top). Again, the production of cytokines was dependent on the presence of FBS in vitro, since no cytokines were produced when splenocytes from either of the 4 DC-treated groups were cultured in NMS-containing medium (Figure 4, bottom). In addition, the effect of FBS-derived DCs was not due to the high concentration of FBS used, since DCs grown in 1.5% FBS had a similar effect (Figure 3). Furthermore, it was evident that any DC exposed to FBS could induce an FBS-specific type 2 response in vivo, since both FBS-pulsed NMS DCs and MACS-purified splenic DCs induced type 2 cytokines (Figure 3).
Finally, whereas only FBS and FBS-IL-10 DCs induced Th2 cytokines, IFN-γ production was detectable in splenocytes from both PBS-treated mice and mice treated with any of the 4 DC subsets, but no difference was found (C.H. and H.M., unpublished data, January 2005).
All together, our data show that FBS-exposed DCs prime CD4+ T cells in vivo toward an FBS-specific Th2 response, whereas NMS-derived DCs do not.
Pretreatment of naive mice with DCs before virus infection strongly influences expansion of virus-specific T cells
We wanted to use immature DCs to reduce virus-specific T cells in an antigen-specific manner. Based on our results, we chose to study the more immature FBS-IL-10 and NMS-IL-10 (Figure 1E) subsets. For comparison, we also included the FBS DC subset. In an assay similar to above, we treated naive mice 2 times with NP118-pulsed or unpulsed DCs before infection with LCMV and examined the NP-specific immune response on day 8 after infection.
Strikingly, mice treated with FBS or FBS-IL-10 DCs had a highly significant reduction in the number of NP-specific T cells independent of antigen pulsing of the DCs in vitro. In contrast to this, NMS-IL-10 DCs could reduce the number of NP-specific T cells only if pulsed with the antigenic peptide in vitro, whereas unpulsed NMS-IL-10 DCs had no effect (Figure 5A-B). The reduction in virus-specific T cells in FBS and FBS-IL-10-treated mice was both due to an impairment of the expansion of spleen cell numbers after infection, as well as a reduction in overall CD8+ T-cell numbers and the percentage of NP-specific IFN-γ+CD8+ T cells. In contrast, NMS-IL-10 DCs reduced the number of virus-specific T cells by reducing overall CD8+ T-cell percentage and the percentage of NP-specific IFN-γ+CD8+ T cells, whereas total spleen cell number was similar to PBS-treated controls (Figure S2). These data suggest that splenocytes in FBS DC- or FBS-IL-10 DC-treated mice were impaired in their expansion in response to the viral infection, most likely due to the high levels of Th2 cytokines in these animals.
On average, peptide-pulsed NMS-IL-10 DCs were not as efficient as FBS or FBS-IL-10 DCs in inhibiting expansion of NP-specific T cells (Figure 5B) but were capable of reducing the number of NP-specific T cells to, on average, less than 40% of PBS-treated controls.
We also analyzed the expression level of CD8 on CD8+ splenocytes in treated animals. The CD8 coreceptor is down-regulated on CD8+ T cells after LCMV infection, since more than 90% of CD8+ T cells were CD8high in uninfected control mice, whereas only approximately 30% were CD8high in LCMV-infected control mice (Figure 5C-D). However, in all 4 FBS DC-treated groups, the mean percentage of CD8high cells was between 50% and 70%, indicating that a smaller fraction of CD8+ T cells had been activated than in the PBS-treated mice. Importantly, animals treated with unpulsed NMS-IL-10 DCs had the same percentage of CD8high cells as the PBS-treated animals, whereas cells from animals treated with NMS-IL-10 DCs pulsed with the relevant NP-antigen displayed a higher percentage of CD8high cells (Figure 5D), suggesting that fewer CD8 cells had become properly activated.
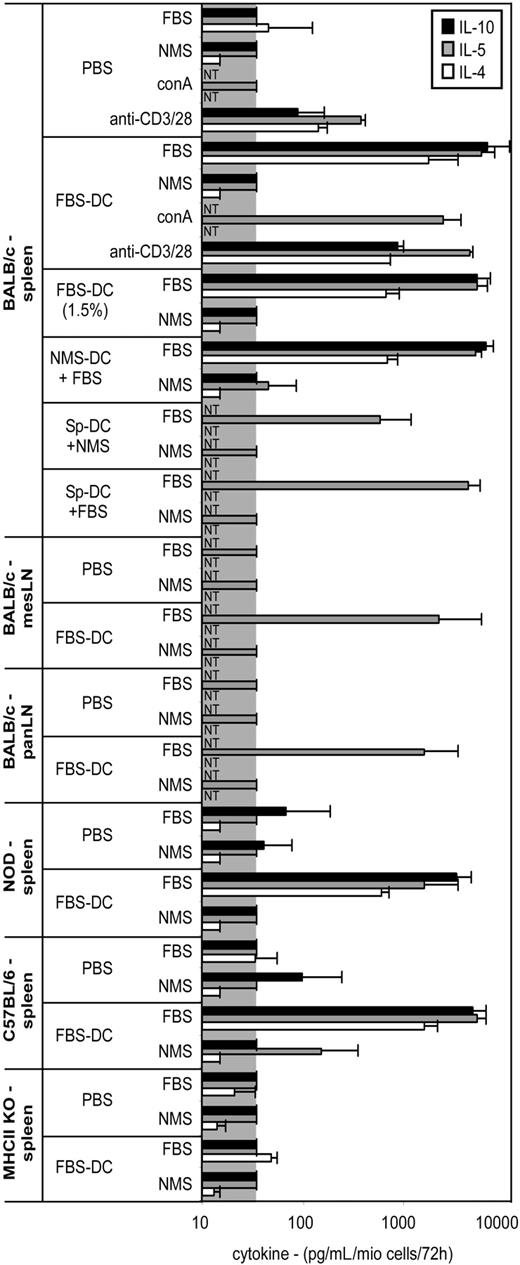
Cytokine production by splenocytes after immunization of naive mice with DCs. Different strains of mice were immunized twice with the indicated DC subset or PBS as described in Figure 2, and production of cytokines by splenocytes or lymph node cells in response to medium containing FBS, NMS, conA, or anti-CD3/28 was analyzed by ELISA. mesLN and panLN indicate mesenteric and pancreatic lymph nodes, respectively. conA is concanavalin A. Signal below detection limit is indicated by the gray box. NT indicates not tested; NMS-DC + FBS, NMS-DCs pulsed with 10% FBS; SpDC, MACS-purified splenic DCs. Note that SpDCs pulsed with NMS induced some cytokines, which was probably due to the presence of BSA in the buffer used for purification of the cells. However, the levels induced by NMS-pulsed SpDCs were still 10-fold lower than the levels induced by FBS-pulsed SpDCs.
Cytokine production by splenocytes after immunization of naive mice with DCs. Different strains of mice were immunized twice with the indicated DC subset or PBS as described in Figure 2, and production of cytokines by splenocytes or lymph node cells in response to medium containing FBS, NMS, conA, or anti-CD3/28 was analyzed by ELISA. mesLN and panLN indicate mesenteric and pancreatic lymph nodes, respectively. conA is concanavalin A. Signal below detection limit is indicated by the gray box. NT indicates not tested; NMS-DC + FBS, NMS-DCs pulsed with 10% FBS; SpDC, MACS-purified splenic DCs. Note that SpDCs pulsed with NMS induced some cytokines, which was probably due to the presence of BSA in the buffer used for purification of the cells. However, the levels induced by NMS-pulsed SpDCs were still 10-fold lower than the levels induced by FBS-pulsed SpDCs.
T-cell activation in vivo by DC subsets. BALB/c mice were treated with 106 syngeneic DCs on days -14 and -7. Splenocytes were harvested on day 0 and 106 cells were cultured in medium containing 10% FBS (top) or 1.5% NMS (bottom) for 72 hours and cytokine production was analyzed. Each dot represents an individual mouse.
T-cell activation in vivo by DC subsets. BALB/c mice were treated with 106 syngeneic DCs on days -14 and -7. Splenocytes were harvested on day 0 and 106 cells were cultured in medium containing 10% FBS (top) or 1.5% NMS (bottom) for 72 hours and cytokine production was analyzed. Each dot represents an individual mouse.
Altogether, these data demonstrate that only DCs generated without the use of FBS reduce the number of NP-specific T cells in LCMV-infected mice in an antigen-dependent manner in vivo.
FBS-derived DCs induce a systemic Th2 response and impair viral clearance in LCMV-infected mice
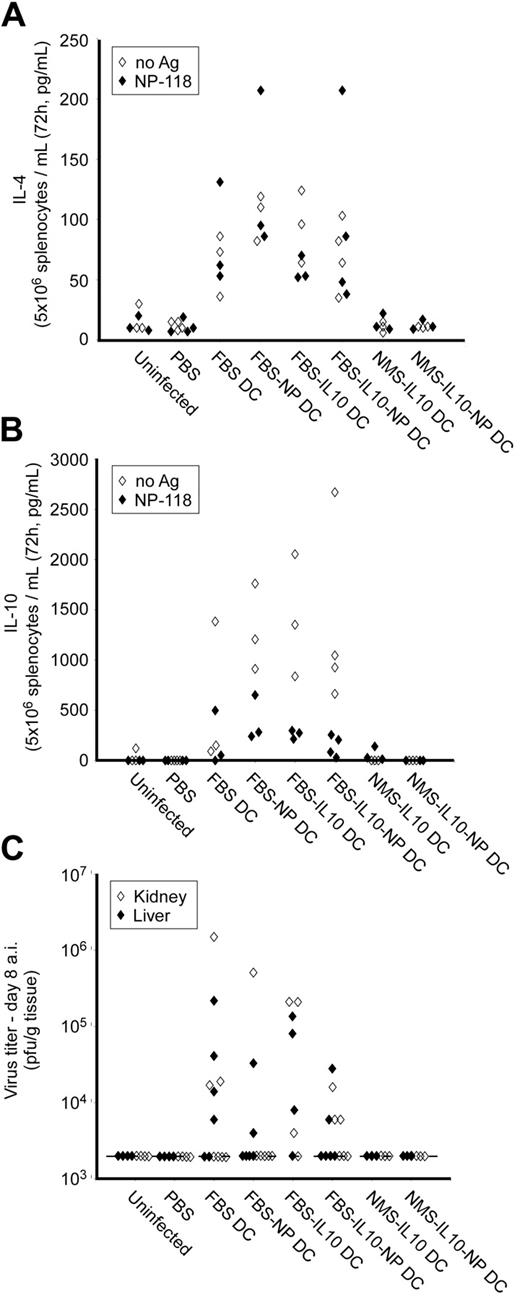
Immunity to LCMV is characterized by a type 1 response with generation of high numbers of IFN-γ-producing cytotoxic CD8+ T cells. In general, IFN-γ induction is inhibited by type 2 cytokines like IL-4 and IL-10 and it was therefore possible that the impaired number of NP-specific T cells seen in the FBS and FBS-IL-10 DC-treated animals was due to the systemic IL-4 and IL-10 production we had observed before (Figure 2). Furthermore, the reduced number of virus-specific T cells could influence viral clearance and we therefore tested spleen cell production of IL-4 and IL-10 of LCMV-infected mice, and virus titer in liver and kidney on day 8.
Indeed, splenocytes from all FBS and FBS-IL-10 DC-treated LCMV-infected animals produced significant amounts of both IL-4 and IL-10 independently of addition of NP-peptide antigen in vitro (Figure 6A-B). Furthermore, several animals treated with FBS or FBS-IL-10 DCs still contained infectious viral particles in liver and/or kidney on day 8 (Figure 6C). In contrast, spleen cells from PBS-injected control animals as well as NMS-IL-10 DC-treated animals did not produce detectable levels of IL-4 or IL-10 and no virus was detectable on day 8 after infection. The impairment in viral clearance in FBS- or FBS-IL-10 DC-treated animals was only transient, since no virus could be detected on day 30 after infection (C.H., T.W., and M.G.v.H., unpublished data). In contrast, although animals treated with NP-pulsed NMS-IL-10 DCs had a reduction in the number of virus-specific T cells (Figure 5B), these mice did not have detectable viral particles on day 8 (Figure 6C), suggesting that the reduced number of NP-specific T cells was still sufficient to clear the virus.
Effect of DC treatment of LCMV-specific immunity in vivo. Naive BALB/c mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. The LCMV-specific T-cell response was analyzed by restimulation of splenocytes in vitro with 10 μg/mL NP-118 peptide for 4 hours and analyzed by ICCS as described. (A) Representative FACS diagrams of the numbers shown in panel B. (B) Total number of NP-118 reactive IFN-γ+CD8+ splenocytes after ICCS. (C) Representative FACS diagrams of CD8 expression as shown in panel D. (D) Quantification of the percent CD8lo and CD8hi spleen cells. In panels B and D, each dot represents an individual mouse. **P < .01; ***P < .005 compared with PBS group.
Effect of DC treatment of LCMV-specific immunity in vivo. Naive BALB/c mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. The LCMV-specific T-cell response was analyzed by restimulation of splenocytes in vitro with 10 μg/mL NP-118 peptide for 4 hours and analyzed by ICCS as described. (A) Representative FACS diagrams of the numbers shown in panel B. (B) Total number of NP-118 reactive IFN-γ+CD8+ splenocytes after ICCS. (C) Representative FACS diagrams of CD8 expression as shown in panel D. (D) Quantification of the percent CD8lo and CD8hi spleen cells. In panels B and D, each dot represents an individual mouse. **P < .01; ***P < .005 compared with PBS group.
Finally, to study the mechanism of CD8+ T-cell tolerance induced by NMS-IL-10 DCs, we tested the activation of OVA-specific OT-I T cells by NMS-IL-10 DCs pulsed with OVA-peptide. Interestingly, NMS-IL-10 DCs induced initial activation of OT-I T cells, followed by apoptosis, resulting in almost complete absence of OVA-specific T cells after 8 days of culture. This was in contrast to OT-I T cells stimulated by mature DCs (NMS-IL-4 DCs stimulated with LPS+IFN-γ) or by NMS-IL-10 DCs in the presence of IL-2 (Figure S3). Thus, these data suggest that NMS-IL-10 DCs induce tolerance by abortive activation of CD8+ T cells followed by apoptosis and deletion.
Influence of DCs on diabetes development in the RIP-LCMV mouse model of type 1 diabetes
To test whether FBS DCs could influence virus-induced autoimmunity, we treated RIP-LCMV mice with FBS DCs before viral infection. Only 16% of the mice treated with unpulsed FBS DCs developed diabetes and no mice treated with NP-peptide-pulsed FBS DCs became diabetic (Figure 7). These observed frequencies were significantly different (P < .01 and P < .005, Fisher exact test) from the high diabetes frequency of 87% observed in the PBS-treated control group. Thus, FBS DC injection changed the normal diabetes incidence dramatically, yet independent of NP pulsing in vitro.
Since NMS-IL-10 DCs were capable of lowering the number of virus-specific T cells (Figure 5) in a manner dependent on antigen pulsing in vitro, we tested whether NP-pulsed NMS-IL-10 DCs could prevent autoimmunity. Although not all animals were protected from disease, disease development was inhibited as compared with controls, since only 45% of the animals developed diabetes (Figure 7A; P < .05 compared with control). Importantly, unpulsed NMS-IL-10 DCs did not affect disease development.
Immunohistochemistry on pancreatic sections showed that islets in PBS-treated control mice and in mice treated with unpulsed NMS-IL-10 DCs were completely destroyed with infiltrates of both CD4+ and CD8+ cells (Figure 7Bi-iv). Sections from nondiabetic mice treated with NP-pulsed NMS-IL-10 DCs demonstrated the presence of many islets with both peri- and intra-islet insulitis (Figure 7Bv-vi), however, it was striking that in these mice many islets were completely free of infiltration, thus offering a possible explanation why these mice remained normoglycemic (Figure 7Bvii-viii). In mice treated with either NP-pulsed or unpulsed FBS DCs, nearly all islets were almost completely free of insulitis (Figure 7Bix-xii). Thus, NMS-IL-10 DCs are capable of inhibiting viral immunity and autoimmunity in an antigen-specific and selective fashion, whereas FBS-exposed DCs exert systemic effects, many of which cannot be attributed to the antigen used for in vitro pulsing.
Discussion
One of our principal new discoveries here is that FBS-exposed DCs induce an FBS-specific Th2 immune response upon injection in naive mice. In contrast, NMS-derived DCs influenced subsequent immune responses only if pulsed with a relevant antigen in vitro and would thus appear to be better suited for antigen-specific immunotherapy. Induction of an FBS-specific Th2 response was caused not only by FBS DCs (MHC IIint), but also by NMS DCs (MHC IIlo) and by magnetic bead purified splenic DCs (MHC IIhi) pulsed with FBS. The divergent MHC II levels on these various DCs would also suggest that the induced Th2 response is not directly related to the different MHC II expression level.
The finding that FBS-exposed DCs can prime T cells against FBS-derived antigens in vivo is important although conceptually not surprising, but it was unexpected that the priming against FBS-derived antigens could exert such a strong influence on the development of an LCMV-specific immune response and virus-induced autoimmunity. We and others have shown that the injection of antigen-pulsed FBS-derived DCs can indeed induce antigen-specific immune response in vivo, but the in vitro recall assay must be performed in NMS-supplemented cultures instead of FBS-supplemented cultures.5,10,31,36 Collectively, these studies and our study demonstrate that FBS-exposed DCs are likely to prime against FBS-derived antigens. Depending on the particular assay this may not always be of concern, but in proof-of-concept protocols involving prevention or treatment of autoimmunity and/or tolerance induction, this might overestimate the tolerogenic potential of in vitro-generated DCs.
Cytokine production and virus titer after DC treatment and LCMV infection. Naive BALB/c mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. The LCMV-specific T-cell response was analyzed on day 8 after infection. (A,B) Spleen cell production of IL-4 (A) and IL-10 (B) was analyzed by ELISA after 72 hours of restimulation with or without NP-118. (C) LCMV viral titer in kidney and liver was analyzed by plaque assay. Lower detection limit of the assay (2000 pfu/g tissue) is indicated by a dotted line. Each dot represents an individual mouse; there were 3 to 6 mice in each group.
Cytokine production and virus titer after DC treatment and LCMV infection. Naive BALB/c mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. The LCMV-specific T-cell response was analyzed on day 8 after infection. (A,B) Spleen cell production of IL-4 (A) and IL-10 (B) was analyzed by ELISA after 72 hours of restimulation with or without NP-118. (C) LCMV viral titer in kidney and liver was analyzed by plaque assay. Lower detection limit of the assay (2000 pfu/g tissue) is indicated by a dotted line. Each dot represents an individual mouse; there were 3 to 6 mice in each group.
In our hands, bone marrow-derived DCs induce a Th2 response, irrespective of IL-12 production or maturation state, and splenic DCs also induce a Th2 response if exposed to FBS in vitro. This shows that, although the DC phenotype clearly can determine the nature of the subsequent immune response in other systems,37 the nature and amount of antigen is also important in this decision and this is in agreement with other reports.38,39 Thus, all FBS-exposed DCs tested in this study induced a vigorous Th2 response.
Our data support the hypothesis that immunosuppression mediated by FBS-exposed DCs is due to the induction of FBS-specific CD4+ T cells producing Th2 cytokines. By contrast, no Th2 cytokines were detectable in mice treated with NMS-IL-10 DCs. In this case, immunosuppression appears to be by a different mechanism—presumably by abortive activation and subsequent deletion of NP-118-specific T cells by NMS-IL-10 DCs since we show that OT-1 T cells stimulated with OVA-pulsed NMS-IL-10 DCs undergo initial activation followed by apoptosis and deletion in vitro. This would be in accordance with other studies showing that immature DCs can induce CD8+ T-cell tolerance by a similar mechanism.40-42
Importantly, spleen cells from mice treated with syngeneic FBS-derived DCs produce Th2 cytokines, even when measured after infection with LCMV. This correlated with an impairment to generate sufficient numbers of NP-118-specific T cells, and with a delay in viral clearance. This was surprising, since LCMV normally drives strong Tc1 and Th1 responses.43 However, the priming of T cells toward FBS-derived antigens and the subsequent skewing toward a Th2 response characterized by IL-4, IL-5, and IL-10 production might impair development of virus-specific IFN-γ+ T cells and indeed in other studies, Th2 cytokines have been shown to prevent the generation of a normal type 1 response.44,45 One tempting implication of this is that the murine immune system is incapable of developing a robust type 1 response when the majority of the T cells—at the same time—are being primed to a type 2 fate due to a systemic response to another antigen, in this case FBS. In some cases this can be unfavorable, as seen by the delayed clearance of viral particles, but in other cases beneficial, as seen by a protection against development of autoimmune diabetes. The latter is also exemplified in infectious tolerance when orally induced T cells can use IL-4 and IL-10 to educate DCs, which subsequently can relay this information to naive cells.46
Our data show that only NMS-IL-10 DCs were capable of significantly reducing the number of NP-specific T cells in a manner that was dependent on in vitro antigen pulsing and to a level where virus is still cleared but where autoimmunity is nevertheless prevented. Such a correlation between the number of antigen-specific T cells and the development of autoimmune diabetes is in correlation with other studies, showing that autoimmunity in this model could be prevented by lowering the number of virus-specific T cells or by inducing a type 2 response.47,48
Although based on murine studies, our observations have important implications for the translation of findings with FBS-exposed murine DCs to the human clinical setting, where not FBS but autologous or no serum will be applied. Since many animal studies involving DC therapy are carried out using FBS-exposed DCs, some of the admittedly striking reports of protection against autoimmunity or transplant rejection after DC treatment might be due to systemic immune deviation rather than antigen-specific immunosuppression. In our opinion, this makes a translational interpretation of such data more difficult. One example is the demonstration that FBS-derived DCs could protect against diabetes development in the NOD mouse model of T1D15 in a manner that was independent of antigen-pulsing of DCs in vitro.17 Subsequent analysis of the protected NOD mice revealed a significant induction of the type 2 cytokines upon culturing of splenocytes in vitro.16 We find it likely that the observed protection from diabetes is due to systemic immunomodulation toward a type 2 response, similar to what we have demonstrated in this study, and not due to antigen-specific immunosuppression.
Taken together, our data demonstrate that the results should be interpreted cautiously when using FBS-derived DCs as a means to induce antigen-specific unresponsiveness in vivo. It is possible that the protective effects of immature DCs in disease models in vivo in some cases should be re-evaluated, since the observed effects may be due to systemic immune deviation rather than antigen-specific immunosuppression. Still, our results support the concept that immature DCs may reduce immunity in an antigen-specific manner, provided that these cells are generated in FBS-free culture systems. The issue of the in vivo use of DCs is important, since DCs are now being used in preclinical proof-of-concept studies conducted for translational purposes. In this respect, studies involving DC treatment in experimental animal models should be done in FBS-free culture systems and results from animal model systems using in vitro-generated DCs grown in heterologous serum should be interpreted with caution.
Diabetes development in RIP-LCMV mice after DC treatment and LCMV infection. RIP-LCMV mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. (A) Diabetes development was followed by measuring blood glucose. (B) Pancreatic sections were stained for CD4 and CD8. Images are representative of 3 to 6 individual animals in each group. The data shown are pooled from 2 independent experiments.
Diabetes development in RIP-LCMV mice after DC treatment and LCMV infection. RIP-LCMV mice were injected intraperitoneally with 106 of the indicated DC subset on days -10 and -3, followed by LCMV infection (105 pfu) on day 0. (A) Diabetes development was followed by measuring blood glucose. (B) Pancreatic sections were stained for CD4 and CD8. Images are representative of 3 to 6 individual animals in each group. The data shown are pooled from 2 independent experiments.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-03-0975.
Supported in part by the Danish Ministry of Science, Technology and Development (C.H.) and institutional funds from Novo Nordisk. M.G.v.H. was supported by R01DK51 091, AI44 451, U19, and P01 awards from the National Institute of Allergy and Infectious Disease (NIAID).
Hagedorn Research Institute is an independent basic research component of Novo Nordisk.
H.M. and M.G.v.H. contributed equally to this work.
C.H., M.E., A.E.J., H.M., and M.G.v.H. designed research; C.H. and T.W. performed research; C.H., H.M., and M.G.v.H. analyzed data; and C.H., H.M., and M.G.v.H. wrote the paper.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Trine Larsen and the employees of our Animal Unit for excellent technical assistance. Dr Urs Christen is thanked for help with preparing the OVA-Kb tetramers.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal