We have taken advantage of the Cre/lox system to generate a mouse model with inducible deficiency of transforming growth factor β receptor II (TβRII). Using this approach, transforming growth factor β (TGF-β) signaling deficiency can be restricted to the hematopoietic system by bone marrow transplantation. Mice that received transplants with TβRII-/- bone marrow develop a lethal inflammatory disorder closely resembling that of TGF-β1-null mice. Previous in vitro studies have suggested multiple roles for TGF-β in T-cell development, including proliferation, apoptosis, and differentiation. We used our transplantation model to ask whether T-cell development is normal in the absence of TGF-β signaling. The findings show for the first time in vivo and in fetal thymus organ culture (FTOC) that TGF-β is not required for thymocytes to differentiate along the entire pathway of thymic T-cell development, as defined by the expression patterns of CD4, CD8, CD25, and CD44. In contrast to previous investigations, no increase of thymocyte apoptosis was observed. However, TβRII-deficient CD8+ thymocytes displayed a 2-fold increase in proliferation rate, as determined by bromodeoxyuridine (BrdU) incorporation in vivo. These results reinforce the importance of TGF-β as an immune regulator critical for T-cell function.
Introduction
Transforming growth factor β (TGF-β) is a group of growth factor isoforms (TGFβ1, TGFβ2, and TGFβ3) involved in a multitude of physiologic functions including the immune system. They primarily interact with the TGF-β type I receptor (TβRI) and type II receptor (TβRII), both essential for propagation of the intracellular signal, including phosphorylation of Smad (small mother against decapentaplegic) proteins regulating transcriptional activation. The development of immature thymocytes to CD4+ or CD8+ single-positive (SP) T cells is defined by their sequential expression of cell surface markers such as CD3, CD4, CD8, CD25, CD44, and the T-cell receptor (TCR).1,2 Involvement of TGF-β in T-cell function has been suggested by in vitro studies demonstrating that TGF-β inhibits interleukin-1 (IL-1)-, IL-2-, and IL-7-dependent thymocyte proliferation.3-6 Expansion of thymocytes may also be indirectly promoted by an inhibitory action of TGF-β on apoptosis.7-9 Inhibitory effect of TGF-β on thymocyte development has been shown in fetal thymus organ culture (FTOC).10 This study demonstrated that TGF-β causes a very early block in development within the CD4-CD8- population, affecting differentiation of the CD44+CD25- into the CD44+CD25+ subset of thymocytes. Further evidence for an inhibitory mode of action on T-cell development derives from the observation that TGF-β inhibits differentiation of CD4+ thymocytes into the T helper 1 (Th1) and Th2 subsets of T helper cells.11,12 A more complex immunoregulatory role of TGF-β emerged by the finding that TGF-β may also have stimulatory effects on immune functions. Thus, at certain stages of T-cell development, TGF-β stimulates, rather than inhibits, proliferation.13
A critical role of TGF-β in T-cell homeostasis and function has been reinforced by studies using knock-out and transgenic mouse models. TGF-β1-null mice showed multifocal autoimmune inflammation in most organs, characterized by tissue lesions and infiltrations of inflammatory cells, primarily lymphocytes, elevated levels of nuclear autoantibodies, and activated peripheral lymphocytes.14-17 These mice, in addition, show increased thymic and peripheral apoptosis.18 Transgenic dominant-negative approaches designed to block TGF-β type II receptor (TβRII) function specifically in T cells showed the importance of TGF-β for these cells to control autoimmunity and T-cell homeostasis. Thus, inactivation of TβRII in CD4+ and CD8+ T cells leads to autoimmune inflammatory disease and spontaneous T-cell activation,19 while CD2+-specific TβRII inactivation rather resulted in expansion of CD8+ T cells in peripheral lymphoid organs.20
The specific roles of TGF-β in T-cell development and function remain, however, unclear. We have previously presented a model of inducible TβRII gene knock-out in mice.21 Using the cre/lox gene targeting approach, we generated mice where TβRII exon 4 was flanked by 2 loxP sites. These short sequences do not interfere with gene function but can excise exon 4 when interacting with cre-recombinase, causing disruption of the gene. Cre-recombinase was expressed from a transgene following crossing with a strain carrying the cre-recombinase gene controlled by the interferon-inducible Mx1 promoter.21,22 These TβRII-/- mice invariably developed a lethal inflammatory disease by 8 to 10 weeks after induction. In addition, mouse recipients of TβRII-/- bone marrow developed inflammation and died by 6 to 9 weeks after transplantation. However, 3-week recipients showed essentially no signs of pathology, rendering the model useful for studies of the immune system with minimal interference with the inflammatory phenotype. This model can be used to study the role of TGF-β in the development of all thymocyte subsets, including the earliest stages, in contrast to the transgenic models of TGF-β signaling deficiency that express the dominant-negative TβRII in late and mature T cells. In the present study, we investigated the role of TGF-β signaling at all stages of thymic T-cell development in vivo as well as in fetal thymus organ culture (FTOC). The findings demonstrate that TGF-β signaling is not required for thymocyte precursors to differentiate into the CD4+ and CD8+ T-cell subsets. Nevertheless, TβRII-/- CD8+ thymocytes showed increased proliferation, while apoptosis in vivo was unaffected.
Materials and methods
Mouse strains and bone marrow transplantation
As the source of TβRII-/- bone marrow cells and thymocytes, we used an inducible TβRII-/- knock-out strain, which is a crossing between a cre-transgenic strain and our TβRIIflox/flox mice (Mx1-cre × TβRIIflox/flox). These mice have mixed genetic background (129SV × C57BL/6), express the Ly5.2 (CD45.2) cell surface marker, and were used for all experiments except the apoptosis and proliferation studies. The genotypes that follow induction (eg, treatment with polyinosinic-polycytidylic acid [polyI: polyC]) of experimental mice (Mx1-cre × TβRIIflox/flox) and controls (Mx1-cre × TβRII+/flox or TβRIIflox/flox, lacking Mx1-cre) are, for simplicity, in the text referred to as TβRII-/-, TβRII+/-, and TβRIIflox/flox, respectively. PolyI:polyC is a modified nucleic acid that induces expression of interferon, which thereby induces the Mx1-cre transgene. For apoptosis and proliferation experiments, the same strain was backcrossed for 7 generations into C57BL/6. The mouse strains were generated, induced, and transplanted as described previously.21 Briefly, flox-mutant mice were induced by injections with polyI:polyC, and bone marrow was harvested one week later for transplantation. As bone marrow recipients for noncompetitive experiments, the B6SJL strain was used, which is congenic with C57BL/6 and has the Ly5.1 (CD45.1) cell surface marker. For competitive transplantations, a crossing between B6SJL and C57BL/6, which is Ly5.1+/Ly5.2+, was used. The animals were kept in a barrier facility free from pathogenic viruses and microorganisms.
TGF-β response in vitro
Flat-bottom wells were coated with 50 μL soluble anti-CD3 antibodies (5 μg/mL in phosphate-buffered saline [PBS]) for 1.5 hours at 37°C, 5% CO2. Cells (100 000) were incubated in triplets with anti-CD28 antibodies (10 μg/mL), and with or without TGF-β (3 ng/mL). Cells (100 000) in triplets were cultured under the same conditions without anti-CD3 antibodies as control. The cells were cultured for 72 hours, pulsed with 1 μCi (0.037 MBq) 3H-thymidine for 4 hours at 37°C, 5% CO2, and harvested with a Tomtec cell harvester (Hamden, CT). 3H-thymidine incorporation was measured using a microbeta liquid scintillation beta-counter.
Fetal thymus organ culture (FTOC)
FTOC was performed as previously described,23,24 with some modifications. In brief, fetal thymic lobes (embryonic day 14.5 [E14.5]) were dissected and placed in Terasaki plates (Nunc A/S, Roskilde, Denmark) in Iscoves modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich, St Louis, MO), 100 μg/mL penicillin and 100 μg/mL streptomycin, 0.05 mM 2-β-mercaptoethanol, 1 mM sodium pyruvate, and 1% of modified essential medium (MEM) nonessential amino acids (Invitrogen, Carlsbad, CA). Lobes were then irradiated (25 Gy) and CD4-CD8- double-negative (DN) thymocytes were added. The Terasaki plates were turned upside down and left in a humidified incubator for 2 days at 37°C, 5% CO2, to allow DN thymocytes to reconstitute the lobes. Subsequently, the lobes were transferred to 25 mm/8 μm Nucleopore Track-Etch Membranes (Whatman, Clifton, NJ) floating on culture medium in 6-well plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) and cultured at 37°C at 5% CO2 for 7 or 14 days. Each lobe was aggregated with 1000 DN thymocytes. In general, 10 to 30 lobes reconstituted with TβRII-/- or control cells (TβRII+/- or TβRIIflox/flox) were used for each experiment.
Semiquantitative PCR analysis
To exclude the possibility of insufficient gene disruption, semiquantitative polymerase chain reaction (SQ-PCR) was performed in all experiments. Template DNA for PCR was prepared from T cells as described previously.21 DNA preparations were quantified by spectrophotometry followed by gel electrophoresis of 50 ng DNA for adjustments using a mouse genomic DNA solution with known (0.098 μg/μL) concentration as a standard (D-4416; Sigma-Aldrich). PCR was run with 2 different primer pairs to detect (1) the flox and wild-type alleles and (2) the null allele.21 Dilution series of template DNA were tested by PCR to determine the interval where gel electrophoresis band intensity was proportional to the amount of template.
Magnetic purification of CD4-CD8- thymocytes for FTOC
CD4-CD8- double-negative (DN) thymocytes for FTOC were enriched by the depletion of CD4+CD8+ double-positive (DP), CD4+ and CD8+ single-positive (SP) cells using unconjugated antibodies against CD4 and CD8. Thymocytes were stained for 15 minutes at 4°C with the unconjugated antibodies anti-CD4 and anti-CD8, washed, and subsequently incubated with antirat κ beads (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 minutes at 4°C. After washing, the cells were magnetically separated using AutoMACS (Miltenyi Biotech). The purity was always in the range of 96% to 100%. All antibodies were purchased from BD Biosciences-Pharmingen (San Diego, CA).
Flow cytometric analysis (FACS)
For flow cytometry, the following fluorochrome or biotin-conjugated antibodies were used: anti-CD4-phycoerythrin (PE), anti-CD4-allophycocyanin (APC), anti-CD4-peridinin chlorophyll-alpha protein (PerCP), anti-CD8α-PerCP, anti-CD8-fluorescein isothiocyanate (FITC), anti-CD8-PE, anti-CD25-FITC, anti-CD44-PE, anti-Ly5.1-PE, anti-Ly5.2-FITC, anti-Ly5.1-biotin, and anti-Ly5.2-biotin. Streptavidin-APC was used to reveal the biotinylated anti-Ly5.1 and anti-Ly5.2 antibodies. For bromodeoxyuridine (BrdU) and annexin V staining, see “Proliferation in vivo” and “Apoptosis in vivo,” respectively. The antibodies against Ly5.1 and Ly5.2 were used to distinguish donor populations (Ly5.2+) from competitors (Ly5.1+) and radioresistant recipient populations (Ly5.1+ or Ly5.1+/Ly5.2+). Stainings were performed as described previously.21 Briefly, 1 × 106 cells were added to round-bottom 96-well plates and stained for 15 minutes on ice with antibodies, washed, and then stained with streptavidin-APC. All antibodies for flow cytometry were purchased from BD Biosciences-Pharmingen. Samples were analyzed using a FACS Calibur (Becton Dickinson, San Jose, CA).
Western blot analysis
Thymocytes were incubated for 2 hours in the presence or absence of 10 ng/mL TGF-β1. Western blot analysis for detection of phosphorylated Smad2 was performed as described previously.25
Proliferation in vivo
Mice were injected intraperitoneally with 1 mg BrdU in a volume of 0.5 mL (BrdU Flow kit; BD Biosciences-Pharmingen) or 0.5 mL buffered saline (PBS) as control and were killed 8 hours later. After cell preparation and counting, 0.5 × 106 cells were stained for CD4, CD8, Ly5.1, Ly5.2, and BrdU (anti-BrdU-FITC) according to the BrdU Flow kit manual (BD Biosciences-Pharmingen).
Apoptosis in vivo
Mice were injected intraperitoneally with 100 μg anti-CD3 antibodies (200 μg/mL) or 0.5 mL PBS and killed after 24 hours. After cell preparation and counting, the cells were assayed for depletion of CD4/CD8 double positives by staining for CD4, CD8, Ly5.1, and Ly5.2. For anti-annexin V-PE and 7-aminoactinomycin D (7-AAD) stainings, 5 × 105 cells were treated according to the annexin V detection kit protocol (BD Biosciences-Pharmingen). Hamster anti-CD3 monoclonal antibodies (clone 145-2C11) for induction of apoptosis in vivo were purified in our laboratory from a hybridoma culture supernatant by affinity chromatography on a Protein G column (Amersham Pharmacia Biotech, Uppsala, Sweden).
Results
Thymocytes from bone marrow transplantations are TβRII-/- and unresponsive to TGF-β
TβRII-/- thymocytes for this study were produced by transplanting TβRII-/- bone marrow cells to lethally irradiated B6SJL wild-type recipient mice. Three weeks later, thymocytes were harvested for analysis. As controls, we used TβRII+/- or TβRIIflox/flox mice throughout this investigation, and we never observed any difference between these and wild-type (TβRII+/+) mice. It was essential to confirm that the thymocytes studied had the TβRII-/- genotype and were not contaminated by nontargeted cells. Equally important was to show that the mutation causes complete TGF-β signaling deficiency. We have used a series of tools to convincingly demonstrate that these criteria were fulfilled.
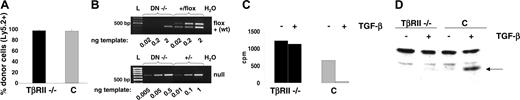
Verification of the TβRII-/- knock out in thymocytes from recipients of TβRII-/- bone marrow at 3 weeks after transplantation. (A) Reconstitution of thymocytes as determined by flow cytometry and staining with anti-Ly5.2 antibodies against cells of donor origin. The diagram shows mean values of reconstitution (% of Ly5.2+ thymocytes, n = 8). C = TβRII+/- controls (n = 8). (B) SQ-PCR analysis of TβRII-/- CD4-CD8- double-negative (DN-/-) thymocytes from bone marrow recipients at 3 weeks after transplantation, to determine Cre/lox recombination efficiency. The top panel shows the absence of the flox allele among DN-/- thymocytes, whereas the bottom panel shows the presence of the null (-) allele. PCR products were generated from 3 dilutions of each DNA template. The PCR reactions are specific for the flox and wild-type (+) alleles (top panel) and the null allele (bottom panel). Both PCRs were run on the same DN-/- DNA preparation. Band intensities among the dilutions of control DNA (+/flox and +/-) indicate that the amount of template chosen is proportional to the amount of PCR product. The wild-type band seen in the DN-/- 2-ng lane of the flox/wild-type PCR is derived from a minor fraction of contaminating wild-type (wt) cells surviving irradiation (< 3% as seen in A). The 2-fold amount of control DNA in the PCR to detect the null allele was used to compensate for heterozygosity. L = 100-bp ladder. (C) Test of antiproliferative response of TβRII-/- or control (C = TβRIIflox/flox) thymocytes to TGFβ1, in the presence of 3H-thymidine. Proliferation is measured by incorporation of 3H-thymidine, which is monitored as radioactive decay (cpm). (D) Western blot analysis to detect the presence of phospho-Smad2 (indicated by the arrow) in thymocytes following stimulation with TGFβ1, indicated by +.C = control thymocytes (TβRIIflox/flox). Trace amounts of phospho-Smad2 in TβRII-/- thymocytes are most likely due to the contamination with approximately 5% wild-type cells.
Verification of the TβRII-/- knock out in thymocytes from recipients of TβRII-/- bone marrow at 3 weeks after transplantation. (A) Reconstitution of thymocytes as determined by flow cytometry and staining with anti-Ly5.2 antibodies against cells of donor origin. The diagram shows mean values of reconstitution (% of Ly5.2+ thymocytes, n = 8). C = TβRII+/- controls (n = 8). (B) SQ-PCR analysis of TβRII-/- CD4-CD8- double-negative (DN-/-) thymocytes from bone marrow recipients at 3 weeks after transplantation, to determine Cre/lox recombination efficiency. The top panel shows the absence of the flox allele among DN-/- thymocytes, whereas the bottom panel shows the presence of the null (-) allele. PCR products were generated from 3 dilutions of each DNA template. The PCR reactions are specific for the flox and wild-type (+) alleles (top panel) and the null allele (bottom panel). Both PCRs were run on the same DN-/- DNA preparation. Band intensities among the dilutions of control DNA (+/flox and +/-) indicate that the amount of template chosen is proportional to the amount of PCR product. The wild-type band seen in the DN-/- 2-ng lane of the flox/wild-type PCR is derived from a minor fraction of contaminating wild-type (wt) cells surviving irradiation (< 3% as seen in A). The 2-fold amount of control DNA in the PCR to detect the null allele was used to compensate for heterozygosity. L = 100-bp ladder. (C) Test of antiproliferative response of TβRII-/- or control (C = TβRIIflox/flox) thymocytes to TGFβ1, in the presence of 3H-thymidine. Proliferation is measured by incorporation of 3H-thymidine, which is monitored as radioactive decay (cpm). (D) Western blot analysis to detect the presence of phospho-Smad2 (indicated by the arrow) in thymocytes following stimulation with TGFβ1, indicated by +.C = control thymocytes (TβRIIflox/flox). Trace amounts of phospho-Smad2 in TβRII-/- thymocytes are most likely due to the contamination with approximately 5% wild-type cells.
Thymocytes of animals that underwent transplantation derive from hematopoietic stem cells (HSCs) produced in TβRIIflox/flox/Mx1-cre donor mice, which have been induced by cre/lox mutagenesis to obtain the TβRII-/- genotype. These HSCs were assessed for cre/lox mutagenesis using SQ-PCR and showed an efficiency of recombination approaching 100%. The fraction of targeted cells was, in addition, analyzed using PCR on colonies derived from single HSCs in tissue culture and out of 60 colonies analyzed, all were TβRII-/-.21,22,26,27 Thus, the HSCs constituting the precursors for thymocytes in animals that underwent transplantation are practically 100% TβRII-/-.
Thymocytes from animals that underwent transplantation should mainly derive from these TβRII-/- HSCs, but there was still a possibility of poor thymic repopulation and that a fraction of recipient-derived thymocytes, surviving irradiation, could contaminate the population. We tested the thymic repopulation of TβRII-/- T cells by transplanting 2 × 106 TβRII-/- (or TβRII+/- control) bone marrow cells in recipient mice. Thymic colonization was determined 3 weeks later by FACS analysis, using antibodies against the donor- and recipient-specific cell surface markers Ly5.2 and Ly5.1, respectively. Among 8 recipients of TβRII-/- bone marrow, 97.4% ± 2.1% of thymus-derived cells were Ly5.2 positive, demonstrating that the vast majority of the thymocytes were derived from the donor animals (Figure 1A). TβRII+/- control donor populations colonized recipients with similar efficiency. Thus, only a minor fraction (1%-5%) of the thymocyte population is radioresistant cells, derived from the recipient mice, and these cells can be excluded from the flow cytometric analysis by appropriate staining and gating using the Ly5.1 and Ly5.2 markers.
The very high colonization of thymus with donor cells was no proof, per se, that these were TβRII-/-. Therefore, we used SQ-PCR to estimate the recombination efficiency and the potential contamination of thymocytes with nonrecombined TβRIIflox/flox cells (Figure 1B). No flox allele was detected after amplification of 2ngTβRII-/- CD4-CD8- DN thymocyte DNA, while a flox band was clearly seen after PCR of 0.02 ng control DNA (TβRII+/flox), suggesting that the fraction of thymocytes having the TβRII-/- genotype is in the range of 99%. In addition, PCR to detect the null allele, using 0.005, 0.05, and 0.5 ng DN-/- template showed similar band intensities as for 0.01, 0.1, and 1 ng control DNA (+/-), respectively, providing further evidence that the major fraction of transplanted thymocytes is TβRII-/-. This analysis was routinely applied in all experiments performed in this study.
We also wanted to functionally verify disruption of the TβRII gene and gene product in TβRII-/- mice. In our initial studies,21 Mx1-cre × TβRIIflox/flox mice, induced to become TβRII-/-, showed a dramatic inflammatory phenotype, which is invariably lethal. The same phenotype develops in animals that have received transplants of TβRII-/- bone marrow and is strikingly similar, if not identical, to that of TGF-β1-/- mice. As expected, TβRII-/- myeloid (granulocyte macrophage colony-forming unit [CFU-GM]) cells were unresponsive in colony assays to the growth inhibitory effect of TGF-β. These data strongly suggest that TGF-β signaling is severely impaired in hematopoietic cells of TβRII-/- mice. We also showed by PCR and Southern blot analysis that exon 4 is deleted in TβRII-/- mice. Exon 4 encodes a majority of the kinase and the entire transmembrane domain, and it has been shown by mutagenesis studies that deletion of any of these domains causes complete ablation of receptor function.28
In order to verify loss of TβRII function specifically in thymocytes, we measured the antiproliferative response, monitored by 3H-thymidine incorporation, of TβRII-/- thymocytes from 3-week recipients to TGF-β1. Nonfractioned TβRII-/- or control (TβRIIflox/flox) thymocytes were costimulated for 72 hours with anti-CD28 and anti-CD3 antibodies in the presence or absence of TGFβ1. Control cells showed, as expected, a dramatic decrease (94%) in 3H-thymidine incorporation, indicating efficient TGFβ1-mediated inhibition of proliferation. In contrast, only a minor inhibition (8%), presumably resulting from contaminating wild-type cells surviving irradiation, was observed in TβRII-/- thymocytes (Figure 1C).
TβRII signaling is mediated by the phosphorylation of a series of Smad proteins, eventually resulting in transcriptional regulation of target genes. We analyzed the phosphorylation of Smad2 in response to receptor activation by TGF-β1, in order to verify the lack of TGF-β signaling in TβRII-/- thymocytes. Western blot analysis clearly showed the presence of phospho-Smad2 in control cultures following TGF-β stimulation, whereas thymocytes isolated from 3-week recipients of TβRII-/- bone marrow do not respond to stimulation with TGF-β.
TβRII-/- thymocyte development is normal in vivo and in FTOC
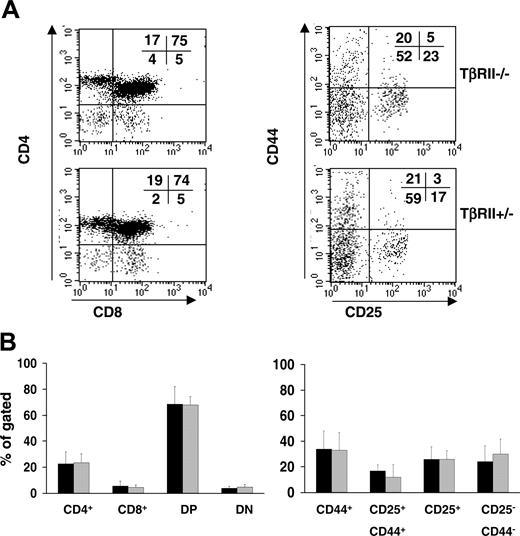
The developmental potential of T cells deficient in TGF-β signaling was assessed in vivo by analyzing a set of cell surface markers of thymocytes. As controls, TβRII+/- thymocytes were used. At the time point for analysis, 3 weeks after transplantation, essentially no signs of inflammation had developed as determined from their normal weight, health condition, thymic cellularity, and histology of multiple organs.21 We analyzed by flow cytometry the expression profiles for CD25 and CD44, which defines the very first developmental stages of thymic T-cell development by the sequential appearance of the CD25-CD44+, CD25+CD44+, CD25+ CD44-, and CD25-CD44- populations.29-31 In addition, CD4 and CD8, which normally are not expressed until CD25 and CD44 have been down-regulated, were analyzed. Surprisingly, no significant differences between TβRII-/- and control populations were observed for any of the T-cell developmental markers examined, suggesting that TGF-β signaling is not required for thymocyte development (Figure 2).
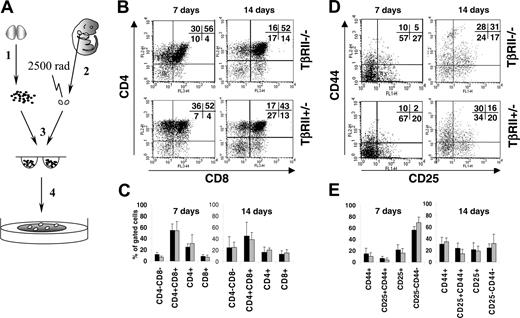
Thymocytes in vivo could be influenced by circulating factors compensating for the loss of TGF-β signaling. Therefore, we also examined thymocyte development by fetal thymus organ culture (FTOC). Briefly, wild-type irradiated (25 Gy) fetal thymus lobes isolated from B6SJL embryos were reconstituted with TβRII-/- or TβRII+/- CD4-CD8- DN cells in vitro. Thymocytes were analyzed for the same developmental markers as in the in vivo experiments at days 7 and 14 of FTOC. In a series of 5 experiments, all TβRII-/- thymocyte subsets developed normally (Figure 3). The relative proportion of some populations in individual flow cytometric dot plots may differ slightly in comparisons between TβRII-/- and controls, but mean values from all experiments performed did not reveal any differences among thymocytes expressing the markers analyzed. These results support our previous conclusion that TGF-β is dispensable for T-cell development.
Flow cytometric analysis of thymocytes from recipients of TβRII-/- or TβRII+/- bone marrow cells at 3 weeks after transplantation. (A) Left panels show thymocytes stained to detect the cell surface antigens CD4 and CD8. Right panels show staining for CD25+ and CD44+ cells among the CD4-CD8- double-negative (DN) fraction. All cells were in addition stained and gated for the marker of donor cells, Ly5.2. The percentage of cells occupying each quadrant is indicated. (B) Mean percentage values of the subpopulations described in panel A. (▪) TβRII-/- (n = 8); (▦)TβRII+/- controls (n = 6). Results are expressed as mean ± SDs.
Flow cytometric analysis of thymocytes from recipients of TβRII-/- or TβRII+/- bone marrow cells at 3 weeks after transplantation. (A) Left panels show thymocytes stained to detect the cell surface antigens CD4 and CD8. Right panels show staining for CD25+ and CD44+ cells among the CD4-CD8- double-negative (DN) fraction. All cells were in addition stained and gated for the marker of donor cells, Ly5.2. The percentage of cells occupying each quadrant is indicated. (B) Mean percentage values of the subpopulations described in panel A. (▪) TβRII-/- (n = 8); (▦)TβRII+/- controls (n = 6). Results are expressed as mean ± SDs.
TβRII-/- CD8+ thymocytes show normal apoptosis in vivo
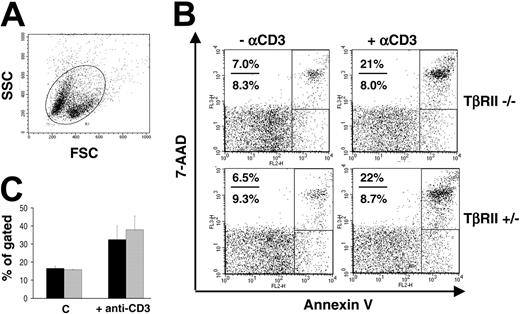
We tested the apoptotic potential of TβRII-/- thymocytes in vivo by treating animals with anti-CD3 antibodies in order to induce apoptosis by overstimulating the T-cell receptor. For these experiments, we used a TβRII strain that was backcrossed to C57BL/6 for 7 generations and, thus, congenic with the recipient strains used. Inductions and transplantations were performed as previously described. Mice that received transplants of TβRII-/- or TβRII+/- bone marrow were injected intraperitoneally with 100 mg anti-CD3 antibodies and killed 24 hours later for flow cytometric analysis of apoptosis. Previous investigations32,33 have shown that DP thymocytes are rapidly depleted upon treatment with antibodies against the CD3/TCR complex both in vivo and in vitro. We analyzed the susceptibility of thymocytes to anti-CD3-mediated depletion in recipients of TβRII-/- or TβRII+/- bone marrow at 3 weeks after transplantation. Mice injected with anti-CD3 antibodies showed typical responses in terms of reduced thymic cellularity (not shown) and an increased population of dead or dying cells, as revealed by the flow cytometric forward and side scatter (FCS and SSC, respectively; Figure 4A). However, staining with anti-CD4 and anti-CD8 antibodies provided no evidence for altered apoptotic response of TβRII-/- DP thymocytes to anti-CD3 antibodies (not shown). This result was supported by staining with annexin V and 7-AAD to detect early and late apoptosis, respectively. No significant difference between TβRII-/- and TβRII+/- thymocytes in total apoptosis (early + late) was observed (Figure 4B-C). The results were consistent in a total of 2 independent experiments analyzing (1) 5 TβRII-/- and 4 TβRII+/- mice and (2) 5 TβRII-/- and 6 TβRII+/- mice. These results suggest that TGF-β is not critical to regulate activation-induced cell death in the total population of thymocytes.
Development of TβRII-/- thymocytes in FTOC. (A) (1) Thymocytes were harvested from bone marrow recipients at 3 weeks after transplantation and enriched for CD4-CD8- thymocytes. (2) Simultaneously, thymic lobes from day-14.5 embryos were dissected. (3) Thymocytes and lobes were put together to aggregate in hanging drop culture for 2 days and (4) subsequently plated into FTOC cultures for 7 or 14 days until flow cytometric analysis. (B,D) Representative dot plots showing flow cytometric analysis of thymocytes at days 7 and 14 of FTOC. CD25/CD44 plots were gated for CD3+ in addition to CD4-CD8- cells to ensure immaturity. Percentage values for each subset are indicated. (C,E) Mean percentage values of T-cell subsets from a number of independent FTOC experiments. (▪) TβRII-/- FTOC thymocytes; (▦) control FTOC thymocytes (TβRII+/- or TβRIIflox/flox). Number of experiments: CD4/CD8 analysis: TβRII-/- (n = 5), control (n = 4); CD25/CD44 analysis: TβRII-/- (n = 5), control (n = 5). The scale represents mean percentage of the Ly5.2+ gated populations indicated. Results are expressed as mean ± SDs.
Development of TβRII-/- thymocytes in FTOC. (A) (1) Thymocytes were harvested from bone marrow recipients at 3 weeks after transplantation and enriched for CD4-CD8- thymocytes. (2) Simultaneously, thymic lobes from day-14.5 embryos were dissected. (3) Thymocytes and lobes were put together to aggregate in hanging drop culture for 2 days and (4) subsequently plated into FTOC cultures for 7 or 14 days until flow cytometric analysis. (B,D) Representative dot plots showing flow cytometric analysis of thymocytes at days 7 and 14 of FTOC. CD25/CD44 plots were gated for CD3+ in addition to CD4-CD8- cells to ensure immaturity. Percentage values for each subset are indicated. (C,E) Mean percentage values of T-cell subsets from a number of independent FTOC experiments. (▪) TβRII-/- FTOC thymocytes; (▦) control FTOC thymocytes (TβRII+/- or TβRIIflox/flox). Number of experiments: CD4/CD8 analysis: TβRII-/- (n = 5), control (n = 4); CD25/CD44 analysis: TβRII-/- (n = 5), control (n = 5). The scale represents mean percentage of the Ly5.2+ gated populations indicated. Results are expressed as mean ± SDs.
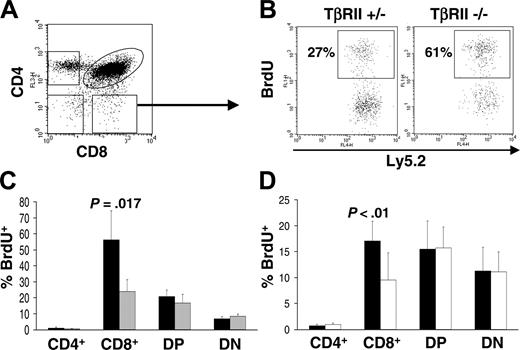
TβRII-/- CD8+ thymocytes display increased proliferation in vivo
In order to examine proliferation in vivo of TβRII-/- thymocyte subsets, we used BrdU incorporation assay. The same backcrossed strain and transplantation protocol were used as for the apoptosis studies. Mice that underwent transplantation were injected intraperitoneally with 1 mg BrdU, and 8 hours later they were killed for purification of thymocytes. These cells were analyzed for incorporation of BrdU in thymic subsets using flow cytometry. Two independent experiments were performed, and staining for BrdU incorporation, CD4, and CD8 expression demonstrated a 2-fold increase of the TβRII-/- CD8+ population compared with TβRII+/- CD8+ thymocytes (experiment 1: P = .017, -/- [n = 4] and +/- [n = 4]; experiment 2: P = .041, -/- [n = 6] and +/- [n = 5]). The other subsets showed only minor differences to the control population that were not consistent in both experiments (Figure 5A-C).
Flow cytometric analysis of thymocyte apoptosis in vivo. (A) Dot plot showing forward-scatter (FFC) and side-scatter (SSC) analysis of thymocytes from an animal treated with anti-CD3 antibodies. The top left population constitutes the apoptotic/dead cells, while the bottom right is alive. The circular region depicts the total population of cells analyzed. (B) Representative dot plots from staining with annexin V and 7-AAD. Early and late apoptosis are shown in the bottom and top rectangular regions, respectively. The total level of apoptosis was determined by summarizing the values of both of these regions. (C) Mean values of total apoptosis from annexin V/7-AAD staining, showing the percentage of thymocytes that are apoptotic when treated with anti-CD3 or PBS (C = control). (▪) TβRII-/- (C: n = 3; +anti-CD3: n = 5); and (▦) TβRII+/- (C: n = 2; +anti CD3: n = 4). Results are expressed as mean ± SDs.
Flow cytometric analysis of thymocyte apoptosis in vivo. (A) Dot plot showing forward-scatter (FFC) and side-scatter (SSC) analysis of thymocytes from an animal treated with anti-CD3 antibodies. The top left population constitutes the apoptotic/dead cells, while the bottom right is alive. The circular region depicts the total population of cells analyzed. (B) Representative dot plots from staining with annexin V and 7-AAD. Early and late apoptosis are shown in the bottom and top rectangular regions, respectively. The total level of apoptosis was determined by summarizing the values of both of these regions. (C) Mean values of total apoptosis from annexin V/7-AAD staining, showing the percentage of thymocytes that are apoptotic when treated with anti-CD3 or PBS (C = control). (▪) TβRII-/- (C: n = 3; +anti-CD3: n = 5); and (▦) TβRII+/- (C: n = 2; +anti CD3: n = 4). Results are expressed as mean ± SDs.
In order to verify that the increased proliferation of CD8+ thymocytes is a primary phenotype (ie, a direct consequence of TβRII-/- deficiency of these cells and not caused by subclinical systemic disease) we performed competitive transplantation experiments. Thus, TβRII-/- or TβRII+/- bone marrow was cotransferred at a 50:50 ratio with B6SJL competitor bone marrow to a recipient strain generated by crossing B6SJL and C57BL/6. This recipient strain can be used to distinguish TβRII mutant cells (Ly5.2+) from competitors (Ly5.1+) and radioresistant recipient cells (Ly5.1+/Ly5.2+). All 3 strains are congenic with C57BL/6. BrdU in vivo analysis was done as described in the previous experiment. In 2 independent experiments TβRII-/- CD8+ thymocytes displayed a 2-fold increase in proliferation in comparison with competitor cells, sharing the same microenvironment (experiment 1: P < .01, -/- [n = 4] and +/- [n = 4]; experiment 2: P < .01, -/- [n = 7] and +/- [n = 7]) (Figure 5D). In contrast, the other TβRII-/- thymocyte subsets analyzed (CD4+, DP, and DN) did not differ from the competitors. Neither were there significant differences when comparing TβRII+/- control cells with competitors (not shown). These results suggest that TβRII-/- CD8+ cells have a higher proliferation rate than thymocytes capable of TGF-β signaling.
Flow cytometric analysis of thymocyte proliferation in vivo. (A) The populations analyzed by anti-BrdU staining are shown. The arrow indicates the CD8+ population analyzed for BrdU incorporation in panel B. (B) Noncompetitive BrdU incorporation in vivo. The dot plots show representative staining with anti-BrdU, gated for the Ly5.2+ and CD8+ populations. The values indicate the percentage of the Ly5.2+/CD8+ cells that are BrdU positive. (C) Graphic representation of noncompetitive BrdU incorporation in CD4/CD8 subsets. (▪) TβRII-/- (n = 4); and (▦) TβRII+/- controls (n = 4). (D) Graphic representation of a competitive transplantation experiment. (▪)TβRII-/- (n = 7); and (□) wild-type competitor cells (n = 7). Results are expressed as mean ± SDs.
Flow cytometric analysis of thymocyte proliferation in vivo. (A) The populations analyzed by anti-BrdU staining are shown. The arrow indicates the CD8+ population analyzed for BrdU incorporation in panel B. (B) Noncompetitive BrdU incorporation in vivo. The dot plots show representative staining with anti-BrdU, gated for the Ly5.2+ and CD8+ populations. The values indicate the percentage of the Ly5.2+/CD8+ cells that are BrdU positive. (C) Graphic representation of noncompetitive BrdU incorporation in CD4/CD8 subsets. (▪) TβRII-/- (n = 4); and (▦) TβRII+/- controls (n = 4). (D) Graphic representation of a competitive transplantation experiment. (▪)TβRII-/- (n = 7); and (□) wild-type competitor cells (n = 7). Results are expressed as mean ± SDs.
Discussion
A complex network of cytokines, chemokines, and cellular interactions strictly regulates the development of T lymphocytes to acquire a specific adaptive immune response. TGF-β possesses a unique position among immune regulatory proteins by its multitude of functions at a variety of levels of differentiation of T cells as well as by the partly opposing effects at different stages of inflammation.34-37 Genetic animal models have convincingly demonstrated a critical role for TGF-β to regulate immune functions, in particular of T cells.14,16,19,38-40 We have investigated the potential role of TGF-β in thymic T-cell development and function using mice deficient in the TGF-β type II receptor. This null mutation affects all developmental stages of T cells causing a complete block of TGF-β signaling in a cell autonomous manner. In addition, using a transplantation approach, the mutation is restricted to cells of the hematopoietic system and since the mutagenesis is inducible, previous limitations such as early lethality and interference of T-cell development with systemic inflammatory disease are circumvented.21
In the present study, we showed that lethally irradiated recipients of TβRII-/- bone marrow cells fully reconstituted the thymus with TβRII-/- thymocytes within 3 weeks after transplantation, before the mice have developed systemic inflammatory disease. Surprisingly, there were no signs of aberrant thymocyte development at this time point based on expression analysis of the developmental markers CD4, CD8, CD25, and CD44. Normal thymocyte development of mice deficient in TGF-β signaling before the appearance of systemic disease was also reported from TGF-β1-null (TGF-β1-/-) mice16 and dominant-negative transgenic models.19,20 The TGF-β1-null mice had, however, circulating TGF-β1 transferred from the mother via the placenta and lactation, which might be sufficient to support normal T-cell development.41 As these mice became cleared from TGF-β1, they developed inflammatory disease precluding them from unbiased T-cell studies. The transgenic models did not achieve block of TGF-β1 signaling during the entire pathway of T-cell development since they depended on tissue-specific promoters that were activated first from the initiation of CD2 or CD4 expression. In addition, it was not clear whether the dominant-negative transgenic approach contributed to a total block of TGF-β receptor signaling since these animals showed a less dramatic immune phenotype. Therefore, we show for the first time that TGF-β is dispensable for differentiation of T-cell precursors into the thymic CD4+ and CD8+ subsets of T cells.
The apparently normal development of thymic T cells in vivo could be explained by the fact that TGF-β has no significant role for T-cell development or, alternatively, that the thymic environment, or intracellular or circulating factors might compensate for the TβRII deficiency. A shared or redundant role of TGF-β with other factors could be revealed using a less complex model than the in vivo transplantation system. We therefore used FTOC in order to exclude redundancy of TGF-β signaling caused by circulating factors or hematopoietic cells and thereby expose effects of TβRII deficiency on T cells not observable in vivo. Using this approach, the fate of TβRII-/- CD4-CD8- DN cells was followed during the course of 2 weeks. This immature population differentiated normally during 14 days into CD4 and CD8 DP as well as SP T-cell subsets. Likewise, TβRII-/- and control cultures showed essentially the same pattern of CD25 and CD44 expression. Thus, TβRII-/- thymocytes develop normally in FTOC, supporting our conclusion from the in vivo experiments that TGF-β is not required for development of the major thymocyte subsets. Furthermore, circulating factors are not likely to compensate for TβRII deficiency and explain the lack of developmental phenotype in the in vivo model.
Several studies have implied a role for TGF-β in regulating programmed cell death of T cells.18,42 Of particular relevance, Chen et al18 showed increased apoptosis of TGF-β1-null thymocytes in vivo in 9- to 12-day-old mice, both with and without exposure to anti-CD3 antibodies (activation-induced cell death). In addition, in situ deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining suggested enhanced spontaneous apoptosis of TGF-β1-null thymocytes in day-18 embryos. In contrast, our activation-induced cell death experiments did not provide evidence for increased apoptosis in the total thymocyte population (live plus dead/apoptotic) of TβRII thymocytes when analyzed by DP depletion or annexinV/7-AAD staining. These results suggest that different pathways may, at least in part, be affected by null mutations of TGF-β1 and TβRII. Alternatively, the discrepancy may result from considerable differences in the experimental models: the animals that underwent transplantation in our study have null mutant cells only in the hematopoietic compartment, enabling TGF-β signaling in other tissues (eg, thymic stroma). In contrast, TGF-β1-null mice have a global disruption of the gene, affecting all tissues. Moreover, the ligand knock-out renders receptor signaling possible through the presence of other members of the TGF-β family.
The TGF-β1-null mice for apoptotic studies were considered as asymptomatic and without inflammatory disease. However, they displayed a decrease in total numbers of thymocytes, which has never been observed among our animals at 3 weeks after transplantation. Reduced numbers of thymocytes were apparent in our mice only when displaying inflammation at 6 to 9 weeks after transplantation.21 Thus, it cannot be excluded that TGF-β1-null mice show an apoptotic phenotype secondary to inflammatory influence. This argument is supported by the fact that TGF-β1-null mice developed a more pronounced apoptosis as the mice became symptomatic. A further difference between the models is that the TGF-β1-null mice carry the mutation in all cells during embryonic development, which potentially could influence thymocyte function.
We examined the thymocyte proliferation rate in vivo using the BrdU incorporation assay. The results demonstrated a 2-fold increase in proliferation of TβRII-/- CD8+ thymocytes, but not of the other subsets analyzed. Although our previous data did not provide evidence for inflammatory disease in recipients at 3 weeks after induction, and the mice in this investigation did not show any clinical signs of illness (abnormal behavior, weight loss, and decreased thymocyte cellularity21 ), it could not be excluded that a subclinical phenotype affected the CD8+ phenotype. We therefore repeated the proliferation study in a competitive setting. The results showed that the TβRII-/- CD8+ thymocytes have a proliferative advantage to the competitor population, suggesting that the increased proliferation observed is not secondary to inflammatory disease. Flow cytometric analysis of the CD4+/CD8+ profile did not, however, show an increased proportion of CD8+ cells, indicating that homeostasis of this subset is maintained in vivo in the absence of TGF-β signaling. Of interest, a study using a dominant-negative transgenic approach to inactivate TβRII in mice showed massive overpopulation of CD8+ T cells in the spleen and lymph nodes,20 supporting our results suggesting that TGF-β is essential to control CD8+ proliferation. The increased proliferation in TβRII-/- CD8+ thymocytes suggests the involvement of a cytokine(s) with enhanced stimulatory potential due to absence of the inhibitory TGF-β pathway. This could potentially be an interleukin such as IL-7 or IL-15,4,43,44 and it would be interesting to investigate whether TβRII-deficient thymocytes are more responsive to these or other cytokines.
In conclusion, we have shown that TGF-β signaling is not required in vivo for thymic development of T-cell populations. Nevertheless, our data suggest that appropriate proliferation of CD8+ thymocytes is dependent on the inhibitory function of TGF-β. Impaired TGF-β signaling could potentially cause uncontrolled expansion of this T-cell subset with profound effects on immune functions.
Prepublished online as Blood First Edition Paper, August 30, 2005; DOI 10.1182/blood-2005-05-1871.
Supported by grants to S.K. from The Swedish Cancer Society, The European Commission (INHERINET), The Swedish Gene Therapy Program, The Swedish Medical Research Council, The Swedish Children Cancer Foundation, A Clinical Research Award from Lund University Hospital, The Joint Program on Stem Cell Research supported by The Juvenile Diabetes Research Foundation, and The Swedish Medical Research Council and to C.M.C. from The Juvenile Diabetes Foundation, The Swedish Medical Research Council, The Swedish Association for Diabetes Research, and Malmö University Hospital Foundations. The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research. P.L. was supported by The Foundation for Strategic Research and Craaford foundation, Sweden.
P.L. and M.C. contributed equally to the project.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lilian Wittman, Ulrika Blank, and Jeanette Arvastsson for valuable technical assistance and Ana Cumano for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal