Previously we reported that we could increase the fraction of carboxylated factor X by reducing the affinity of the propeptide for its binding site on human gamma glutamyl carboxylase. We attributed this to an increased turnover rate. However, even with the reduced affinity propeptide, when sufficient overproduction of factor X is achieved, there is still a significant fraction of uncarboxylated recombinant factor X. We report here that the factor X of such a cell line was only 52% carboxylated but that the fraction of carboxylated factor X could be increased to 92% by coexpressing the recently identified gene for vitamin K epoxide reductase. Because vitamin K is in excess in both the untransfected and vitamin K epoxide reductase (VKOR)–transfected cells, the simplest explanation for this result is that VKOR catalyzes both the reduction of vitamin K epoxide to vitamin K and the conversion of vitamin K to vitamin K hydroquinone. In addition to its mechanistic relevance, this observation has practical implications for overproducing recombinant vitamin K–dependent proteins for therapeutic use.
Introduction
Posttranslational modification of glutamic acid to gamma carboxyl glutamic acid (Gla) is required for the activity of a few proteins, most of which are related to coagulation. These modified proteins are often called vitamin K–dependent proteins because they require reduced vitamin K for their Gla modification. The enzyme that catalyzes the modification of glutamate (Glu) to Gla is the gamma-glutamyl carboxylase (GGCX), an 87.542-kDa1 integral membrane protein with 5 transmembrane domains.2 For the carboxylation reaction, a propeptide on the substrate binds to an exosite on the vitamin K–dependent gamma-carboxylase, holding the substrate in place while multiple glutamic acids are modified to Gla.3 The carboxylation also requires CO2, O2, and reduced vitamin K. It has been observed that for each Glu modified, at least 1 molecule of vitamin K epoxide is formed.4 It is thought that an intermediate of vitamin K is a sufficiently strong base to abstract a proton from the gamma position of the glutamates in the Gla domain,5 and that during this process the reduced vitamin K is converted to vitamin K epoxide (KO). Vitamin K epoxide reductase (VKOR), the target of warfarin anticoagulants, is an 18.2-kDa protein6,7 with 3 transmembrane domains.8 The role of VKOR is to convert the vitamin K epoxide back to vitamin K and, perhaps, vitamin K to reduced vitamin K.
We proposed that efficient carboxylation depends upon a balance between the half-life of the enzyme-substrate complex and the rate of carboxylation of the bound substrate. The half-life of the enzyme-substrate complex is primarily dependent upon the affinity of the propeptide of the substrate for the carboxylase. And, the rate of carboxylation is dependent upon the concentration of reduced vitamin K available for the reaction.3
Initially, it seemed apparent that if a sufficient supply of vitamin K were available, the limiting factor for carboxylation would be GGCX. This rationale seemed warranted because warfarin poisoning can be overcome by large doses of vitamin K,9,10 indicating that alternative enzymes for converting vitamin K to reduced vitamin K are present.11 However, after the purification and cloning of GGCX,1,12 it was reported that coexpression of factor IX (FIX) and GGCX failed to improve the degree of carboxylation in a Chinese hamster ovary (CHO) cell line overexpressing human FIX.13 This leads to the question of what is the limiting step in carboxylation of vitamin K–dependent proteins in a cell. To obtain a clear answer to this question we need an appropriate system. Previously, we observed that the percentage of carboxylated factor X (FX) in the human embryonic kidney (HEK) 293 cell line could be dramatically increased by reducing the affinity of the FX propeptide for GGCX14 ; we attributed this increased carboxylation to an increase in the steady state turnover. However, if the level of expression of FX bearing the prothrombin propeptide is sufficiently high, the level of expression still exceeds the ability of the cell to achieve complete posttranslational modification of the intended Gla residues (R.M.C., unpublished observations, November 2002). Therefore these cells provide a superb system to evaluate whether GGCX, VKOR, or VKOR plus GGCX can enhance carboxylation in vivo. In this study, using this HEK 293 cell line overproducing FX, we demonstrate that the percentage of carboxylated material can be dramatically increased, from approximately 50% to approximately 95%, by coexpression of the VKOR.
Materials and methods
Materials
All restriction enzymes were from New England Biolabs (Ipswich, MA). Pyrococcus furiosus (Pfu) DNA polymerase was obtained from Stratagene (La Jolla, CA). Lipofectin, hygromycin B, pcDNA3.1/Hygro vector, Trizol reagent, and Moloney murine leukemia virus reverse transcriptase (M-MLV RT) were from Invitrogen (Carlsbad, CA). Trypsin-EDTA (ethylenediaminetetraacetic acid), fetal bovine serum, and Dulbecco phosphate-buffered saline were from Sigma (St Louis, MO). Antibiotic-antimycotic, G418 (Geneticin, Grand Island, NY) and Dulbecco modified Eagle medium (DMEM) F-12 were from GIBCO (Grand Island, NY). Puromycin and pIRESpuro3 vector were from BD Biosciences–Clontech (Mountain View, CA). Rabbit anti–human FX (affinity-purified IgG) and Rabbit anti–human FX (HRP conjugate) were from Dako Corporation (Carpinteria, CA). Peroxidase-conjugated AffiniPure rabbit anti–goat immunoglobulin G (IgG) was from Jackson ImmunoResearch Laboratories (West Grove, PA). Q-sepharose Fast Flow was obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Calcium-dependent monoclonal human FX antibody [MAb; 4G3] was obtained from Dr Harold James (University of Texas, Tyler). Bio-Scale CHT5-I Hydroxyapatite was from Bio-Rad Laboratories (Hercules, CA). RQ1 (RNA-qualified) RNase-Free DNase was from Promega (Madison, WI). DyNAmo SYBR Green qPCR kit was from Finnzymes (Keilaranta, Espoo, Finland). DNA Engine An MJ Research Opticon 2 PCR thermal cycler (MJ Research, Alameda, CA) was used for real-time polymerase chain reaction (PCR).
Methods
Construction of expression vectors. All constructs were made in a cell line, HEK293-FX (A6), expressing human FX with 1 mutation, Ile16Leu, and the prothrombin propeptide.14 We selected the FX-expressing cells with the neomycin analog G418. This particular cell line expresses FX at such high levels (7-9 μg/106 cells/24 hours) that only about 50% of the protein is carboxylated even though the FX propeptide was replaced by that of prothrombin.
HEK293-FX (A6) expressing VKOR. 2 primers were designed to amplify the VKOR cDNA.6,7 Primer 1: 5′-CCGGAATTCGCCGCCACCATGGGCAGCACCTGGGGGAGCCCTGGCTGGGTGCGG introduced a Kozak sequence15 (underlined) and a 5′ EcoRI site. Primer 2: 5′-CGGGCGGCCGCTCAGTGCCTCTTAGCCTTGCC introduced a NotI site at the 3′ terminus of the cDNA. After PCR amplification and digestion with EcoRI and NotI, we inserted the PCR product into pIRESpuro3 which has a CMV virus major immediate early promoter/enhancer and confers puromycin resistance upon the transformed cells. HEK293-FX (A6) was transfected with the plasmid pIRESpuro3-VKOR using lipofectin according to the manufacturer's protocol. Selection was done with 450 μg/mL G418 and 1.75 μg/mL puromycin. Resistant colonies were picked and screened for VKOR activity as previously described.6 The colony with highest VKOR activity was selected for further analysis.
HEK293-FX (A6) expressing GGCX. Two primers were designed to amplify the GGCX cDNA.1 Primer 3: 5′-CGCGGATCCGCCGCCACCATGGCGGTGTCTGCCGGGTCCGCGCGGACCTCGCCC, introduced a BamH1 site and a Kozak sequence (underlined) at the 5′ terminus and Primer 4: 5′-CGGGCGGCCGCTCAGAACTCTGAGTGGACAGGATCAGGATTTGACTC that introduced a NotI site at the 3′ terminus. After digestion with BamHI and NotI, we inserted the PCR product into pcDNA3.1/Hygro. pcDNA3.1/Hygro has a cytomegalovirus (CMV) promoter and confers hygromycin resistance upon the transformed cell. Transformed colonies were selected with 300 μg/mL hygromycin and 450 μg/mL of G418. Resistant colonies were picked and screened for GGCX activity with the small peptide substrate phenylalanine-leucine-glutamate-glutamate-leucine (FLEEL).1 The colony with highest GGCX activity was selected for further studies.
HEK293-FX (A6) coexpressing VKOR and GGCX. To obtain a HEK293-FX (A6) cell line overexpressing both VKOR and GGCX, we transfected HEK293-FX (A6)–VKOR with plasmid pcDNA3.1/Hygro-GGCX; 18 resistant colonies were selected for analysis. We also transfected HEK293-FX (A6)–GGCX with pIRESpuro3-VKOR; from this selection only 1 resistant colony was obtained. Finally, we transfected HEK293-FX (A6) simultaneously with both pIRESpuro3-VKOR and pcDNA3.1/Hygro-GGCX; in this case, we got 10 resistant colonies. The 29 isolated colonies were then assayed for both VKOR and GGCX activity and the colony with the highest levels of both activities was selected for further analysis.
Expression of FX from each cell line in roller bottles. We grew the 4 stable cell lines HEK293-FX (A6), HEK293-FX (A6)–VKOR, HEK293-FX (A6)–GGCX, and HEK293-FX (A6)–VKOR-GGCX to confluency in T 225 flasks and transferred them into roller bottles. At 24 and 36 hours, we replaced the media with serum-free media containing vitamin K1 at 6 μg/mL. We then collected the medium from each cell line every 24 hours until a total of 3 liters was obtained.
Purification of FX from each cell line. FX was purified from conditioned media of each cell line using a 3-step chromatographic method (Q-sepharose Fast Flow, FX immunoaffinity [G3], and Bio-Scale CHT5-I Hydroxyapatite) as described.14,16
Gla analysis of pooled fractions from each cell line. The Gla content of each peak of the hydroxyapatite column for all 4 cell lines were analyzed as described.14
Analysis of mRNA expression levels for VKOR, GGCX, and FX among each cell line by using real-time quantitative PCR. Total RNA extraction and real-time quantitative (Q)–PCR amplification were performed as described.6 All samples were repeated in quadruplicate. Melting curves were analyzed for all samples. Five-fold serial dilutions were used for each recombinant plasmid (pIRESpuro3-VKOR, pcDNA3.1/Hygro-GGCX, and pCMV4-ss-pro-II-hFX) to generate standard curves. We used the following primers: VKOR forward primer: 5′-CAGCTATTGTTAGGTTGCCTGCGG; VKOR reverse primer: 5′-GCTCACGTTGATAGCATAGGTGGTG; GGCX forward primer: 5′-CCATAGGAGGAATGCCCACG; GGCX reverse primer: 5′-AGCCAGTGCCGGGACAAATA; HFX forward primer: 5′-AGGGGACCGGAACACGGAGC; and HFX reverse primer: 5′-GGTGGACTGCCGGCCCTTCT. β-actin was used as the internal control. Its primers used were those suggested by MJ Research.17
Results
FX expression
Because it was necessary to select for cells overproducing VKOR, GGCX, or both VKOR and GGCX, it is possible that the level of FX expressed in the selected cells might differ from that of the starting cells. To circumvent this, these cDNAs were introduced into the same FX cell line (clone A6) that was overexpressing this protein (6-9 μg FX/106 cells/24 hours). Each of the resulting established cell lines (FX (A6), FX (A6)–VKOR, FX (A6)–GGCX, and FX (A6)–VKOR-GGCX) were expressing relatively equal amount of FX protein (data not shown).
VKOR and GGCX activities for each cell line
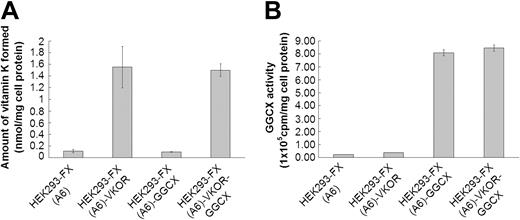
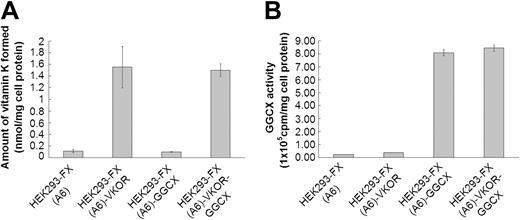
Figure 1 show VKOR and GGCX activities for the 4 cell lines selected for high expression (HEK293-FX (A6), HEK293-FX (A6)–VKOR, HEK293-FX (A6)–GGCX, HEK293-FX (A6)–VKOR-GGCX), respectively. These data demonstrate that there is at least a 10-fold overexpression of the introduced genes in each of the cell lines.
Analysis of mRNA expression levels of VKOR, GGCX, and FX by real-time Q-PCR
To confirm the results of our enzyme-linked immunosorbent assay (ELISA) and activity measurements, the relative amounts of VKOR, GGCX, and FX mRNA were determined for each cell line. The amount of mRNA expressed was normalized to β-actin mRNA. Table 1 shows that the mRNA for FX (A6) is similar for all 4 cell lines; this is consistent with our results on the expression levels determined by ELISA. The cell lines transfected with VKOR cDNA contained 10 times more VKOR mRNA than did the starting cell line. In the case of transfection with GGCX cDNA, both cell lines had 86 times more mRNA than the endogenous level.
Analysis of the efficiency of carboxylation in the differentcell lines
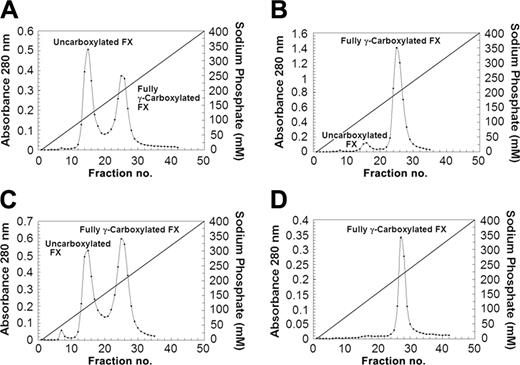
We have previously shown that FX may be separated into carboxylated and uncarboxylated species by chromatography on hydroxyapatite.14 For analysis of the carboxylation state of the FX in the different cell lines, 3 L media were collected from cells grown in roller bottles and the FX from each cell line was purified by Q-sepharose and antibody affinity chromatography as described in “Materials and methods.” Three to 4 mg/L FX was recovered from each liter of conditioned medium. We then used hydroxyapatite chromatography to separate carboxylated FX from noncarboxylated FX. Two protein peaks were obtained. As previously observed, the first peak contains uncarboxylated FX, while the second pool is composed of fully γ-carboxylated human FX. Figure 2 depicts the separation of uncarboxylated and fully γ-carboxylated human FX from each of the 4 cell lines. Fifty-two percent of the FX was carboxylated in the starting HEK293-FX (A6) cell line. VKOR alone was able to increase the percentage of carboxylated FX to 92%. GGCX was only marginally improved (57%) while the cell line with both VKOR and GGCX was essentially fully carboxylated.
VKOR and GGCX activity for the various cell lines. (A) Vitamin K reduced from vitamin K epoxide by the VKOR was measured as previously described (n = 3).6 HEK293-FX (A6): HEK293 cells over-expressing factor X. HEK293-FX (A6)–VKOR: HEK293 cells overexpressing both factor X and the VKOR. HEK293-FX (A6)–GGCX: HEK293 cells overexpressing both factor X and GGCX. HEK293-FX (A6)–VKOR-GGCX: HEK293 cells overexpressing factor X, VKOR, and GGCX. (B) GGCX activity for the following cell lines as defined in panel A: HEK293-FX (A6), HEK293-FX (A6)–VKOR, HEK293-FX (A6)–GGCX, HEK293-FX (A6)–VKOR-GGCX. GGCX activity toward the pentapeptide substrate FLEEL was measured as described (n = 3). Data are presented as mean ± SD.1
VKOR and GGCX activity for the various cell lines. (A) Vitamin K reduced from vitamin K epoxide by the VKOR was measured as previously described (n = 3).6 HEK293-FX (A6): HEK293 cells over-expressing factor X. HEK293-FX (A6)–VKOR: HEK293 cells overexpressing both factor X and the VKOR. HEK293-FX (A6)–GGCX: HEK293 cells overexpressing both factor X and GGCX. HEK293-FX (A6)–VKOR-GGCX: HEK293 cells overexpressing factor X, VKOR, and GGCX. (B) GGCX activity for the following cell lines as defined in panel A: HEK293-FX (A6), HEK293-FX (A6)–VKOR, HEK293-FX (A6)–GGCX, HEK293-FX (A6)–VKOR-GGCX. GGCX activity toward the pentapeptide substrate FLEEL was measured as described (n = 3). Data are presented as mean ± SD.1
Gla analysis of each pool from 4 kinds of cell line
To confirm the γ-carboxylation state of FX of the fractions eluting from the hydroxyapatite column, Gla analysis was performed. As expected, in the first peak essentially no Gla was detected, while the Gla content of the second peak was comparable to plasma-derived FX (Table 2). FX from HEK293-FX (A6)–VKOR-GGCX cells eluted from hydroxyapatite as one peak, whose Gla content was comparable with the Gla content of plasma-derived factor X.
Discussion
In this study, we investigated the effect of coexpressing VKOR and GGCX on the efficiency of carboxylation of vitamin K–dependent proteins expressed in HEK 293 cells. For each transfection, we selected clones that expressed similar levels of FX and that exhibited significant overexpression of VKOR, GGCX, or VKOR and GGCX. Our goal is to extend our understanding of the mechanism of carboxylation to an in vivo system. According to our present understanding, GGCX is the enzyme that accomplishes the carboxylation reaction.18 The role of VKOR is to convert vitamin K epoxide back to vitamin K and, perhaps, vitamin K hydroquinone.19
Separation of γ-carboxylated and uncarboxylated FX by hydroxyapatite chromatography. Separation of the γ-carboxylated and uncarboxylated factor X is as described in “Materials and methods” and as previously described.14 Cell line abbreviations are as defined in Figure 1. In each panel the first peak represents the uncarboxylated factor X and the second peak is fully γ-carboxylated factor X. (A) HEK293-FX (A6). (B) HEK293-FX (A6)–VKOR. (C) HEK293-FX (A6)–GGCX. (D) HEK293-FX (A6)–VKOR-GGCX. Diagonal lines indicate sodium phosphate concentration gradient of elution.
Separation of γ-carboxylated and uncarboxylated FX by hydroxyapatite chromatography. Separation of the γ-carboxylated and uncarboxylated factor X is as described in “Materials and methods” and as previously described.14 Cell line abbreviations are as defined in Figure 1. In each panel the first peak represents the uncarboxylated factor X and the second peak is fully γ-carboxylated factor X. (A) HEK293-FX (A6). (B) HEK293-FX (A6)–VKOR. (C) HEK293-FX (A6)–GGCX. (D) HEK293-FX (A6)–VKOR-GGCX. Diagonal lines indicate sodium phosphate concentration gradient of elution.
Patients who overdose on warfarin can be rescued by treatment with vitamin K.9,10 Because VKOR is inactivated by warfarin, this indicates that other enzymes not sensitive to warfarin, such as the nicotinamide adenine dinucleotide (NAD)–dependent deoxythymidine (DT)–diaphorase,11 can convert vitamin K to reduced vitamin K. Therefore, it was expected that in the presence of adequate vitamin K, coexpression of GGCX would greatly improve the amount of fully carboxylated vitamin K recombinant protein produced in cell culture because the alternative pathway could provide sufficient reduced vitamin K for the reaction. However, after the purification and cloning of GGCX,1,12 Rehemtulla et al13 reported that coexpression of factor IX and GGCX in cell culture did not improve the fraction of fully carboxylated FIX. Recently, Wajih et al20 concluded that VKOR catalyzed the rate-limiting step in the carboxylation reaction. This conclusion was based on experiments using extracts from cell lines overexpressing exogenous VKOR, GGCX, or VKOR and GGCX. The in vitro carboxylation of the small substrate FLEEL was increased in extracts from cells overexpressing VKOR only. To determine if these results held true in a system mimicking the in vivo situation, we used a HEK 293 cell line expressing chimeric FX whose propeptide was that of prothrombin.14 We characterized FX from cell lines transfected with the substrate and either VKOR, GGCX, or both enzymes.
In the HEK 293 cell line expressing only basal levels of VKOR and GGCX activity (no transfection), 52% of the FX was carboxylated. Transfecting the same cell lines with GGCX increased the level of carboxylated FX marginally to 57%. In contrast, cotransfection of these cells with VKOR increased the fraction of carboxylated FX to 92%. Cotransfection with both VKOR and GGCX resulted in essentially complete carboxylation of the secreted FX. The FX expression levels for each of these cell lines were essentially equivalent based on an FX-specific ELISA and based on mRNA levels also. However, when both VKOR and GGCX were transfected into the HEK 293 cell line overproducing factor X, we recovered less purified factor X compared with the other 3 cell lines. This may be attributed to the fact that we measured FX in cells grown in T25 flasks, but the protein was collected from cells grown in roller bottles. These cells (HEK 293-FX (A6)–VKOR-GGCX) did not adhere well to the roller bottles and significant numbers of cells were lost each time we collected medium. While our paper was under review, Wajih et al reported that factor IX expression in baby hamster kidney (BHK) cells could also be helped by coexpressing VKOR.21 Both this work and that of Hallgren et al22 and Rehemtulla et al13 report that GGCX expression actually decreases the amount of functional, carboxylated, vitamin K–dependent protein secreted from the cells. In fact, we recovered slightly more factor X from the cell line overexpressing GGCX than from any of the other cell lines. Our only conclusion from this is that, in our system, GGCX does not reduce the production of a vitamin K–dependent protein.
The results presented here indicate that coexpressing VKOR and FX dramatically improved the extent of carboxylation in cell culture. In our experiments, the simplest explanation for the dramatic increase in carboxylation observed when VKOR is coexpressed with the cell line overproducing FX is that VKOR is responsible for both the conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin K hydroquinone. Since we provide vitamin K to the cells, it seems unlikely that the conversion of vitamin K epoxide to vitamin K is rate limiting. The simplest interpretation of our results is that, at least for the conditions used in this study, the conversion of vitamin K to vitamin K hydroquinone by VKOR is the rate-limiting step in the vitamin K cycle. This conclusion is consistent with several earlier publications. Gardill and Suttie23 concluded that both vitamin K epoxide and vitamin K are reduced by a common enzyme. It is also consistent with the report of Preusch and Smalley11 that the rate of conversion of vitamin K to reduced vitamin K is much faster with a dithiothreitol (DTT) catalyzed reaction (VKOR?) than with DT-diaphorase, which can convert vitamin K to reduced vitamin K. An alternative, although we feel less likely, explanation of our results is that sufficient vitamin K epoxide is produced during carboxylation and conversion of vitamin K to vitamin K hydroquinone is fast. In this scenario conversion of vitamin K epoxide produced during carboxylation to vitamin K would be rate limiting.
In addition to the mechanistic implications of this research, there are also practical implications. At present substantial needs exist for (1) recombinant human FIX to treat hemophilia B patients; (2) FVIIa for treating patients with autoantibodies (inhibitors) to either FIX or FVIII and for bleeding that results from general trauma24 ; and (3) activated protein C, for the treatment of sepsis.25,26 To date, these vitamin K–dependent proteins are produced in cell cultures with CHO, BHK, and human embryo kidney cells (HEK 293). A common problem for all these cell lines is that, if significant overproduction is achieved, a significant fraction of the recombinant protein produced is undercarboxylated and therefore inactive. Including VKOR or both VKOR and GGCX in the cell lines expressing these important enzymes should greatly increase the yield of active enzyme.
In summary, VKOR appears to be the rate-limiting enzyme for carboxylation of FX in the system that was used for these experiments. However, there will undoubtedly be circumstances where the amount of GGCX will be limiting. For example, the particular cell system that we used for this study should exhibit a relatively rapid steady-state turnover because our FX bears the propeptide of prothrombin and the affinity of the propeptide of prothrombin for GGCX is about 40-fold less than that of the FX propeptide. It seems likely, then, that when overproduction of a particular vitamin K protein is achieved, and sufficient reduced vitamin K (as opposed to vitamin K) is available, there will still be undercarboxylated substrate and the percentage of carboxylated product can be increased by concurrently overproducing GGCX. A logical way to test this hypothesis is to use one of the cell lines described in our previous paper14 that overexpresses FX with its own propeptide. Because the difference in affinity translates into a difference in steady-state turnover, we expect that coexpressing GGCX will have a significant effect on in vivo carboxylation of an FX bearing its own propeptide.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-06-2495.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.