Comment on Warkentin et al, page 3791
In this issue of Blood, Warkentin and colleagues demonstrate that fondaparinux elicits an immune response to PF4/heparin. Antibodies induced by the drug do not recognize complexes of PF4/fondaparinux but, paradoxically, bind complexes of PF4/heparin and PF4/LMWH.
In 1979, Rosenberg and Lam1 noted that the anticoagulant activity of heparin resides in only a subpopulation of heparin molecules that bind antithrombin. They subsequently identified an invariant tetrasaccharide sequence responsible for heparin's biologic activity.1 A modified version of this sequence has since been developed as a synthetic pentasaccharide compound (fondaparinux; molecular weight [MW] = 1728) with potent factor Xa inhibitory activity. In several large clinical trials, fondaparinux has proven to be comparable to low-molecular-weight heparin (LMWH) in safety and efficacy for the prevention and treatment of venous thromboembolism.
The clinical trials of fondaparinux, however, did not directly address one important biologic aspect of this heparin-like drug, its immunogenicity and potential for initiating heparin-induced thrombocytopenia (HIT), a life-threatening complication caused by platelet factor 4 (PF4)/heparin antibodies. PF4/heparin antibodies are frequently induced by heparin therapy and, depending on the clinical setting, occur in approximately 8% to 21% of medical patients exposed to unfractionated heparin (UFH) and in 2% to 8% of patients treated with LMWH.2 Although the immune pathogenesis of HIT is not well understood, it is recognized that a subset of seropositive patients will develop clinical disease.FIG1
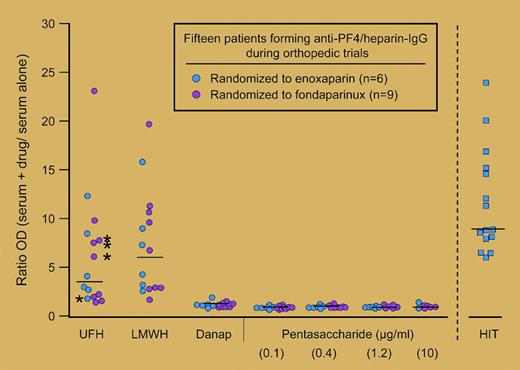
Ratio of antibody binding to PF4/polysaccharide complexes compared to PF4 alone by fluid-phase EIA. See the complete figure in the article beginning on page 3791.
Ratio of antibody binding to PF4/polysaccharide complexes compared to PF4 alone by fluid-phase EIA. See the complete figure in the article beginning on page 3791.
The limited interactions of fondaparinux with PF43 and lack of serologic cross-reactivity of drug with preformed HIT antibodies4 have led to the general perception that fondaparinux is likely nonimmunogenic. In this issue of Blood, Warkentin and colleagues dispel this notion through serologic investigations of patients enrolled in 2 large orthopedic trials (Pentathlon5 and Pentamaks6 ) comparing enoxaparin to fondaparinux for thromboprophylaxis after surgery. For their analysis, samples from over 2700 patients (or ∼80% of the overall study population from the 2 trials) were assayed for PF4/heparin antibodies at baseline and at days 5 to 9 of anticoagulant therapy and correlated with clinical outcomes.
There were several surprising but clinically noteworthy findings in this study. The authors document similar seroconversion rates in both treatment groups. PF4/heparin antibodies were detected in 1.5% and 2.8% of fondaparinux-treated patients compared with 1.1% and 5.2% of the enoxaparin-treated patients (Pentathlon and Pentamaks trials, respectively; P value not significant for fondaparinux vs enoxaparin). Antibodies induced by fondaparinux were comparable to those induced by enoxaparin with respect to immunoglobulin G (IgG) isotype and capacity to trigger UFH-dependent platelet activation. There were no cases of clinical HIT among the seropositive patients in either treatment group. The most intriguing findings of this study were related to the serologic specificities of fondaparinux-induced antibodies. As shown in the figure, fondaparinux-induced antibodies do not bind complexes of PF4/fondaparinux but, rather, show reactivity toward complexes of PF4/UFH and PF4/enoxaparin.
Based on these findings, the authors conclude that fondaparinux-sensitized patients are at low risk for developing HIT, as antibodies do not bind PF4/fondaparinux complexes and therefore cannot induce drug-dependent platelet activation. This conclusion is likely premature for several reasons. In the original reports of the 2 clinical trials,5,6 thrombocytopenia (< 100 × 109/L) was reported in 2% to 4% of patients receiving either drug. Whether thrombocytopenia in these 2 clinical trials was drug induced or associated with development of PF4/heparin antibodies was not addressed by Warkentin et al. This lack of clinical and serologic information on thrombocytopenic patients is an important limitation of this study and makes it difficult to estimate the true risk of clinical HIT associated with either fondaparinux or enoxaparin. Because of the cross-reactivity profile of fondaparinux-induced PF4/heparin antibodies, it is clear that sensitized patients are at risk for developing HIT if they are subsequently exposed to UFH or LMWH. Fondaparinux-sensitized patients are also theoretically at risk for developing subacute or delayed-onset HIT, as PF4/heparin antibodies can often elicit disease in the absence of circulating drug.7 Additional prospective studies and experience with fondaparinux are needed to understand the true clinical significance of the immune response invoked by this new agent. Until then, the sensitizing effects of fondaparinux and its potential for inducing clinical HIT must be taken seriously. ▪