Abstract
Natural killer (NK) cells are critical in the first-line defense against viral infections. Chronic HIV-1 infection leads to a perturbation in the NK cell compartment, yet the kinetics of this deregulation and the functional consequences are unclear. Here, we characterized changes in the NK cell compartment longitudinally by multiparameter flow cytometry, starting in acute HIV-1 infection. Acute HIV-1 infection was associated with elevated NK cell numbers, with an expansion of CD3negCD56dimCD16pos NK cells and an early depletion of CD3negCD56brightCD16neg NK cells. Ongoing viral replication resulted in a depletion of CD3negCD56dimCD16pos NK cells with a paralleled increase in functionally anergic CD3negCD56negCD16pos NK cells, accompanied by reduced functional activity, as measured by CD107a expression and cytokine secretion. Taken together, these studies demonstrate a sequential impairment of NK cell function with persistent viral replication resulting from a progressive deregulation of NK cell subsets with distinct functional properties.
Introduction
Natural killer (NK) cells are critical in the first-line defense of the immune system against viral infections.1 Three subsets of NK cells can be defined by their differential expression of CD56 and CD16. The largest subset is comprised of cytolytic CD3negCD56dimCD16pos NK cells.2 A second subset includes the immunoregulatory CD3negCD56brightCD16neg NK cells able to secrete large quantities of cytokines and chemokines.3 Finally, the last subset consists of the recently described CD3negCD56negCD16pos NK cells.4,5
Significant changes have been observed within the NK cell compartment during chronic HIV-1 infection, such as a decline in the proportion of CD3negCD56pos cells as well as an expansion of CD3negCD56negCD16pos NK cells.4,6-8 In addition, several reports have demonstrated functional impairment of NK cells, including reduced cc-chemokine production,9-11 antibody-dependent cellular cytotoxicity (ADCC),12,13 and changes in cytokine secretion.6,8,14,15 Overall, these data suggest significant changes within the NK cell compartment in chronic viremic HIV-1 infection. However, little is known about the NK cell compartment during acute infection and the kinetics of these deregulations. Here, we report that distinct changes in the NK cell compartment start in acute HIV-1 infection, progressively leading to a depletion of functionally active NK cells.
Study design
Study subjects
Sixty-nine subjects were included in this study, including 14 control subjects not infected with HIV-1, 45 subjects with chronic HIV-1 infection, and 10 with acute HIV-1 infection (Table 1). Acute infection was defined by a negative HIV-1 enzyme-linked immunosorbent assay (ELISA) or a positive ELISA but fewer than 3 bands in an HIV-1 Western blot in the presence of detectable HIV-1 RNA. Of the 45 subjects with chronic infection, 11 were successfully treated with highly active antiretroviral therapy (HAART) for at least 6 months (“treated aviremic”), 9 individuals were treated with HAART but not fully virologically suppressed (“treated viremic”), 14 subjects were untreated and had progressive HIV-1 infection (“untreated viremic”), and 11 subjects had nonprogressive chronic infection defined by controlled viral replication in the absence of HAART for more than 6 years (Table 1).
The Massachusetts General Hospital (MGH) Institutional Review Board approved the study, and each subject gave informed consent for participation in the study.
Flow cytometric analysis of intracellular cytokine production and CD107a
NK cell subpopulations were defined by the expression of CD3, CD56, and CD16. The frequency of degranulating (CD107a expression) and interferon γ (IFN-γ)–secreting NK cells was quantified by multiparameter intracellular cytokine staining, as described previously.8,16 A response was considered positive if the frequency of CD107a expressing or cytokine secreting stimulated NK cells was at least 3-fold greater than in unstimulated controls.16
Statistical analysis
Differences between multiple groups were performed by using a one-way analysis of variance (ANOVA) with a Tukey correction.
Results and discussion
Total NK cell numbers are expanded in acute HIV-1 infection
HIV-1 infection has been shown to lead to a loss of CD3negCD56pos NK cell numbers.4,6-8 Recently, the accumulation of CD3neg CD56negCD16pos NK cells has been described in chronic HIV-1 infection.5 We therefore compared changes in the NK cell compartment during different stages of HIV-1 infection and in control subjects not infected with HIV-1. No differences in the total proportion of NK cells, including all 3 subsets of CD3negCD56bright CD16neg, CD3negCD56dimCD16pos, and CD3negCD56negCD16pos NK cells, were observed among the groups of subjects with chronic infection or control subjects without infection (Figure 1A). In contrast, the total proportion of NK cells in subjects with acute HIV-1 infection was significantly increased (Figure 1A; P < .05 for all comparisons). Taken together, these data suggest that the overall NK cell population is expanded during acute HIV-1 infection, but it returns to levels similar to those seen in control subjects not infected with HIV-1 in subjects with chronic HIV-1 infection.
Loss of CD3negCD56pos and accumulation of CD3negCD56negCD16pos NK cells in chronic HIV-1 infection results in an overall stability of the NK cell pool
To gain a more comprehensive understanding of the individual contribution of the 3 NK cell subsets to the total number of NK cells, we studied the relative proportions of these different subsets during acute and chronic HIV-1 infection. CD3negCD56brightCD16neg and CD3negCD56dimCD16pos NK cells were significantly reduced in individuals with chronic HIV-1 infection with viremic progressive infection (Figure 1B) compared with negative control subjects (P < .001 and P < .05, respectively). In contrast, CD3negCD56neg CD16pos NK cells were significantly expanded in individuals with chronic progressive HIV-1 infection compared with subjects negative for HIV-1 (P < .05). However, subset distributions were similar in individuals with nonprogressive HIV-1 infection and negative control subjects and could be partially reconstituted in individuals successfully treated with HAART. These data demonstrate dramatic redistributions in the NK cell compartment during chronic progressive HIV-1 infection but not in subjects with nonprogressive infection. Additionally, in contrast to previous studies describing an overall loss of NK cells in chronic HIV-1 infection, the more detailed assessment of NK cells, including the subset of CD56negCD16pos NK cells, confirms a loss of CD3negCD56pos NK cells, yet the overall number of NK cells remains stable in chronic HIV-1 infection because of a concurrent accumulation of CD3negCD56negCD16pos NK cells.
Deregulations within the NK cell compartment that are initiated in acute HIV-1 infection are partially recovered by early initiation of HAART
To characterize the kinetics by which the redistributions within the NK cell compartment occur, we extended our studies to 10 individuals identified during acute HIV-1 infection. Compared with negative control subjects and individuals with progressive HIV-1 infection, subjects with acute infection experienced a significant expansion of CD3negCD56dimCD16pos NK cells during acute infection (Figure 1B). In contrast, the CD3negCD56brightCD16neg NK cells were significantly reduced in acute infection as opposed to negative control subjects (P = .01). Although there was a slight increase in the proportion of the CD3negCD56negCD16pos NK cells in acute infection, this difference was not significant when compared with control subjects negative for HIV-1 and significantly lower than in subjects with untreated chronic HIV-1 infection (P = .005), suggesting the accumulation of this subset of cells occurs with protracted exposure to viremia.
Distribution and longitudinal changes in the NK cell subsets. (A) The dot plot represents the proportion of total NK cells (including CD3negCD56brightCD16neg, CD3negCD56dimCD16pos, and CD3negCD56negCD16pos) for subjects with acute infection, all groups with chronic infection, and control subjects without HIV-1 infection. (*The percentage of NK cells was significantly [P < .05 using ANOVA] increased in individuals with acute HIV-1 infection). (B) The whisker box plots represent the proportion of each subset of CD3negCD56brightCD16neg (▧), CD3negCD56dimCD16pos (▦), and CD3negCD56negCD16pos (□) NK cells for all groups with chronic HIV-1 infection, control subjects, and subjects with acute HIV-1 infection. Numbers indicate the median percentage. (C) Whisker box plots depict the distribution of the 3 NK cell subsets after 1 year of follow-up for 5 subjects that received antiretroviral therapy in acute HIV-1 infection and 5 subjects that remained off therapy. Error bars represent 95% confidence intervals about the mean.
Distribution and longitudinal changes in the NK cell subsets. (A) The dot plot represents the proportion of total NK cells (including CD3negCD56brightCD16neg, CD3negCD56dimCD16pos, and CD3negCD56negCD16pos) for subjects with acute infection, all groups with chronic infection, and control subjects without HIV-1 infection. (*The percentage of NK cells was significantly [P < .05 using ANOVA] increased in individuals with acute HIV-1 infection). (B) The whisker box plots represent the proportion of each subset of CD3negCD56brightCD16neg (▧), CD3negCD56dimCD16pos (▦), and CD3negCD56negCD16pos (□) NK cells for all groups with chronic HIV-1 infection, control subjects, and subjects with acute HIV-1 infection. Numbers indicate the median percentage. (C) Whisker box plots depict the distribution of the 3 NK cell subsets after 1 year of follow-up for 5 subjects that received antiretroviral therapy in acute HIV-1 infection and 5 subjects that remained off therapy. Error bars represent 95% confidence intervals about the mean.
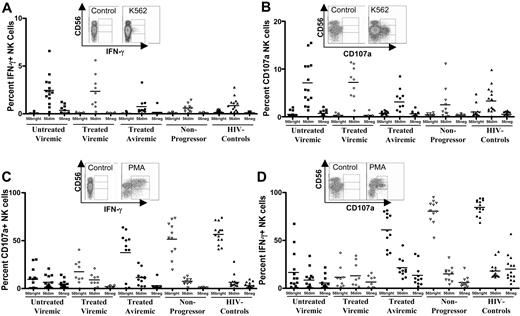
Functional capacity of NK cell subsets in chronic HIV-1 infection. Primary flow data and dot plots represent pattern of IFN-γ secretion (A) and CD107a expression (B) following stimulation with MHC-devoid K562 cells. Similarly, primary flow cytometry results and dot plots represent the level of IFN-γ secretion (C) and CD107a up-regulation (D) following stimulation with phorbol 12-myristate-13-acetate (PMA)/ionomycin for the 3 subsets of NK cells for the untreated viremic (▪), treated viremic (⋄), treated aviremic (•), nonprogressors (▿), and subjects without HIV-1 infection (▴).
Functional capacity of NK cell subsets in chronic HIV-1 infection. Primary flow data and dot plots represent pattern of IFN-γ secretion (A) and CD107a expression (B) following stimulation with MHC-devoid K562 cells. Similarly, primary flow cytometry results and dot plots represent the level of IFN-γ secretion (C) and CD107a up-regulation (D) following stimulation with phorbol 12-myristate-13-acetate (PMA)/ionomycin for the 3 subsets of NK cells for the untreated viremic (▪), treated viremic (⋄), treated aviremic (•), nonprogressors (▿), and subjects without HIV-1 infection (▴).
The effect of persistent viral replication on the composition of the NK cell compartment was further monitored longitudinally over 1 year in these 10 subjects with acute infection. Five subjects initiated HAART at baseline, resulting in the suppression of viral replication below the level of detection, whereas the remaining 5 individuals did not initiate HAART during the 12-month study period. The proportion of CD3negCD56brightCD16neg NK cells remained reduced in subjects with persistent viral replication but increased significantly in individuals treated during acute infection (P = .01) (Figure 1C). In contrast, the proportion of CD3negCD56neg CD16pos NK cells significantly increased in the untreated acute subjects after 1 year of follow-up (P = .01) (Figure 1C) but did not change significantly in subjects who received therapy (P = .45) (Figure 1C). Taken together, these data demonstrate that the early depletion of CD3negCD56bright NK cells during acute HIV-1 infection is followed by a delayed secondary loss of CD3negCD56dim CD16pos NK cells and an accumulation of CD3negCD56negCD16pos NK cells in the setting of persistent viral replication. In contrast, initiation of HAART during acute infection resulted in a partial normalization within the NK cell compartment.
Functional differences among the 3 subsets of NK cells in chronic HIV-1 infection
To further elucidate the functional properties of these 3 subsets of NK cells, we evaluated the ability of the different subsets to recognize major histocompatability complex (MHC)–devoid target cells (Figure 2A-B) and to respond to mitogenic stimulation in chronic infection (Figure 2C-D) by measuring intracellular IFN-γ production and CD107a expression. Following stimulation with MHC-devoid K562 cells, CD107a expression and cytokine secretion were up-regulated only in the killer immunoglobulin-like receptor (KIR)–expressing CD3negCD56dimCD16pos NK cells but not in the CD3negCD56brightCD16neg NK cells (Figure 2A-B). In contrast, both CD3negCD56dimCD16pos and CD3negCD56brightCD16neg NK cells responded to stimulation with PMA/ionomycin (Figure 2C-D). Interestingly, the CD3negCD56negCD16pos NK cells were not able to secrete cytokine or express CD107a following stimulation with MHC-devoid target cells and responded only to a very limited extent to stimulation with PMA/ionomycin (Figure 2A-D).
Comparison of the different study groups demonstrated that CD107a expression and cytokine secretion by CD3negCD56dim CD16pos NK cells following stimulation with K562 cells were significantly elevated in subjects infected with HIV-1 with ongoing viral replication compared with subjects infected with HIV-1 with undetectable viral loads, nonprogressors, or uninfected control subjects (P < .05 for all comparisons; Figure 1A-B). This observation was associated with an elevated expression of KIR molecules on NK cells in viremic as compared with aviremic subjects (63% versus 34%; P < .001).4 In contrast, the ability of NK cells to respond to PMA/ionomycin was severely compromised in subjects with chronic progressive infection, whereas the ability to respond to this stimulus appeared to be unimpaired in nonprogressors (P < .001). These data demonstrate a differential effect of persistent viremia on the NK cell response, resulting in an increased ability to respond to stimulation with K562 cells associated with increased KIR expression, but an overall reduced responsiveness to stimulation with mitogen. Overall, we observed an early loss of immune-regulatory, cytokine-secreting CD56bright NK cells, followed by a contraction of the cytolytic CD56dim NK effector cells, paralleled by an accumulation of a functionally anergic subset of CD56neg NK cells in progressive chronic infection. These changes in NK cell distributions had an important effect on the deregulation in the functional activity of this cell compartment at different stages of infection. Finally, individuals with nonprogressive chronic HIV-1 infection who maintain normal NK cell subset distributions also manifest adequate functional NK cell responses to stimulation with PMA/ionomycin or K562 cells.
In summary, these data demonstrate that HIV-1 infection leads to an initial expansion of NK cells during acute infection, followed by sequential depletions of functional NK cell subsets with a concurrent expansion of functionally anergic NK cells. The early expansion of cytolytic CD3negCD56dim NK cells during acute infection, prior to the development of strong adaptive T-cell responses,17 may contribute to the dramatic reduction in viral load observed during that period and emphasizes the need for more detailed studies of the role of the innate immune system in the control of HIV-1 replication. Furthermore, our data demonstrate that chronic immune activation may not only result in the impairment of adaptive T- and B-cell responses18-20 but also has a severe effect on the effector cells of the innate immune system.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-03-1100.
Supported by the Harvard Medical School Center for AIDS Research (CFAR).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Distribution and longitudinal changes in the NK cell subsets. (A) The dot plot represents the proportion of total NK cells (including CD3negCD56brightCD16neg, CD3negCD56dimCD16pos, and CD3negCD56negCD16pos) for subjects with acute infection, all groups with chronic infection, and control subjects without HIV-1 infection. (*The percentage of NK cells was significantly [P < .05 using ANOVA] increased in individuals with acute HIV-1 infection). (B) The whisker box plots represent the proportion of each subset of CD3negCD56brightCD16neg (▧), CD3negCD56dimCD16pos (▦), and CD3negCD56negCD16pos (□) NK cells for all groups with chronic HIV-1 infection, control subjects, and subjects with acute HIV-1 infection. Numbers indicate the median percentage. (C) Whisker box plots depict the distribution of the 3 NK cell subsets after 1 year of follow-up for 5 subjects that received antiretroviral therapy in acute HIV-1 infection and 5 subjects that remained off therapy. Error bars represent 95% confidence intervals about the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-03-1100/6/m_zh80220586520001.jpeg?Expires=1769377380&Signature=oMjeEY9rCs6tTskyhHZXvLkPmVpJFWGw2vT5Nc7imulYFMV3pGLgDS3eoyXvjNRmNpVOdPiXL2eUD5dOkJ5hZNPapFDy~N1XodRb2dcAPDRPN6UbYjkDPFG6hmlfTIMpbJAcG9d6PuyIEcVPm6TQzxCohCm9uhgZBYgOR-0pD1s8YsQl5dUQ1AB0FgIVHMkfSkKidFD1ZtvS4WDHxrC1OhbyERWIF6NtD3GsZEGXBrw-blGR5~Tdz2-u-QbaFZ2xX5A8QDKCkP4Vf2g0cTJGjnZ~zL8PW0N3pJcV~UKVfjV2SJDxqKXgwm1lmu8YsWkupGVWrZhUvAEG19RJpVecHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)