Abstract
Imatinib has revolutionized drug therapy of chronic myeloid leukemia (CML). Preclinical studies were promising but the results of clinical trials by far exceeded expectations. Responses in chronic phase are unprecedented, with rates of complete cytogenetic response (CCR) of more than 40% in patients after failure of interferon-α (IFN) and more than 80% in newly diagnosed patients, a level of efficacy that led to regulatory approval in record time. While most of these responses are stable, resistance to treatment after an initial response is common in more advanced phases of the disease. Mutations in the kinase domain (KD) of BCR-ABL that impair imatinib binding have been identified as the leading cause of resistance. Patients with CCR who achieve a profound reduction of BCR-ABL mRNA have a very low risk of disease progression. However, residual disease usually remains detectable with reverse transcription–polymerase chain reaction (RT-PCR), indicating that disease eradication may pose a significant challenge. The mechanisms underlying the persistence of minimal residual disease are unknown. In this manuscript, we review the preclinical and clinical development of imatinib for the therapy of CML, resistance and strategies that may help to eliminate resistant or residual leukemia.
BCR-ABL as a therapeutic target
Chronic myeloid leukemia (CML) is characterized by the presence of a BCR-ABL fusion gene, which is the result of a reciprocal translocation between chromosomes 9 and 22, cytogenetically visible as a shortened chromosome 22 (Philadelphia [Ph] chromosome).1,2 Rare patients with a clinical presentation indistinguishable from CML do not express BCR-ABL and were previously diagnosed as “BCR-ABL– CML.” Because this group of diseases is biologically different, the World Health Organization has defined CML as the BCR-ABL+ disease.3 To avoid confusion, patients without BCR-ABL expression should be diagnosed with “myeloproliferative syndrome, unclassifiable” if they do not fulfill diagnostic criteria of another entity.
Research in the past 2 decades has established that BCR-ABL is the causal to the pathogenesis of CML, and that constitutive tyrosine kinase activity is central to BCR-ABL's capacity to transform hematopoietic cells in vitro and in vivo.4,5 The activation of multiple signal transduction pathways in BCR-ABL–transformed cells leads to increased proliferation, reduced growth-factor dependence and apoptosis, and perturbed interaction with extracellular matrix and stroma. It is thought that the expression of BCR-ABL endows a pluripotent hematopoietic progenitor cell and/or its progeny with a growth and survival advantage over normal cells, which in time leads to the clinical manifestation of CML. An in-depth appraisal of the molecular and cellular biology of CML is beyond the scope of this review, and the reader is referred to recent comprehensive publications.6,7 The fact that it proved difficult to identify “essential” components downstream of BCR-ABL indicates that from the therapeutic standpoint BCR-ABL is by far the most attractive drug target.
Development of an ABL tyrosine kinase inhibitor
The essential role of BCR-ABL tyrosine kinase activity for cellular transformation provided the rational for targeting this function therapeutically.5 In 1992, Anafi and colleagues8,9 reported a tyrphostin, related to erbstatin, that inhibited the tyrosine kinase activity of BCR-ABL and suggested that it might be possible to design specific compounds for the treatment of ABL-associated human leukemias. Subsequently, the tyrphostins AG568, AG957, and AG1112 were identified as the most specific compounds. Growth inhibition of the CML cell line K562 occurred at micromolar concentrations and was associated with inhibition of BCR-ABL tyrosine kinase activity.10 Tyrphostins are competitive toward adenosine triphosphate (ATP) or substrate, or both. Although active in vitro, they have not been developed for clinical use. Another early compound with activity toward BCR-ABL is herbimycin A, an antibiotic derived from Streptomyces hygroscopicus.11 Herbimycin A was originally thought to inhibit BCR-ABL tyrosine kinase12 but it was subsequently shown to promote BCR-ABL protein degradation.13 Selective inhibition of primary CML cells was also shown for genistein, a flavonoid.14
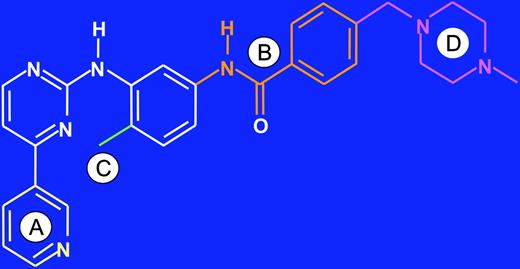
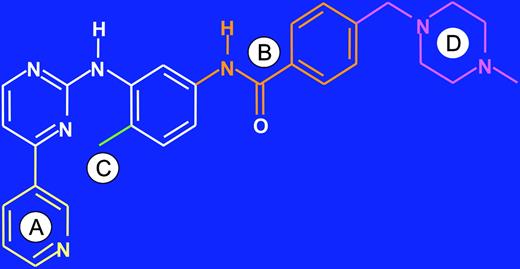
Starting in the late 1980s, scientists at Ciba Geigy (now Novartis), under the direction of N. Lydon and A. Matter, initiated projects on the identification of compounds with inhibitory activity against protein kinases. In one medicinal chemistry project focusing on protein kinase C (PKC) as a target, a 2-phenylaminopyrimidine derivative was identified as a lead compound (Figure 1).15,16 This compound had low potency and poor specificity, inhibiting both serine/threonine and tyrosine kinases, but from this starting point, a series of derivatives were synthesized. The addition of a 3′-pyridyl group at the 3′-position of the pyrimidine (Figure 1A) enhanced the cellular activity of the derivatives. Activity against tyrosine kinases was enhanced by introduction of a benzamide group at the phenyl ring (Figure 1B). A key observation from analysis of structure activity relationships was that substitutions at the 6-position of the anilino phenyl ring led to loss of PKC inhibition. However, the introduction of a “flag-methyl” group at this position retained or enhanced activity against tyrosine kinases (Figure 1C). Profiling of the compounds showed that they also inhibited the ABL tyrosine kinase. The first series of compounds had poor oral bioavailability, with low water solubility. The attachment of a highly polar side chain, N-methylpiperazine, markedly improved solubility and oral bioavailability (Figure 1D). STI571 (formerly CGP57148B, now imatinib mesylate; Gleevec or Glivec, Novartis, Basel, Switzerland) emerged as the most promising compound for clinical development, since it had the highest selectivity for growth inhibition of BCR-ABL–expressing cells.
Lead optimization. Development of imatinib from a 2-phenylaminopyrimidine backbone (shown in white). (A) Activity in cellular assays was improved by introduction of a 3′ pyridyl group (yellow) at the 3′ position of the pyrimidine. (B) Activity against tyrosine kinases was further enhanced by addition of a benzamide group (orange) to the phenyl ring. (C) Attachment of a “flag-methyl” group (green) ortho to the diaminophenyl ring strongly reduced activity against PKC. (D) Addition of an N-methylpiperazine (purple) increased water solubility and oral bioavailability.
Lead optimization. Development of imatinib from a 2-phenylaminopyrimidine backbone (shown in white). (A) Activity in cellular assays was improved by introduction of a 3′ pyridyl group (yellow) at the 3′ position of the pyrimidine. (B) Activity against tyrosine kinases was further enhanced by addition of a benzamide group (orange) to the phenyl ring. (C) Attachment of a “flag-methyl” group (green) ortho to the diaminophenyl ring strongly reduced activity against PKC. (D) Addition of an N-methylpiperazine (purple) increased water solubility and oral bioavailability.
In vitro profile of imatinib
Inhibition of kinase activity
Studies using purified enzymes expressed as bacterial fusion proteins or using immunoprecipitations of intact proteins showed that imatinib potently inhibits all of the ABL tyrosine kinases. This includes cellular ABL, viral ABL (v-ABL), and BCR-ABL (Table 1).17-19 In contrast, the compound was inactive against serine/threonine kinases, did not inhibit the epidermal growth factor (EGF) receptor intracellular domain, and showed weak or no inhibition of the kinase activity of the receptors for vascular endothelial growth factor (VEGF-R1 and VEGF-R2), fibroblast growth factor receptor 1 (FGF-R1), tyrosine kinase with immunoglobulin and EGF homology-2 (TIE-2 [TEK]), c-MET, and nonreceptor tyrosine kinases of the SRC family (FGR, LYN, and LCK).
The results of the kinase assays were confirmed in cell lines expressing constitutively active forms of ABL such as v-ABL,17 p210BCR-ABL,18 p185 BCR-ABL,20,21 and translocated ets leukemia (TEL)–ABL,20 where imatinib was found to inhibit ABL kinase activity with 50% inhibitory concentration (IC50) values ranging between 0.1 and 0.35 μM. Numerous Ph+ cell lines derived from patients with CML or acute lymphoblastic leukemia (ALL) have subsequently been tested. In most of these lines, the IC50 values were also in the range of 0.1 to 0.5 μM, indicating that the compound effectively penetrates the cell membrane.21-23
Consistent with its in vitro profile, imatinib inhibited signaling of the ligand-activated platelet-derived growth factor receptor (PDGFR),19,24 as determined by ligand-stimulated PDGFR autophosphorylation, at an IC50 of 0.1 to 1 μM. Inhibition of the constitutively active TEL-PDGFR fusion protein was observed at an IC50 of 0.15 μM.20 Furthermore, the compound potently inhibited autophosphorylation of the KIT receptor upon binding of its cognate ligand, stem-cell factor (SCF),19,25 and to suppress KIT autophosphorylation in a cell line established from a patient with a gastrointestinal stromal tumor (GIST) with an activating Kit mutation.26
In contrast, signal transduction mediated by EGF, insulin, insulin-like growth factor I (IGF-I), FGF, and phorbol ester was insensitive to imatinib.17 Furthermore, imatinib did not affect FLT-3 or the receptor for colony-stimulating factor 1 (CSF-1, FMS), or the nonreceptor tyrosine kinases SRC and JAK-2. The latter mediates signaling from a number of cytokine receptors, including the receptors for IL-3, G-CSF, and erythropoietin.19
Effects upon cell proliferation and apoptosis in vitro
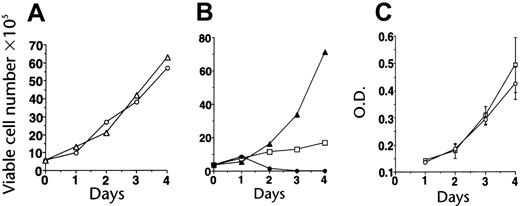
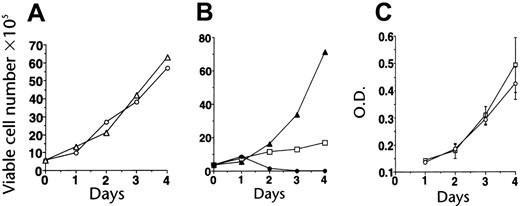
Effects on cells with activated ABL signaling. Imatinib was tested for its antiproliferative activity against a variety of cell lines expressing activated ABL proteins, including lines that had been transduced to factor independence by p210BCR-ABL as well as their growth factor–dependent parental cells.18 Imatinib potently inhibited the growth of cells expressing p210BCR-ABL, while up to 10 μM did not inhibit growth of parental or v-SRC–transformed cells (Figure 2). Similar activity was seen in p185BCR-ABL– and TEL-ABL–transformed cells.20 The selective growth-inibitory effect was confirmed in many cell lines derived from CML or Ph+ ALL,18,21,22,27,28 while Ph– cell lines were unaffected.21,27 The in vitro IC50 for inhibition of proliferation generally paralleled the IC50 values for inhibition of BCR-ABL kinase activity seen in cellular assays. Exposure to imatinib led to apoptotic cell death.18,27 Additional studies demonstrated activity in fresh leukemic cells from patients with CML and Ph+21,22 and selective inhibition of colony formation by committed progenitor cells from patients with CML, with little effect on normal hematopoiesis at concentrations of up to 1 μM.18,27 The latter findings were confirmed in stroma-dependent long-term cultures.29
Establishment of imatinib selectivity. (A) Imatinib (CGP57148) at a concentration of 10 μM does not inhibit the growth of 32D cells (32D + IL-3, ○; 32D + IL-3 + imatinib, ▵). (B) Imatinib at 1 μM inhibits the growth of 32D cells expressing BCR-ABL (32Dp210); the effect is partially reversible with IL-3 (32Dp210 alone, ▴; + imatinib, ○; + imatinib + IL-3, □). (C) Imatinib does not affect the growth of 32D cells transformed by SRC (32Dv-SRC alone, □; + 10 μM imatinib, ○). Reproduced from Druker et al18 with permission from Nature, copyright 1996. O.D. indicates optical density. Error bars indicate mean ± standard deviation.
Establishment of imatinib selectivity. (A) Imatinib (CGP57148) at a concentration of 10 μM does not inhibit the growth of 32D cells (32D + IL-3, ○; 32D + IL-3 + imatinib, ▵). (B) Imatinib at 1 μM inhibits the growth of 32D cells expressing BCR-ABL (32Dp210); the effect is partially reversible with IL-3 (32Dp210 alone, ▴; + imatinib, ○; + imatinib + IL-3, □). (C) Imatinib does not affect the growth of 32D cells transformed by SRC (32Dv-SRC alone, □; + 10 μM imatinib, ○). Reproduced from Druker et al18 with permission from Nature, copyright 1996. O.D. indicates optical density. Error bars indicate mean ± standard deviation.
Effects on cell lines with activated PDGFR or c-KIT signaling. Imatinib strongly inhibited proliferation of v-sis–transformed BALB/c 3T3 mouse fibroblasts, which proliferate autonomously due to autocrine PDGF production.24 Furthermore, the compound dose-dependently suppressed PDGF-stimulated proliferation of A10 rat aorta smooth muscle cells but did not affect serum-induced growth.19 Cells expressing a TEL-PDGF receptor fusion protein were also imatinib sensitive.20
Growth inhibitory activity was also seen against a cell line established from a patient with a GIST, which contained an activating point mutation in KIT.26 In contrast, no activity was seen in a cell line derived from human malignant mastocytosis with a mutation in the activation loop (A-loop) of the kinase (D816V).30 Thus, this specific mutation activates the kinase while conferring imatinib resistance at the same time. Imatinib was also found to inhibit SCF-mediated growth of small-cell lung cancer cell lines. The IC50 values for inhibition of KIT autophosphorylation and proliferation were 0.1 and 0.3 μM, respectively, in serum-free media, whereas growth inhibition in serum-containing media required higher drug levels and was not strictly correlated with KIT expression (IC50 in the range of 5 μM), suggesting that additional targets were modified.31,32
In vivo profile of imatinib: animal models
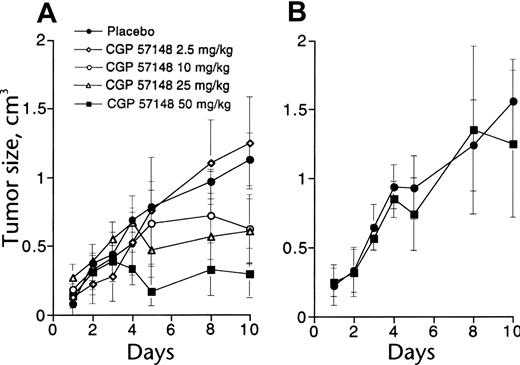
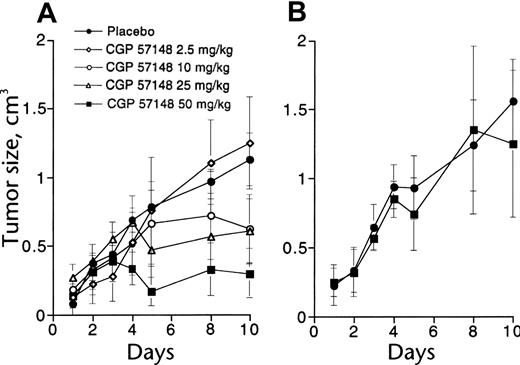
To test the effects of imatinib on tumor growth, BCR-ABL–transformed 32D cells were injected into syngeneic mice.18 Once-daily intraperitoneal treatment using doses of imatinib from 2.5 to 50 mg/kg, starting 1 week after cell injection, caused dose-dependent inhibition of tumor growth (Figure 3), while 50 mg/kg intraperitoneal treatment was inactive against tumors derived from v-SRC–transformed 32D cells, consistent with the lack of inhibition of SRC kinase activity by imatinib. Similar experiments in nude mice using KU812 cells, a BCR-ABL+ human cell line, demonstrated the need for continuous inhibition of BCR-ABL kinase activity to achieve maximal antitumor effects.33 Early pharmacokinetic studies at Novartis had demonstrated that imatinib is rapidly absorbed following oral administration to mice and pharmacologically relevant concentrations are achieved in the plasma, with a half-life of approximately 1.3 hours.
In vivo antitumor activity of imatinib. (A) Mice injected with 32D cells expressing BCR-ABL were treated with graded doses of intraperitoneal imatinib from days 11 to 18 (○, placebo; ♦, 2.5 mg/kg; ○, 10 mg/kg; ▵, 25 mg/kg; ▪, 60 mg/kg). There was a significant difference in tumor size between controls and mice treated with at least 10 mg/kg. (B) No effect was seen in mice injected with 32D cells expressing v-SRC. Reproduced from Druker et al18 with permission from Nature, copyright 1996. Error bars indicate mean ± standard deviation.
In vivo antitumor activity of imatinib. (A) Mice injected with 32D cells expressing BCR-ABL were treated with graded doses of intraperitoneal imatinib from days 11 to 18 (○, placebo; ♦, 2.5 mg/kg; ○, 10 mg/kg; ▵, 25 mg/kg; ▪, 60 mg/kg). There was a significant difference in tumor size between controls and mice treated with at least 10 mg/kg. (B) No effect was seen in mice injected with 32D cells expressing v-SRC. Reproduced from Druker et al18 with permission from Nature, copyright 1996. Error bars indicate mean ± standard deviation.
Optimization of the treatment schedule to 3 times daily administration of 50 mg/kg intraperitoneally or 160 mg/kg orally for 11 consecutive days, assuring continuous blockage of p210BCR-ABL tyrosine kinase, resulted in tumor-free survival of mice injected with KU812 cells. With the same schedule, established tumor nodules began to regress 48 hours after beginning treatment; by day 8, no treated animal had measurable disease. Eight of 12 animals remained tumor free with over 200 days of follow-up, while 4 relapsed on days 48 through 60. The antitumor effect of imatinib was specific for p210BCR-ABL expressing cells, as no growth inhibition occurred in mice injected with U937, a BCR-ABL– myeloid cell line.
Imatinib was also tested in the transduction-transplantation model of CML.4 In this system, lethally irradiated syngeneic mice receive marrow infected with a BCR-ABL retrovirus and consistently die within 3 weeks from an aggressive CML.34 Treatment with imatinib (50 mg/kg in the morning, 100 mg/kg in the evening) led to prolonged survival.35 Responses were quite variable, with 25% of animals having refractory disease. Imatinib was not capable of preventing CML, even if started as early as 48 hours after transplantation, but none of the responders progressed on therapy. No consistent association between response and BCR-ABL mRNA and protein levels was seen, and no other cause underlying refractoriness could be determined. Notably, there was a trend toward “clonal depletion” in responding animals, suggesting that imatinib was able to successfully target some, but not all leukemic clones.
Clinical studies: imatinib monotherapy
Phase 1 studies
Phase 1 trials with imatinib began in June of 1998 and were designed to determine the maximally tolerated dose, with clinical benefit as a secondary endpoint. Patients in the chronic phase of CML who had failed therapy with interferon-α (IFN) were eligible.36 Imatinib, given as daily oral therapy, was well tolerated, without dose-limiting toxicity. Hematologic responses were seen at doses of 140 mg and greater. Most remarkable was that 53 of 54 patients treated with at least 300 mg showed complete hematologic responses (CHR). Moreover, at doses of 300 mg and higher, cytogenetic responses were achieved in 31% of patients, with 13% achieving complete response. Pharmacokinetic studies revealed that above 300 mg, plasma levels equivalent to the effective in vitro level (1 μM) were achieved. At 400 mg, the current standard dose for CML in chronic phase, peak levels at steady state were approximately 4.6 μM and trough levels approximately 2.13 μM, with a half-life of 19.3 hours, indicating that once-daily dosing was sufficient to provide continuous kinase inhibition.37 Side effects were generally mild and included nausea, diarrhea, periorbital edema, and skin rashes. Myelosuppression was frequent, likely reflecting efficacy due to suppression of Ph+ hematopoiesis rather than toxicity.
Based on these extremely promising results, the phase 1 study was expanded to patients with myeloid and lymphoid blast crisis of CML and patients with relapsed or refractory Ph+ ALL.38 With doses of 300 to 1000 mg/d, 11% of patients with myeloid blast crisis achieved CHR, and another 10% achieved a reduction of bone marrow blasts to less than 5%, without complete recovery of peripheral blood counts. In patients with lymphoid disease, the corresponding remission rates were 20% and 15%, respectively. Unfortunately, most patients with myeloid and all but 1 patient with lymphoid blast crisis relapsed within weeks to months.
Phase 2 studies
Phase 2 studies began in late 1999 using imatinib as a single agent for all stages of CML. For patients in blast crisis and with Ph+ ALL, these studies confirmed the results of the phase 1 trial (Table 2).39,40 Patients in chronic phase who had failed IFN therapy did much better than expected, with CCR rates of 41% and major cytogenetic remission (MCR) of 60%.41 Importantly, these responses were usually durable, resulting in a progression-free survival of 89.2% at 18 months. Not surprisingly, the efficacy in patients with accelerated phase was intermediate between chronic phase and blast crisis.42 The results of the phase 1 and 2 trials led to the approval by the Food and Drug Administration of imatinib for the treatment of CML in advanced phase and after failure of IFN therapy. A recent retrospective study compared the survival of 143 patients who received imatinib after failure of IFN-based therapy with 246 historical controls managed with conventional therapy and found a significant overall survival benefit for patients treated with imatinib, which was, however, confined to those patients who achieved a cytogenetic response.43 In contrast, a subsequent study showed a survival benefit for all patients treated with imatinib, regardless of cytogenetic response, a discordance that may be due to the statistical methods used.44
Phase 3 study
Imatinib and the combination of IFN plus cytarabine were compared in a randomized trial, which showed imatinib to be vastly superior compared with CHR, MCR, and CCR, as well as progression-free survival (Table 3).45 A recent update showed a CCR rate of 81% at 30 months. Since the design of this study allowed crossover for lack of efficacy or intolerance, a large proportion of patients crossed from IFN/cytarabine to imatinib. As imatinib effectively rescued these individuals, the true differences between the groups were certainly underestimated, and no difference in overall survival was observed. However, a retrospective comparison with a historical control group treated with IFN showed superior survival for patients on imatinib.46 In addition to its superior efficacy, patients on imatinib have a far better quality of life.47 Based on these results, the drug was approved by the regulatory authorities for first-line treatment of CML in the United States and Europe.
Monitoring of residual disease by quantitative RT-PCR in complete cytogenetic responders showed that the risk of disease progression was inversely correlated with the reduction of BCR-ABL mRNA compared with pretherapeutic levels. Progression-free survival was 100% at 24 months in patients who achieved a reduction of their BCR-ABL transcripts by 3 orders of magnitude or more by 12 months, a state termed major molecular response. In contrast, the risk of disease progression at 24 months was 5% in patients with a less than 3-log reduction of BCR-ABL transcripts, and 15% in those who failed to achieve CCR.48 It should be noted that the average BCR-ABL expression of a group of untreated patients was used as the reference in this study, while other studies have used individual pretherapeutic levels.49 It is possible that the use of individual pretherapeutic levels would lead to a slightly different predictive model.
Clinical studies: imatinib in combination with other agents
A number of studies were initiated to improve the efficacy of imatinib monotherapy by combining the drug with other active agents, such as IFN, homoharringtonine, and cytarabine, or by increasing the dose. The study populations included either newly diagnosed patients or patients after failure of IFN therapy. The general theme is that CCR tends to occur earlier, but at the expense of more toxicity, particularly myelosuppression. In a recently published phase 2 study with an imatinib/cytarabine combination, the rate of grades 3/4 hematologic toxicity was 53%,50 compared with approximately 20% with imatinib monotherapy.45 After 3 months, the rate of MCR was 70%, compared with approximately 60% for imatinib monotherapy. However, after 12 months, the gap had practically closed, with 83% major cytogenetic responses, compared with approximately 84% with imatinib alone. Similar observations were made with a combination of imatinib and pegylated IFN.51 In a single-institution study of IFN-refractory patients, the rate of CCR on 800 mg imatinib daily was 89%, compared with 41% with 400 mg in the phase 2 multicenter trial.52 Due to the fact that the risk profile in the single-center study was more favorable than in the phase 2 study, a direct comparison of the data is impossible. Nonetheless, together with the observation that dose escalation in cytogenetically refractory patients leads to cytogenetic responses in some patients,53 these data lend support to the notion that response rates may increase with higher doses of imatinib. Several large phase 3 studies have been initiated in Europe that compare standard-dose imatinib versus higher doses and combinations with IFN or cytarabine, and a Southwest Oncology Group (SWOG) trial comparing 400 versus 800 mg imatinib daily is expected to start in early 2005. These studies should clarify whether combination treatment or higher doses of imatinib are superior to standard therapy. Given the high rates of CCR and progression-free survival with standard therapy, it might take many years to detect significant differences. Thus, novel surrogate endpoints such as negativity by RT-PCR are needed.
Resistance to imatinib
Distinguishing refractory and resistant disease
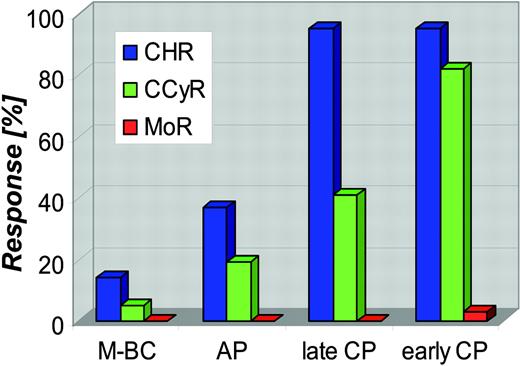
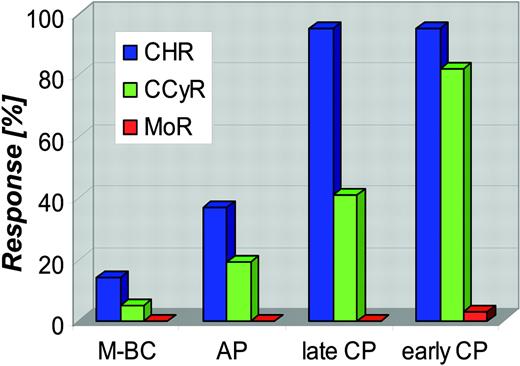
Response rates and the durability of responses to imatinib are highly dependent on the stage of disease at which treatment is initiated (Figure 4).39,41,42,45 Refractoriness (primary resistance) should be distinguished from secondary resistance that develops after an initial response, as the underlying mechanisms may be different. Potentially, the greatest long-term concern is the high rate of molecular refractoriness. In the phase 3 trial, less than 4% of patients with CCR were negative for BCR-ABL on at least 1 occasion48,49 and another study found no molecular responders among 120 patients with late chronic phase.54 The much higher rates of RT-PCR negativity reported in some other studies may be due to the use of less sensitive assays, such as quantitative RT-PCR only, without confirmation by nested RT-PCR.52
Rates of complete hematologic remission, complete cytogenetic remission, and molecular remission (defined as RT-PCR negativity) in the phase 2 and 3 trials with imatinib monotherapy. Blue bars indicate complete hematologic remission (CHR); green bars, complete cytogenetic remission (CCyR); red bars, molecular remission (MoR). M-BC indicates myeloid blast crisis; and AP, accelerated phase.
Rates of complete hematologic remission, complete cytogenetic remission, and molecular remission (defined as RT-PCR negativity) in the phase 2 and 3 trials with imatinib monotherapy. Blue bars indicate complete hematologic remission (CHR); green bars, complete cytogenetic remission (CCyR); red bars, molecular remission (MoR). M-BC indicates myeloid blast crisis; and AP, accelerated phase.
It is not yet known whether patients in CCR with minimal residual disease will invariably relapse after discontinuation of imatinib or remain in remission. Anecdotal cases suggest that both are possible,55,56 similar to patients on IFN.57 Notably, IFN-induced CCR involves cytotoxic T-cell responses against leukemia-specific antigens, similar to allografting, whereas no such response is demonstrable in patients with imatinib-induced CCR. This may imply that continuous imatinib therapy is required to maintain CCR, as long as minimal residual disease persists.58,59 In patients after allografting, RT-PCR negativity is a therapeutic goal because of its strong predictive power for freedom from progression,60 and longer follow-up will be necessary to establish whether this holds true for patients on imatinib.61 Another unresolved issue is therapy-induced cytopenia, which is an adverse prognostic feature.62 If cytopenia is the result of less intensive treatment because of dose interruptions or reflects more advanced disease is not yet clear, but G-CSF and/or erythropoietin to allow full dosing is widely used in such patients.
Laboratory studies
Most BCR-ABL+ human cell lines are sensitive to imatinib at concentrations of less than 0.5 μM. Exceptions include SD-1 and KCL-22. Both lines proliferate in the presence of 0.5 to 1 μM imatinib, despite the fact that BCR-ABL autophosphorylation is inhibited.27,63,64 In SD-1 cells, resistance may be explained by their being Epstein Barr virus (EBV)–transformed,65 suggesting that their proliferation and survival are dependent on EBV-driven signals rather than BCR-ABL. In KCL-22 cells, comparison of gene expression by microarray showed marked differences between an imatinib-sensitive and an imatinib-resistant clone of this cell line, although it is not clear how exactly this relates to their resistant phenotype.66 To model resistance in vitro, several groups have generated imatinib-resistant cell lines by culture in gradually increasing concentrations of imatinib, which usually required several months.63,67,68 The most common mechanism of resistance in these lines is a significant increase of BCR-ABL mRNA and protein, sometimes associated with gene amplification.
In addition to overexpression of BCR-ABL, increased expression of MDR1 has been observed in imatinib-resistant Lama-84 cells, and resistance was partially reversible by verapamil, a P-glycoprotein (PGP) inhibitor.63 Several other studies have since reported on the role of PGP as a transporter of imatinib in cell lines, indicating that MDR1 expression confers resistance,69-73 although this was not universially confirmed.74
Overexpression of LYN, an SRC kinase, was demonstrated in an imatinib-resistant clone of the K562 cell line.75 Compared with the parental line, these cells expressed reduced amounts of BCR-ABL, and inhibition of BCR-ABL by imatinib did not affect growth and viability. In contrast, treatment with an SRC kinase inhibitor affected the resistant but not the parental line.
The in vitro selection conditions for resistant cell clones greatly influence the type of resistance. Mutations of the ABL kinase domain (KD) were only rarely found in imatinib-resistant lines,76,77 although a recent study was able to reproducibly generate KD mutant BaF/3BCR-ABL clones with a limiting dilution strategy.78
The human BCR-ABL+ leukemia cell line KU812 injected into nude mice was used to study the mechanisms underlying in vivo resistance to imatinib.33 If imatinib treatment was started in mice with measurable tumors, there was a high rate of relapse after an initial response. Surprisingly, the tumor cells retained sensitivity to imatinib in vitro, despite their apparent resistance in vivo. Additional studies showed that the plasma concentrations of alpha-1–acidic glycoprotein (AAG) in the animals with resistance were elevated and that AAG avidly binds imatinib, reducing its bioavailability.79 The ability of AAG to mediate in vitro resistance to imatinib is still controversial.80-82
Clinical resistance
Amplification and overexpression of BCR-ABL. In a study by Gorre et al,83 fluorescence in situ hybridization demonstrated amplification of BCR-ABL at the genomic level in 3 of 9 patients with resistant disease. In a subsequent report, an increase in the level of BCR-ABL mRNA was seen in 4 of 37 patients.84 Both mechanisms are thought to lead to increased expression of BCR-ABL protein, although, due to the difficulties analyzing BCR-ABL protein in clinical specimens, this has not yet been formally demonstrated.
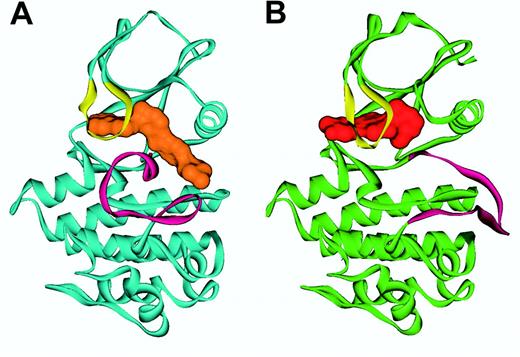
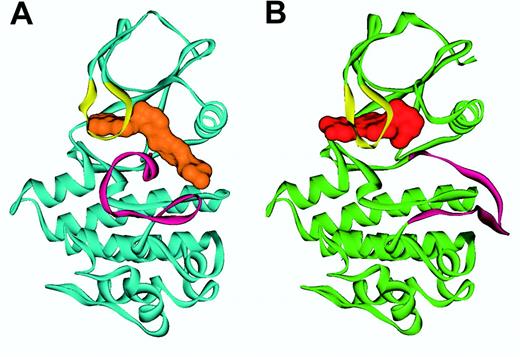
Mutations of the BCR-ABL KD. Enzyme kinetics suggest that imatinib functions as a competitive inhibitor of ATP binding to the ABL kinase with a inhibition constant (Ki) value of 85 nM for c-ABL.85 The crystal structure of the catalytic region of both the murine and human ABL kinase in complex with an imatinib analog86 and with imatinib87,88 has been solved (Figure 5). Imatinib intimately interacts with the KD, engaging no less than 21 amino acid residues.87 The most important finding is that imatinib binds ABL in the kinase-inactive conformation, in which the A-loop, the major regulatory element, occludes the catalytic center. In contrast, steric hindrance prevents imatinib binding to the open, active conformation of the A-loop. This mode of binding is critical to imatinib's high selectivity for ABL over other kinases, since the structural diversity of the A-loops of different kinases is largely restricted to their inactive conformations, while the A-loops of active kinases are very similar.
Ribbon representation of ABL in complex with imatinib and ID180970. Shown is (A) the conformation of Abl (blue) in complex with imatinib (orange), with the A-loop (magenta) in a “closed” conformation, and (B) the Abl conformation (green) in the PD180970 (red) complex with the A-loop (magenta) in an “open” conformation. The P-loop (yellow) folds down over the inhibitor in both cases. Figure prepared by Sandra W. Cowan-Jacob based on reported structures.85,88
Ribbon representation of ABL in complex with imatinib and ID180970. Shown is (A) the conformation of Abl (blue) in complex with imatinib (orange), with the A-loop (magenta) in a “closed” conformation, and (B) the Abl conformation (green) in the PD180970 (red) complex with the A-loop (magenta) in an “open” conformation. The P-loop (yellow) folds down over the inhibitor in both cases. Figure prepared by Sandra W. Cowan-Jacob based on reported structures.85,88
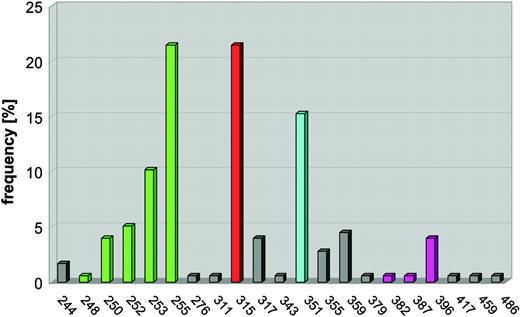
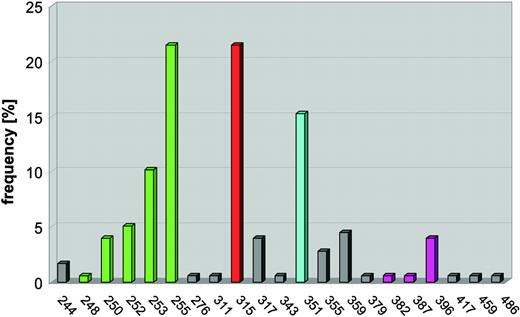
Mutations of the KD are found in 50% to 90% of patients with secondary resistance.83-85,89-91 Characterization of the mutants by in vitro kinase assays, quantification of intracellular BCR-ABL phosphorylation, and proliferation assays generally revealed an IC50 shift toward higher concentrations of imatinib, although the degree of resistance varies (Table 4). Mutations were detected in many different amino acids, but there are 4 distinguishable clusters: ATP binding loop (P-loop), T315, M351, and A-loop (Figure 6).
Frequency of BCR-ABL mutations detected in clinical specimens (n = 177). Mutations cluster in 4 distinct regions of the kinase domain. Mutations of the P-loop (amino acids 248-255, green) are most common, followed by mutations of T315 (red), which forms a hydrogen bond with imatinib. M351 (turquoise) interacts with the SH2 domain and participates in autoregulation of kinase activity. The fourth cluster (magenta) encompasses the A-loop (amino acids 379-398).
Frequency of BCR-ABL mutations detected in clinical specimens (n = 177). Mutations cluster in 4 distinct regions of the kinase domain. Mutations of the P-loop (amino acids 248-255, green) are most common, followed by mutations of T315 (red), which forms a hydrogen bond with imatinib. M351 (turquoise) interacts with the SH2 domain and participates in autoregulation of kinase activity. The fourth cluster (magenta) encompasses the A-loop (amino acids 379-398).
P-loop mutations. The P-loop is an extremely flexible, glycine-rich structure encompassing amino acids 244 to 255 that normally accommodates the phosphate groups of ATP. Upon binding of imatinib, the P-loop undergoes a major downward displacement. This “induced fit” interaction with imatinib is stabilized by a water-mediated hydrogen bond between Y253 and N32286 and the formation of a hydrophobic cage surrounding part of imatinib. Compared with wild-type BCR-ABL, P-loop mutants are 70- to more than 100-fold less sensitive in kinase assays and approximately 10-fold less sensitive in cell proliferation assays.99 In the case of mutant Y253, the main consequence of the mutation may be the disruption of the hydrogen bond to N322.86 Other P-loop mutations may shift the equilibrium toward an active state by disfavoring the induced fit conformation of the P-loop required for imatinib binding.85 An additional level of complexity arises from physiologic regulatory mechanisms that are operational within cells. For example, the Y253F mutant of BCR-ABL exhibits increased kinase activity compared with wild type when expressed in cells, but not in in vitro kinase assays using purified ABL, suggesting that this mutation may disrupt the binding of a physiologic ABL inhibitor.100 For yet-unknown reasons, patients with P-loop mutations appear to have a particularly bad prognosis compared with patients with other types of mutations.90
Mutations of T315. A second cluster of mutations affects T315, which forms a hydrogen bond with imatinib, and much less frequently F317, which binds imatinib via hydrophobic interactions. While the F317L mutant exhibits moderate imatinib resistance, the more frequent T315I mutant is largely insensitive to the drug.83,99 Substitution of threonine with the bulky isoleucine is expected not only to disrupt the hydrogen bond to imatinib but also to allosterically interfere with drug binding. Consistent with this, the insulin receptor kinase, which contains methionine in the position corresponding to T315 in ABL, is resistant to imatinib.17 Notably, the homologous residue in the PDGFR alpha kinase was found mutated in a patient with hypereosinophilic syndrome, who had developed resistance to imatinib.101
Mutations of M351. The mechanism underlying the resistance of this mutant had not been clear until recently, when the crystal structure of the N-terminal region of ABL was solved and the physiologic regulation of the ABL kinase was revealed.102,103 M351 contacts the ABL SH2 domain, an interaction that helps to stabilize the autoinhibited conformation of ABL. Although BCR-ABL is constitutively active, the mechanisms responsible for autoinhibition of ABL are partially maintained in the chimeric protein. Disruption of the interaction between the SH2 and the KD impairs autoinhibition, shifting the equilibrium toward the active conformation, to which imatinib cannot bind.
A-loop mutations. The fourth cluster of mutations is localized in the A-loop, which comprises residues 381 to 402 of ABL. As mentioned, the position of the A-loop regulates kinase activity. The 3 residues at the N-terminal form a DFG (aspartate-phenylalanine-glycine) motif that is highly conserved among tyrosine kinases. In its active “open” position, the A-loop swings away from the catalytic center of the kinase, allowing D381 to bind Mg2+, which coordinates the phosphate groups of ATP, while the C-terminal part of the A-loop provides a platform for substrate binding. Under physiologic circumstances, (auto-) phosphorylation of Y393 is important for activation, as it stabilizes the open conformation of the A-loop. Consistent with this, ABL phosphorylated on tyrosine 393 is less sensitive to imatinib.86,100 Thus, it is likely that mutations in the A-loop prevent the kinase from adopting its inactive conformation, which is required for imatinib binding. A-loop mutants exhibit moderate resistance in cellular and in vitro kinase assays.92,99
Mutations in other regions. Apart from the hotspots, mutations have been observed in many other positions in the KD. Some of these mutants have a sensitivity to imatinib that is not significantly different from wild-type BCR-ABL.93 Thus, it seems unlikely that these mutants are able to confer imatinib resistance in vivo, unless accompanied by yet another mechanism. Consistent with this, some of these mutants (eg, F311L and F359V) have been observed in conjunction with other mutants that exhibit high-level resistance. Importantly, dose escalation of imatinib may be able to recapture responses in patients who harbor mutants with only marginally reduced sensitivity. Many additional KD mutants were recovered from an in vitro mutagenesis screen that used a mutation-prone strain of Escherichia coli,94 which were frequently located in the C-helix or SH2 and SH3 domain contact sites of the KD. Generally, these mutations confer only moderate resistance and their relevance to clinical resistance is currently unknown. In the same experiment, several mutations outside the KD were also detected. As a rule, they affected amino acids that are critical for autoinhibition of ABL102,103 and exhibit a lower degree of imatinib resistance than KD mutants.94 It is conceivable that such mutations further impair the residual autoinhibition of ABL within the BCR-ABL fusion protein, shifting the equilibrium to the active state, to which imatinib cannot bind. Information regarding the frequency of these atypical mutations in resistant patients is limited, as most studies sequenced only the KD. One report failed to detect mutations in the ABL SH2 and SH3 domains of 43 patients with clinical resistance to imatinib.95
Mutations prior to imatinib therapy. In some patients, mutations recovered at relapse were detectable in samples taken prior to imatinib treatment, in one case even at diagnosis.91,104,105 This is consistent with the assumption that the selective pressure exerted by imatinib favors the outgrowth of pre-existing resistant cell clones. The failure to consistently detect mutations in imatinibnaive samples from patients who went on to relapse is likely due to the limited sensitivity of the assays used. An intriguing possibility is that the mutations confer some growth advantage regardless of imatinib and are related to disease progression. This would be consistent with the relatively high proportion of mutated alleles (20%-40%) that was observed in 3 imatinib-naive patients with myeloid blast crisis.91 One explanation could be increased kinase activity that has been ascribed to some BCR-ABL mutants (eg, Y253F100 and T315M106 ), although these data have not yet been confirmed in biochemical assays using full-length protein. It is also conceivable that certain mutants modify the disease by other mechanisms, such as altered downstream signaling.
Point mutations of KIT and PDGFRα have also been described in patients with imatinib-resistant GISTs107 and hypereosinophilic syndrome, respectively.101 If point-mutation–induced resistance is typical of imatinib therapy or typical of cancer therapy with tyrosine kinase inhibitors in general remains to be determined. A striking analogy exists to resistance of HIV to protease and reverse transcriptase inhibitors. As with imatinib in CML, disease eradication is extremely difficult, if not impossible, and point mutations that interfere with drug binding are the leading cause of resistance.108
Clonal evolution. Karyotypic abnormalities in addition to the Ph chromosome have been detected in some in patients at the time of resistance.84,96,109 There is no evidence that molecular changes related to specific abnormalities directly confer resistance, as the pattern of clonal evolution (CE) appears to be similar to that seen in patients on IFN therapy.110 CE was associated with a high risk of disease progression in patients with CHR, suggesting that it should be regarded as a marker of relapse risk rather than a mechanism of resistance, per se.109
Strategies to overcome resistance
Reactivation of BCR-ABL signaling is an almost universal finding in patients with imatinib resistance. This implies that the cells continue to depend on the specific signaling output of the genetic event that initiated the malignant transformation in the first place, and that BCR-ABL remains the best therapeutic target.111
Dose escalation of imatinib
Retrospective data suggest that dose escalation can overcome hematologic or cytogenetic resistance in some patients,53 although these responses may not be maintained.112 An important consideration is the specific type of mutation. Dose escalation is likely to be effective in the case of mutants with a low or moderate level of resistance to imatinib, such as H386P, but not in highly resistant mutants such as T315I or E255K.93
Combination with conventional cytotoxic drugs
Many drugs have been tested for synergism with imatinib (recently reviewed in La Rosee et al113 ). The use of different cell lines and different models of data analysis may explain why the results are not always consistent among the various studies. Altogether, it appears that most combinations are synergistic or at least additive. Since one of the pillars of BCR-ABL–mediated malignant transformation is inhibition of apoptosis,114 this is consistent with the concept that inhibition of BCR-ABL function resensitizes the cells to the induction of apoptosis by conventional agents. Naturally, drugs with established activity in CML have attracted particular attention. Cytarabine, homoharringtonine, and IFN are synergistic in vitro, and combinations with imatinib are currently tested in clinical trials. Promising results have also been obtained for decitabine, a novel hypomethylating agent with activity in CML blast crisis.115 In contrast, the data are conflicting for hydroxyurea,116-118 while methotrexate and topotecan are antagonistic in combination with imatinib. Given the requirement for many dose points, drug combination assessments are difficult to perform in primary cells. Thus, most of the data are based on cell lines, although in a strict sense, any combination would have to be tested in primary cells to confirm that the differential between CML and normal cells afforded by imatinib is maintained.
Alternative inhibitors of ABL
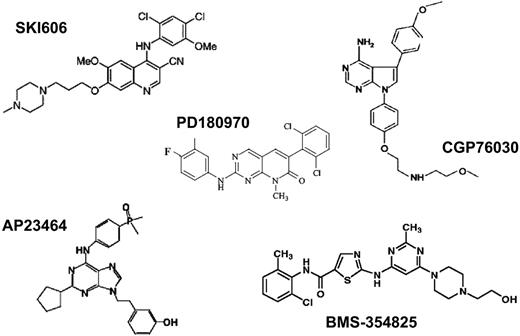
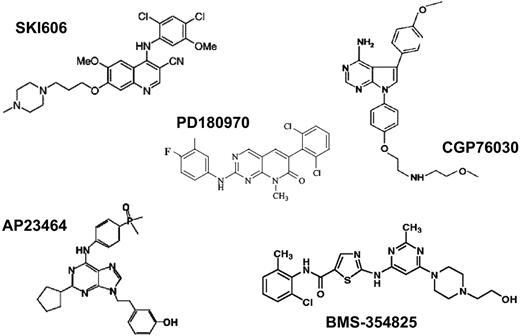
Several compounds with inhibitory activity toward ABL kinase at nanomolar concentrations have been identified (Figure 7; Table 5). Compared with imatinib, these compounds are less specific and have activity against SRC family kinases as well as various other targets. The crystal structure of the ABL KD in complex with the pyridopyrimidines PD173955 and PD180970 has been solved and revealed striking differences compared with imatinib (Figure 5).85,87
In contrast to imatinib, PD173955 is capable of binding to both inactive and active ABL conformations, regardless of the position of the A-loop. Given the structural similarity of active tyrosine kinases, it is not surprising that this is associated with some loss of specificity. One advantage of these potent ABL inhibitors over imatinib may be that the IC50 and IC90 values for inhibition of kinase activity and cellular proliferation of BCR-ABL–transformed cells are lower by 1 to 2 orders of magnitude. Thus, more complete inhibition of BCR-ABL tyrosine phosphorylation should be achievable in patients. Since there is evidence that higher doses of imatinib are superior to lower doses,42,53,123 stronger suppression of BCR-ABL kinase activity might lead to a more profound suppression of the leukemic clone. An even more important feature is the activity of alternative ABL inhibitors against imatinib-resistant mutants of BCR-ABL. One study showed that PD180970 inhibited the kinase activity of most clinically relevant BCR-ABL mutants, with the exception of T315I, with IC50 values equal or only slightly higher than for wild-type ABL, and suppressed cell proliferation of BaF3 cells expressing the various mutants.99 These results were subsequently confirmed.119,124 Unfortunately, the unfavorable pharmacokinetic profile of pyridopyrimidines precludes their clinical development. Another group of compounds with potent inhibitory activity toward SRC- and KD-mutant ABL are trisubstituted purines.120 The most active derivative, AP23464, inhibits the proliferation of BaF3 cells expressing wild-type BCR-ABL with an IC50 of 14 nM. BCR-ABL mutants except T315I and, to a lesser extent E255K, are inhibited at comparable concentrations. The pharmacologic properties of this class of compounds are not yet known. The most promising of the alternative ABL inhibitors is BMS-354825, which has a similar spectrum of activity against wild-type and mutant BCR-ABL and high oral bioavailability. Excellent responses were seen in severe combined immunodeficient (SCID) mice injected with BaF3 cells expressing wild-type or M351T mutant but not T315I mutant BCR-ABL.121 A phase 1 study in patients with imatinib resistance is currently in progress. The fact that BMS-354825 and similar compounds inhibit SRC may be an advantage, as SRC kinases may be activated in rare patients with imatinib resistance75 and play a role in signal transduction downstream of BCR-ABL.125-127 The disadvantage of anti-SRC activity may be an increased rate of side effects.128
Agents that down-regulate BCR-ABL protein
Geldanamycin and its less-toxic analog 17-AAG (17-allylaminogeldanamycin) lead to degradation of BCR-ABL protein by inhibiting heat shock protein 90 (Hsp90), a molecular chaperone required for stabilization of BCR-ABL.13,129-131 A recent study showed that BCR-ABL proteins with E255K and T315I mutations may be even more sensitive to 17-AAG than wild-type BCR-ABL.111 Phase 1 studies with 17-AAG are in progress. The histone deacetylase inhibitor LAQ824 exerts similar effects as 17-AAG by acetylation of Hsp90, which in turn interferes with the protein's chaperone function.132 In addition, this compound down-regulates BCR-ABL mRNA by an as-yet-unknown mechanism. Lastly, arsenic trioxide has been shown to induce down-regulation of BCR-ABL, although the precise mechanism is not known.39,133,134 In a small phase 1 study, the combination of imatinib and arsenic trioxide was well tolerated but no therapeutic activity was observed. This may be due to the fact that the imatinib dose had been reduced at study entry because of concerns about toxicity.135
Combinations with other signal transduction inhibitors
Extensive in vitro studies have tested specific inhibitors of signal transduction pathways downstream of BCR-ABL alone and in combination with imatinib. In most cases, synergistic or additive effects were demonstrable, in some instances also in imatinib-resistant cell lines. Several compounds, including farnesyl transferase inhibitors (FTIs), mammalian target of rapamycin (mTOR) inhibitors (rapamycin, RAD001), and flavopiridol, an inhibitor of cyclin-dependent kinases, are in early clinical development, alone or in combination with imatinib (Table 6). Although there is a rationale for these agents, BCR-ABL itself remains the best target, as resistance to imatinib is almost universally associated with reactivation of BCR-ABL kinase activity, and it is doubtful whether disrupting individual pathways downstream of BCR-ABL will be effective. Studies in mice with homozygous deletions of IL-3, GM-SCF,146 or STAT5147 revealed that BCR-ABL remained fully leukemogenic, indicating that targeting one of these pathways would not be sufficient. Another important lesson from preclinical studies is that synergism between imatinib and other agents depends on residual sensitivity of the leukemic cells to imatinib.134 Thus, combinations of imatinib with decitabine, arsenic trioxide, or FTIs are synergistic in resistant cell lines with overexpression of BCR-ABL but not in lines expressing the T315I mutant.134,136,137 This emphasizes the need to establish the mechanism of resistance to allow for a rational choice of salvage therapy.
Disease persistence
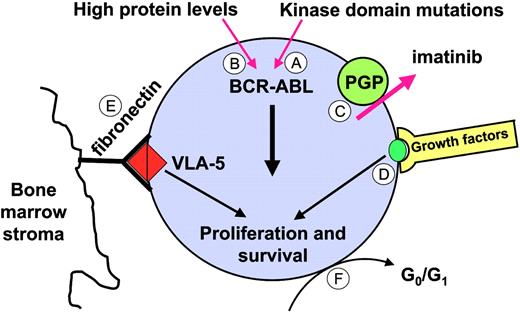
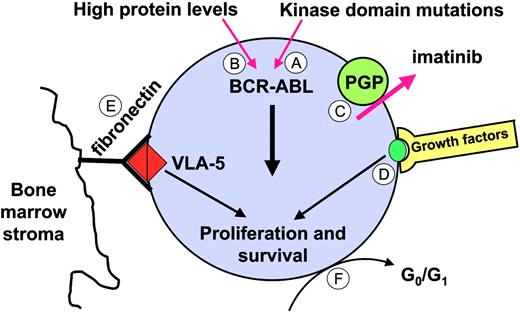
There is a strong correlation between disease phase and the incidence of refractoriness at the hematologic, cytogenetic, and molecular levels (Figure 4). It is evident that only a small fraction even of newly diagnosed patients achieve “molecular remission” (ie, become negative for BCR-ABL by RT-PCR).48 Even in those individuals, more sensitive tests revealed that the underlying disease burden is greater than in patients who become RT-PCR negative after an allogeneic transplantation, where molecular remission is associated with long-term freedom from progression.61 It is at present unclear which mechanisms underlie the persistence of BCR-ABL+ cells and whether these mechanisms are similar to those responsible for acquired resistance. In vitro studies suggest that the primary effect of imatinib on BCR-ABL+ primitive progenitor cells is inhibition of proliferation, without induction of apoptosis.148,149 Hence, imatinib may be able to prevent stem cell proliferation but unable to eliminate “quiescent” cells, even if combined with other agents such as FTIs, cytarabine, PI3 kinase inhibitors, or Hsp90 inhibitors.150 It is unknown though whether the quiescent cells in these ex vivo assays correspond to the cells that persist in vivo. Several mechanisms might allow primitive progenitor cells to survive in the presence of imatinib (Figure 8). One explanation is that survival of persisting leukemia cells is dependent on physiological stimuli, rather than on BCR-ABL kinase activity. Circumstantial evidence for this comes from the observation that CD34+-selected CML cells treated in vitro with imatinib activate mitogen-activated protein kinase in a growth-factor–dependent manner, suggesting that they are capable of switching to an alternative survival and growth signal upon BCR-ABL inactivation.151 Survival signals could also be derived from interaction with stroma cells and extracellular matrix. Studies in BCR-ABL+ cell lines treated with imatinib in combination with cytotoxic agents suggest that adhesion to integrins inhibits apoptosis, even in the absence of active BCR-ABL kinase,152 although it remains to be seen whether these findings are relevant to primary CML cells. A second possibility is that imatinib is not able to completely inhibit BCR-ABL kinase activity, and that the residual activity is sufficient to promote survival but not proliferation. As discussed earlier, data from several groups indicate that imatinib is a substrate of PGP, and high levels of PGP in leukemic stem cells may efficiently reduce intracellular drug levels. If this is the case, then higher doses of imatinib should be more effective, which is in agreement with higher rates of RT-PCR negativity seen in chronic-phase patients treated with 800 mg rather than 400 mg imatinib daily.52 Similarly, ABL inhibitors with greater potency may be able to improve the rates of molecular response by completely inhibiting BCR-ABL. Another mechanism for persistence is mutations in the BCR-ABL KD. A high rate of “unusual” KD mutations was recently reported in a small series of patient in CCR.153 In most instances, the specific mutations had been detected in the aforementioned in vitro mutagenesis screen94 and are predicted to confer only low to moderate resistance. This would explain why these patients achieve CCR but fail to eradicate the disease, and would provide a rationale for therapy with alternative ABL inhibitors. Furthermore, a recent study showed that the levels of BCR-ABL mRNA in early hematopoietic progenitors from CML patients were much higher than in more mature cells, which may render such cells resistant to imatinib.154
Potential mechanisms underlying disease persistence (molecular refractoriness). (A) Kinase domain mutations that confer moderate resistance to imatinib. (B) BCR-ABL levels may be particularly high in the most primitive leukemic stem cells. (C) Inadequate intracellular levels of imatinib as a result of PGP expression. (D) Physiologic growth factor signaling or (E) integrin signals may maintain viability even with BCR-ABL kinase activity completely inhibited. (F) Quiescent (dormant) cells may be protected against imatinib. VLA-5 indicates very late activation antigen-5.
Potential mechanisms underlying disease persistence (molecular refractoriness). (A) Kinase domain mutations that confer moderate resistance to imatinib. (B) BCR-ABL levels may be particularly high in the most primitive leukemic stem cells. (C) Inadequate intracellular levels of imatinib as a result of PGP expression. (D) Physiologic growth factor signaling or (E) integrin signals may maintain viability even with BCR-ABL kinase activity completely inhibited. (F) Quiescent (dormant) cells may be protected against imatinib. VLA-5 indicates very late activation antigen-5.
Future directions
Imatinib and allogeneic transplantation
One of the biggest challenges in the management of CML is the integration of imatinib therapy and allogeneic stem cell transplantation. There appears to be consensus that patients who progressed beyond chronic phase should proceed to an allograft, if this is feasible, using imatinib as an induction therapy with low toxicity. In newly diagnosed patients in chronic phase, one option is to start all patients on imatinib, reserving allografting for those without adequate response. The value of this approach will ultimately depend on whether imatinib compromises the outcome of a subsequent allograft. Retrospective analyses, predominantly of high-risk patients, have yielded conflicting results, and the issue needs to be clarified prospectively.155,156 The other option is to define a group of patients with high-risk disease and a low risk of transplantation-related mortality, in whom allografting is performed as soon as possible, regardless of their response to imatinib. Given the relatively short follow-up of patients on imatinib, it is presently impossible to provide an unequivocal rationale for either approach. Under these circumstances, the patient's individual preference is a crucial factor in finding the optimal course of action.
Predicting response to imatinib
Obviously, therapeutic decisions would be greatly facilitated if it were possible to accurately predict cytogenetic or molecular response prior to imatinib therapy. Although several baseline factors predictive of subsequent MCR have been identified, these variables are of little use for decision-making in individual patients.41 Classification according to the Sokal score defines 3 distinct risk groups157 but other factors must be taken into account. For example, patients with deletions flanking the ABL or BCR breakpoint have lower rates of CCR and a shorter time to progression.158 Given the success of gene profiling by microarray for prediction of treatment outcomes, several groups have used this technology to define expression signatures associated with MCR. In one study of 18 CML patients, most of them in chronic phase, 79 genes were identified with differential expression between peripheral blood mononuclear cells from cytogenetic responders and nonresponders.159 In another study of newly diagnosed patients, mRNA isolated from unselected blood cells was used for expression profiling.160 Again, a number of differentially expressed genes were identified. Unfortunately, since these results were not validated in independent cohorts, their significance is unclear. In our own experience, the differences in the gene expression profiles of white cells from responders and nonresponders are very small and may not predict cytogenetic response.161 In contrast, microarrays or quantitative PCR on rationally chosen candidate genes were successfully used to predict hematological response to imatinib in patients with Ph+ ALL162 or myeloid blast crisis of CML.163
Unexpected late side effects
Another issue affecting therapeutic decisions is late side effects. Generally, imatinib is well tolerated, particularly in newly diagnosed patients, where most adverse events are grade 1 or 2. However, some side effects may become apparent only with longer follow-up. For example, gynecomastia and low testosterone levels are frequent in men treated with imatinib.164 In addition, reduced sperm counts were observed in preclinical studies in dogs and monkeys.165 Only limited data are available regarding the fertility of men treated with imatinib. A total of 18 pregnancies in partners of men taking imatinib had been reported to Novartis by spring 2003. At this time, 6 pregnancies were ongoing and 4 had no or limited information. Among the remaining 8, there were 4 healthy infants, 2 therapeutic abortions (on social grounds), 1 spontaneous abortion, and 1 death in utero. Imatinib is teratogenic in rats, and thus women are advised not to conceive while on treatment. Nonetheless, 26 pregnancies have been reported to Novartis. Eleven patients elected to have therapeutic abortions, 5 had spontaneous abortions, and 7 pregnancies were either ongoing or without sufficient information at the time of last follow-up. Only 3 infants had been carried to term, 2 of which were healthy, while 1 had a hypospadia. Given these very limited data, it is currently impossible to exclude even a major teratogenic effect in humans, and women of childbearing age should not conceive while on imatinib. For long-term therapy of younger women, this is evidently an important issue. If a pregnancy does occur, decisions must be individualized, until more data have been acquired.165
A recent study at Novartis showed an increased frequency of genitourinary tumors in rats treated with 30 to 60 mg/kg imatinib daily for 24 months (G. R. Paul, Novartis Pharma, unpublished data, September 2004). A preliminary analysis of the safety data from clinical trials and spontaneous adverse event reports did not show evidence of an increased overall incidence of malignancies nor a specific increase in the incidence of bladder, kidney, or prostate tumors in patients treated with imatinib compared with that of the general population, but watchful monitoring of patients on imatinib seems nonetheless advisable.
Another concern is chromosomal abnormalities in the Ph– cells of patients with an MCR, in some cases associated with a myelodysplastic syndrome.166 This finding may be an indication that the hematopoiesis of some CML patients has sustained genetic damage in addition to the Philadelphia translocation, for example, as a result of prior cytotoxic chemotherapy. For adequate counseling, it will be important to determine the prognosis of such patients.
Conclusion
Imatinib is proof of principle that rationally designed, molecularly targeted therapy works. The clinical trials in CML, where imatinib produced an unprecedented rate of hematologic and cytogenetic remissions, confirm the essential role of the BCR-ABL tyrosine kinase. Equally important, though not the subject of this review, is the efficacy of imatinib in GISTs, chronic myelomonocytic leukemia with rearrangements of PDGFR, and the hypereosinophilic syndrome, all diseases where the activation of an imatinib-sensitive kinase is central to the pathogenesis.101,167,168 Imatinib represents a paradigm shift in cancer drug development. It is hoped that this will pave the way for a new generation of specific, targeted therapies for the many malignant conditions for which no efficient drug therapy is currently available.
Prepublished online as Blood First Edition Paper, December 23, 2004; DOI 10.1182/blood-2004-08-3097.
Supported by grants from the Doris Duke Charitable Foundation (B.J.D.), and the Leukemia and Lymphoma Society (B.J.D.). M.D. is a Junior Faculty Scholar of the American Society of Hematology.
One of the authors (E.B.) is employed by a company (Novartis Pharma) whose product was studied in the present work.
We thank Sandra Cowan-Jacob (Novartis) for generating Figure 5, Thomas Meyer (Novartis) for sharing unpublished in vitro kinase data, and Chris Koontz (Oregon Health and Science University [OHSU]) for editorial assistance.