Abstract
Present studies show that LBH589, a novel cinnamic hydroxamic acid analog histone deacetylase inhibitor, induces acetylation of histone H3 and H4 and of heat shock protein 90 (hsp90), increases p21 levels, as well as induces cell-cycle G1 phase accumulation and apoptosis of the human chronic myeloid leukemia blast crisis (CML-BC) K562 cells and acute leukemia MV4-11 cells with the activating length mutation of FLT-3. In MV4-11 cells, this was associated with marked attenuation of the protein levels of p-FLT-3, FLT-3, p-AKT, and p-ERK1/2. In K562 cells, exposure to LBH589 attenuated Bcr-Abl, p-AKT, and p-ERK1/2. Treatment with LBH589 inhibited the DNA binding activity of signal transducers and activators of transcription 5 (STAT5) in both K562 and MV4-11 cells. The hsp90 inhibitor 17-allyl-amino-demethoxy geldanamycin (17-AAG) also induced polyubiquitylation and proteasomal degradation of FLT-3 and Bcr-Abl by reducing their chaperone association with hsp90. Cotreatment with LBH589 and 17-AAG exerted synergistic apoptosis of MV4-11 and K562 cells. In the imatinib mesylate (IM)-refractory leukemia cells expressing Bcr-Abl with the T315I mutation, treatment with the combination attenuated the levels of the mutant Bcr-Abl and induced apoptosis. Finally, cotreatment with LBH589 and 17-AAG also induced more apoptosis of IM-resistant primary CML-BC and acute myeloid leukemia (AML) cells (with activating mutation of FLT-3) than treatment with either agent alone. (Blood. 2005;105:1768-1776)
Introduction
In chronic myeloid leukemia in blast crisis (CML-BC) cells, the deregulated activity of the oncoprotein tyrosine kinase (TK) Bcr-Abl promotes differentiation arrest, cell-cycle growth, and resistance to apoptosis.1 Bcr-Abl activates several molecular mechanisms that inhibit apoptosis.2,3 These include increased phosphorylation and activity of signal transducer and activator of transcription 5 (STAT5), resulting in increased expression of the antiapoptotic PBX interaction motif-2 (Pim-2) and Bcl-xL protein.4,5 Bcr-Abl also induces alpha serine/threonine (AKT), Ras/Raf/ERK1/2, and nuclear factor kappa Β (NFκB) activities, which together inhibit apoptosis through multiple mechanisms.6-8 Hence, down-regulating Bcr-Abl levels or activity is a rational strategy for treating CML.9 FLT-3 (FMS-like TK-3) is a member of the class III receptor tyrosine kinases.10 It is activated by FLT-3 ligand-dependent dimerization and autophosphorylation, resulting in pro-growth and pro-survival signaling, also through the STAT5-, Ras/Raf/ERK1/2-, and AKT-mediated pathways.10,11 Activating length mutation (LM)—for example, internal tandem duplication (ITD) of the juxtamembrane domain, or point mutation within the tyrosine kinase domain (TKD) of FLT-3—has been identified in approximately one-third of patients with AML.12,13 These lead to autophosphorylation and constitutive and ligand-independent activity of FLT-3.13 The presence of FLT-3 LM or TKD mutations may confer poor prognosis in AML.14 Based on the success of IM against Bcr-Abl, several inhibitors of FLT-3, including PKC412, have been developed and are being tested in patients with AML.15 But unlike IM in CML,16 so far, as single agents, FLT-3 inhibitors have yielded limited clinical success.13,17
FLT-3 and Bcr-Abl are bonafide client proteins for the chaperone, heat shock protein (hsp) 90.18-21 Treatment with hsp90 inhibitor 17-allylamino-demethoxy geldanamycin (17-AAG) was shown to disrupt the chaperone association of hsp90 with FLT-3 and Bcr-Abl, directing them to polyubiquitylation and proteasomal degradation.19,20 A number of well-characterized client proteins, including c-Raf, AKT, Bcr-Abl, and FLT-3, require interaction with hsp90 to maintain a mature, stable, and functional conformation.21 This is achieved through the binding of adenosine triphosphate (ATP) to the hydrophobic N-terminus pocket of hsp90, which is promoted by the binding of a set of cochaperones, p23 and p50cdc37, as well as of the client proteins.21-23 Adenosine diphosphate (ADP)-bound hsp90 interacts with an alternative subset of cochaperones, including hsp70 that directs the client proteins to a covalent linkage with polyubiquitin and subsequent degradation by the 26S proteasome.21 17-AAG interacts with the ATP binding pocket of hsp90, thereby inhibiting ATP binding and chaperone function of hsp90.21 This recruits the hsp70-based cochaperone complex to hsp90, which results in the ubiquitin-dependent proteasomal degradation of the client proteins.
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) reciprocally catalyze acetylation and deacetylation of lysine residues, respectively, in the amino terminal tails of the core nucleosomal histones in the chromatin and in several transcription factors. This modulates the expression of genes involved in cell-cycle regulation, differentiation, and apoptosis.24-26 Hence, treatment with HDAC inhibitors (HDIs) induces hyperacetylation of the nucleosomal histones and several transcription factors, which increases the levels of p21WAF1 and pro-death molecules (eg, Bak, Bax and Bim), resulting in cell-cycle arrest and apoptosis.26,27 The hydroxamic acid-analog (HA) class of HDIs (eg, SAHA and LAQ824) are known to induce acetylation of hsp90, diminish the binding of ATP to hsp90, and inhibit the chaperone association of hsp90 with its client proteins (eg, Bcr-Abl, mutant FLT-3, and AKT), resulting in attenuation of the levels of the client proteins.28,29 This is associated with growth arrest and apoptosis of leukemia cells expressing Bcr-Abl or mutant FLT-3.28,29 Recently, LBH589 has also been demonstrated to act as a potent HDI at submicromolar levels and is undergoing phase 1 evaluation.30 In the present studies, we determined the effects of LBH589 on the acetylation of hsp90 and on the levels of its client proteins AKT, Bcr-Abl, and mutant FLT-3 in cultured and primary CML-BC and AML cells. Additionally, since 17-AAG also inhibits hsp90 and promotes the proteasomal degradation of its client proteins, we determined whether cotreatment with 17-AAG and LBH589 compared with treatment with either agent alone would be more effective in attenuating the client proteins and exert synergistic cytotoxic effects against human AML or CML-BC cells.
Materials and methods
Reagents and antibodies
17-AAG was obtained from the Developmental Therapeutics Branch of Cancer Therapy Evaluation Program/National Cancer Institute/National Institutes of Health (CTEP/NCI/NIH) (Bethesda, MD). LBH589 was provided by Novartis Pharmaceuticals (East Hanover, NJ). Anti-hsp90 antibody was purchased from StressGen Biotechnologies (Victoria, BC, Canada). Polyclonal anti-poly(ADP-ribose) polymerase (PARP), caspase-9, caspase-3, p-FLT-3, and anti-p-ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Polyclonal anti-STAT5, anti-FLT-3, and goat polyclonal anti-Pim-2 antibody, as well as monoclonal anti-c-Myc and anti-Abl antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-p-STAT5 antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Antibodies for the immunoprecipitation and/or immunoblot analyses of hsp90, p-AKT, AKT, Bcl-xL, and ERK1/2 were obtained, as previously described.19,28,29
Cell culture
Acute leukemia MV4-11 (containing a 30-base-pair-long ITD in the exon 14 of FLT-3) and K562 (containing Bcr-Abl) cells were obtained from American Tissue Culture Collection (Manassas, VA) and maintained in culture in RPMI medium containing 10% fetal bovine serum and passaged twice a week, as previously described.19,28 Logarithmically growing cells were exposed to the designated concentrations of 17-AAG and/or LBH589. Following these treatments, cells were pelleted and washed free of the drug(s) prior to the performance of the studies.
Primary leukemia blasts from patients with CML-BC and AML
Leukemia blasts were procured from the peripheral blood of 5 patients who had met the clinical criteria of imatinib mesylate (IM)-resistant Philadelphia (Ph) chromosome-positive CML-BC and 4 patients with relapsed AML with FLT-3 ITD or point mutation were harvested and purified, as previously described.31 Informed consents were signed by all patients to allow use of their cells for these experiments, as part of a clinical protocol approved by the University of South Florida Institutional Review Board.
Isolation of CD34+ cells
CD34+ hematopoietic progenitors were isolated from the marrow of healthy donors after obtaining institutional review board informed consent. Mononuclear cells (MNCs) were prepared using Ficoll-Hypaque and then CD34+ cells isolated to more than 98% purity using fluorescence activated cell sorting, as previously described.28
Flow cytometry for cell-cycle status
Assessment of percentage of nonviable cells
Apoptosis assessment by annexin-V staining
Untreated and drug-treated cells were stained with annexin-V and propidium iodide (PI) and the percentage of apoptotic cells were determined by flow cytometry, as described previously.32 Analysis of synergism between 17-AAG and LBH589 in inducing apoptosis of MV4-11 and K562 cells was performed by median dose-effect analysis using commercially available software (Calcusyn; Biosoft, Ferguson, MO).19,29
Morphology of apoptotic cells
After drug treatment, 50 × 103 cells were washed with phosphate-buffered saline (PBS; pH 7.2) and resuspended in the same buffer. Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined by light microscopy. The percentage of apoptotic cells was calculated for each experiment.31,32
Western analyses of proteins
Immunoprecipitation of hsp90 or Bcr-Abl and immunoblot analyses
Following the designated treatments, cells were lysed in the lysis buffer (20 mM Tris [pH 8], 150 nM sodium chloride, 1% nonidet P40 (NP40), 0.1 M sodium fluoride, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium orthovanadate, 2.5 μg/mL leupeptin, 5 μg/mL aprotinin) for 30 minutes on ice, and the nuclear and cellular debris were cleared by centrifugation. Cell lysates (200 μg) were incubated with the hsp90- or Abl-specific monoclonal antibody for 1 hour at 4°C. To this, washed Protein G agarose beads were added and incubated overnight at 4°C. The immunoprecipitates were washed 3 times in the lysis buffer and proteins were eluted with the sodium dodecyl sulfate (SDS) sample loading buffer prior to the immunoblot analyses with specific antibodies against hsp90, anti-abl, anti-FLT-3, anti-acetyl lysine, or anti-ubiquitin antibody.28,31
Preparation of detergent-soluble and -insoluble fractions
After the designated drug treatments, cells were lysed with TNSEV buffer (50 mM Tris-HCl, pH 7.5, 2 mM EDTA [ethylenediaminetetraacetic acid], 100 mM NaCl, 1 mM sodium orthovanadate, 1% NP-40 containing 20 μg/mL aprotonin, 20 μg/mL leupeptin, 1 mM PMSF, 25 mM sodium fluoride (NaF), and 5 mM N-ethylmaleimide).16 The insoluble fraction (pellet) was solubilized with SDS buffer (80 mM Tris, pH 6.8, 2% SDS, 100 mM dithiothreitol [DTT], and 10% glycerol). Proteins (50 μg) from the NP-40 soluble and insoluble fractions were separated on 7.5% SDS-polyacrylamide gel and analyzed by Western blotting.28,31
Electrophoretic mobility shift analysis (EMSA) for STAT5
For EMSA for STAT5, a previously described method was used.19,28 Cells were lysed and the nuclei were pelleted by centrifugation, as previously described.14,28 The nuclear extracts (4 μ g to 50 μg) were incubated with 2.0 μg poly(dI-dC) for 15 minutes on ice, followed by 15 minutes of incubation with 1.0 ng Klenow-labeled DNA harboring the STAT5 optimal double-stranded DNA binding sequence: 5′-GATCCGAATTCCAGGAATTCA-3′. For supershift analysis, prior to the addition of poly(dI-dC) the nuclear extracts were incubated with anti-STAT5 antibody (Santa Cruz), or mouse IgG as a control, for 30 minutes on ice. DNA-protein complexes were separated on a 5% nondenaturing polyacrylamide gel in 0.25 × TBE (1 × TBE = 50 mM Tris-borate, 1.0 mM EDTA) and analyzed by autoradiography.
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined using the Student t test. P values of less than .05 were assigned significance.
Results
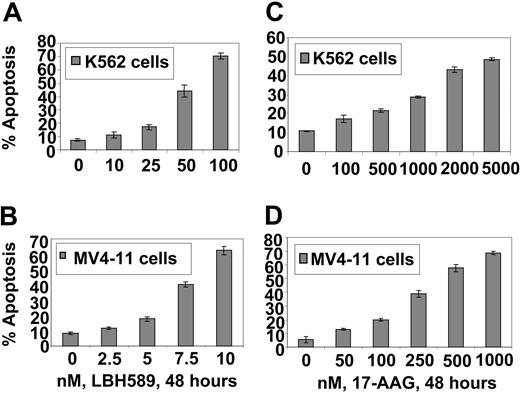
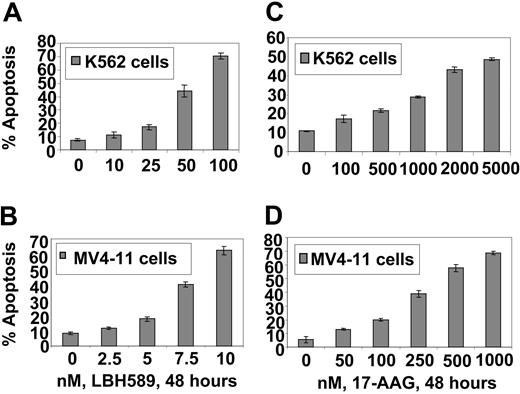
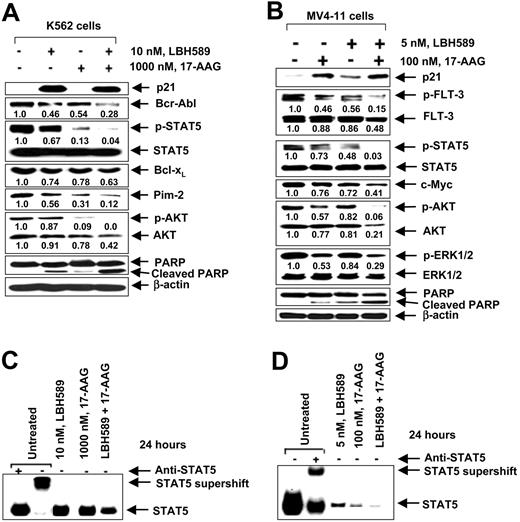
17-AAG and LBH589 attenuate Bcr-Abl and FLT-3 levels and induce apoptosis of K562 and MV4-11 cells. We have previously reported that both Bcr-Abl and FLT-3 are client proteins chaperoned by hsp90, and treatment with a histone deacetylase or hsp90 inhibitor results in down-regulation of the levels of Bcr-Abl and FLT-3 and apoptosis of leukemia cells. Therefore, we first determined the effect of LBH589 and 17-AAG alone on the levels of Bcr-Abl and FLT-3, downstream pro-growth and pro-survival signaling proteins, as well as on the cell-cycle status and apoptosis of K562 and MV4-11 cells. Figure 1A-B demonstrates that exposure to LBH589 induces apoptosis of K562 and MV4-11 cells in a dose-dependent manner. MV411 cells were 5- to 10-fold more sensitive than K562 cells, which may be due to the potent antiapoptotic activity of Bcr-Abl expressed in K562 but not MV4-11 cells. MV4-11 cells were also more sensitive than K562 cells to 17-AAG-induced apoptosis (approximately 4- to 5-fold; Figure 1C-D). Treatment with LBH589 induced hyperacetylation of histones H3 and H4 (Figure 2A,C) and up-regulated p21 levels in K562 and MV4-11 cells (Figure 2B,D). In contrast, LBH589 down-regulated the levels of Bcr-Abl and FLT-3 in K562 and MV4-11 cells, respectively (Figure 2B,D). This was also associated with attenuation of p-FLT-3 (Figure 2D) and p-Bcr-Abl levels (not shown) in the 2 cell types, respectively. Treatment of K562 and MV4-11 cells with LBH589 also attenuated the levels of the signaling proteins downstream of Bcr-Abl and mutant FLT-3 (eg, p-STAT5, p-AKT, and p-ERK1/2 [not shown for K562 cells]), with MV4-11 cells demonstrating greater sensitivity to LBH589 (Figure 2B,D). LBH589 mediated attenuation of p-STAT5 levels was also associated with inhibition of the DNA binding of STAT5 in both K562 and MV4-11 cells, again with more profound effect at much lower concentration of LBH589 in MV4-11 cells (Figure 2E-F). This resulted in the attenuation of the levels of c-Myc, Bcl-xL, and Pim-2, which are pro-growth or pro-survival genes known to be transactivated by STAT5 (Figure 2B,D). In both cell types, LBH589 treatment generated PARP cleavage induced by the activity of the effector caspase-3 and/or caspase-7 (Figure 2B,D).
LBH589 induces apoptosis of K562 and MV4-11 cells. Cells were treated with indicated concentrations of LBH589 (A, B) or 17-AAG (C, D) for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. Values represented as bar graphs are the mean of 3 experiments plus or minus the standard error (SE).
LBH589 induces apoptosis of K562 and MV4-11 cells. Cells were treated with indicated concentrations of LBH589 (A, B) or 17-AAG (C, D) for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. Values represented as bar graphs are the mean of 3 experiments plus or minus the standard error (SE).
LBH589 induces hyper-acetylation of histones H3 and H4, increases p21, but depletes Bcr-Abl in K562 and p-FLT-3 and FLT-3 in MV4-11 cells. Cells were treated with indicated concentrations of LBH589 for 24 hours. After this, histones were isolated and Western blot analyses of acetylated histones H3 and H4 were performed in K562 cells (A) and MV4-11 cells (C). Ponceau staining served as the loading control. Western blot analyses of p21, Bcr-Abl, p-AKT, AKT, p-STAT5, STAT5, c-Myc, Bcl-xL, Pim-2, and PARP were performed on the cell lysates from K562 cells (B), and Western blot analyses of p21, p-FLT-3, FLT-3, p-AKT, AKT, p-ERK1/2, ERK1/2, p-STAT5, STAT5, c-Myc, and PARP were performed on the cell lysates from MV4-11 cells (D). The levels of β-actin served as the loading control. LBH589 inhibits DNA binding activity of STAT5 in K562 and MV4-11 cells. Following treatment of K562 (E) or MV4-11 cells (F) with the indicated concentrations of LBH589 for 24 hours, nuclear extracts were tested for the DNA binding activity of STAT5 by EMSA. For supershift analysis, the nuclear extracts were treated with anti-STAT5 antibody before the addition of the poly(dI-dC) and the labeled DNA probe (see “Materials and methods”).
LBH589 induces hyper-acetylation of histones H3 and H4, increases p21, but depletes Bcr-Abl in K562 and p-FLT-3 and FLT-3 in MV4-11 cells. Cells were treated with indicated concentrations of LBH589 for 24 hours. After this, histones were isolated and Western blot analyses of acetylated histones H3 and H4 were performed in K562 cells (A) and MV4-11 cells (C). Ponceau staining served as the loading control. Western blot analyses of p21, Bcr-Abl, p-AKT, AKT, p-STAT5, STAT5, c-Myc, Bcl-xL, Pim-2, and PARP were performed on the cell lysates from K562 cells (B), and Western blot analyses of p21, p-FLT-3, FLT-3, p-AKT, AKT, p-ERK1/2, ERK1/2, p-STAT5, STAT5, c-Myc, and PARP were performed on the cell lysates from MV4-11 cells (D). The levels of β-actin served as the loading control. LBH589 inhibits DNA binding activity of STAT5 in K562 and MV4-11 cells. Following treatment of K562 (E) or MV4-11 cells (F) with the indicated concentrations of LBH589 for 24 hours, nuclear extracts were tested for the DNA binding activity of STAT5 by EMSA. For supershift analysis, the nuclear extracts were treated with anti-STAT5 antibody before the addition of the poly(dI-dC) and the labeled DNA probe (see “Materials and methods”).
Cotreatment with LBH589 and 17-AAG exerts superior anti-Bcr-Abl and FLT-3 activity and markedly attenuates STAT5 DNA binding activity in MV4-11 cells. Previous reports have indicated that exposure to 17-AAG markedly attenuates the mutant FLT-3 and p-FLT-3 levels in MV4-11, as well as attenuates Bcr-Abl levels in K562 cells.19,20 These studies have also shown that treatment with 17-AAG attenuates p-STAT5, p-AKT, and p-ERK1/2 levels, as well as inhibits the DNA binding activity of STAT5a in MV4-11 and K562 cells.19,31 Therefore, in the present studies, we determined the effects of cotreatment with LBH589 and 17-AAG on Bcr-Abl and FLT-3 levels, as well as on the levels of the signaling proteins downstream of Bcr-Abl and FLT-3. Figure 3A demonstrates that combined treatment of K562 cells with 10 nM LBH589 and 1.0 μM 17-AAG for 24 hours caused more down-regulation of the levels of Bcr-Abl and the downstream p-AKT, AKT, and p-STAT5 levels than treatment with either agent alone. This was associated with more PARP cleavage activity of the caspases. Cotreatment with 17-AAG did not augment LBH589-mediated induction in p21 levels, and STAT5 levels were unaffected by either treatment (Figure 3A). Cotreatment of MV4-11 cells with LBH589 and 17-AAG, each at lower concentration than those used in K562 cells, also caused more down-regulation of the levels of p-FLT-3, FLT-3, and the downstream signaling molecules p-AKT, AKT, p-STAT5, and p-ERK1/2 than treatment with either agent alone (Figure 3B). This was associated with more processing of PARP in MV4-11 cells. Although treatment with 17-AAG alone induced p21 levels, cotreatment with 17-AAG did not augment LBH589-mediated induction of p21 in MV4-11 cells. STAT5 and ERK1/2 levels were unaffected by any of the treatments in either cell type (Figure 3B). As compared with exposure to either agent alone, cotreatment of MV4-11 cells with LBH589 and 17-AAG also inhibited the DNA binding activity of STAT5 in K562 and MV4-11 cells (Figure 3C-D). This was again seen following treatment with lower concentration of each agent in MV4-11 versus K562 cells. More inhibition of the DNA binding activity of STAT5 by cotreatment with LBH589 and 17-AAG was also associated with more attenuation of c-Myc, as well as of Bcl-xL and Pim-2 (Figure 3A-B, and data not shown).
Cotreatment with LBH589 and 17-AAG causes greater attenuation of the levels of Bcr-Abl and FLT-3, and inhibits STAT5 DNA binding in K562 and MV4-11 cells. (A) Following treatment with 10 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p21, Bcr-Abl, p-STAT5, STAT5, Bcl-xL, Pim-2, p-AKT, AKT, p-ERK1/2, ERK1/2, and PARP were performed on the cell lysates from K562 cells. The levels of β-actin served as the loading control. (B) Following treatment with 5 nM of LBH589 and/or 100 nM 17-AAG for 24 hours, Western blot analyses of p21, p-FLT-3, FLT-3, p-AKT, AKT, p-ERK1/2, ERK1/2, p-STAT5, STAT5, and PARP were performed on the cell lysates from MV4-11 cells. The levels of β-actin served as the loading control. (C,D) Cotreatment with LBH589 and/or 17-AAG inhibits STAT5 DNA binding activity more than either agent alone in K562 (C) and MV4-11 cells (D). Following treatment of K562 or MV4-11 cells with indicated concentrations of LBH589 and/or 17-AAG for 24 hours, nuclear extracts were tested for the DNA binding activity of STAT5 by EMSA. For supershift analysis, the nuclear extracts were treated with anti-STAT5 antibody before the addition of the poly(dI-dC) and the labeled DNA probe.
Cotreatment with LBH589 and 17-AAG causes greater attenuation of the levels of Bcr-Abl and FLT-3, and inhibits STAT5 DNA binding in K562 and MV4-11 cells. (A) Following treatment with 10 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p21, Bcr-Abl, p-STAT5, STAT5, Bcl-xL, Pim-2, p-AKT, AKT, p-ERK1/2, ERK1/2, and PARP were performed on the cell lysates from K562 cells. The levels of β-actin served as the loading control. (B) Following treatment with 5 nM of LBH589 and/or 100 nM 17-AAG for 24 hours, Western blot analyses of p21, p-FLT-3, FLT-3, p-AKT, AKT, p-ERK1/2, ERK1/2, p-STAT5, STAT5, and PARP were performed on the cell lysates from MV4-11 cells. The levels of β-actin served as the loading control. (C,D) Cotreatment with LBH589 and/or 17-AAG inhibits STAT5 DNA binding activity more than either agent alone in K562 (C) and MV4-11 cells (D). Following treatment of K562 or MV4-11 cells with indicated concentrations of LBH589 and/or 17-AAG for 24 hours, nuclear extracts were tested for the DNA binding activity of STAT5 by EMSA. For supershift analysis, the nuclear extracts were treated with anti-STAT5 antibody before the addition of the poly(dI-dC) and the labeled DNA probe.
Cotreatment with LBH589 and 17-AAG exerts synergistic apoptotic effects against K562 and MV4-11 cells
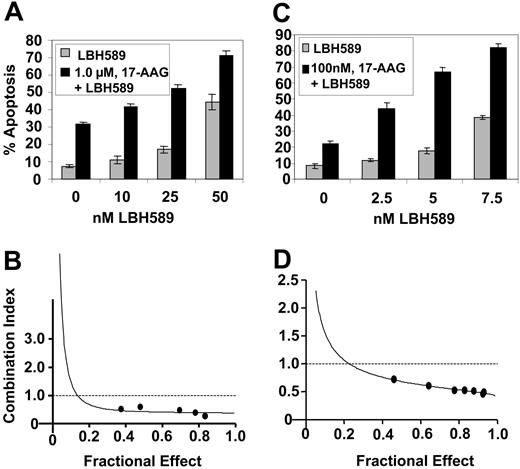
Next, we determined the effect of cotreatment with 17-AAG on LBH589-induced apoptosis of K562 and MV4-11 cells. Combined treatment of K562 cells with 1.0 μM 17-AAG for 48 hours significantly enhanced apoptosis (increase in the percent of annexin-V-positive cells) induced by 10, 25, and 50 nM LBH589 (P < .05; Figure 4A). Similarly, cotreatment of MV4-11 cells with 0.1 μM 17-AAG for 48 hours significantly enhanced apoptosis (increase in the percentage of annexin-V-positive cells) induced by 2.5, 5.0, and 7.5 nM LBH589 (P < .05; Figure 4C). These findings were also associated with increased PARP cleavage activity of the caspases in K562 and MV4-11 cells (data not shown). Importantly, exposure to the combination of 17-AAG and LBH589 exerted synergistic apoptotic effect in K562 and MV4-11 cells, as determined by the median dose-effect isobologram analysis described by Chou and Talalay,33 which revealed the combination index values of less than 1.0 for the combination in each cell type (Figure 4B,D).
Cotreatment with LBH589 and 17-AAG induces more apoptosis and exerts synergistic cytotoxic effects in K562 and MV4-11 cells. (A, C) K562 (A) and MV4-11 cells (C) were treated with the indicated concentrations of LBH589 and/or 17-AAG for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. (B, D) Using Calcusyn software (Biosoft), the analysis of the dose-effect relationship for LBH589 and/or 17-AAG-induced apoptosis of K562 (B) and MV4-11 (D) cells was performed according to the median effect method of Chou and Talalay.33 The combination index (CI) values were calculated for 3 independent experiments. CI<1, CI=1, and CI1 represent synergism, additivity, and antagonism of the 2 agents, respectively.
Cotreatment with LBH589 and 17-AAG induces more apoptosis and exerts synergistic cytotoxic effects in K562 and MV4-11 cells. (A, C) K562 (A) and MV4-11 cells (C) were treated with the indicated concentrations of LBH589 and/or 17-AAG for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. (B, D) Using Calcusyn software (Biosoft), the analysis of the dose-effect relationship for LBH589 and/or 17-AAG-induced apoptosis of K562 (B) and MV4-11 (D) cells was performed according to the median effect method of Chou and Talalay.33 The combination index (CI) values were calculated for 3 independent experiments. CI<1, CI=1, and CI1 represent synergism, additivity, and antagonism of the 2 agents, respectively.
LBH589 depletes mutant Bcr-Abl levels and induces apoptosis of imatinib-refractory leukemia cells
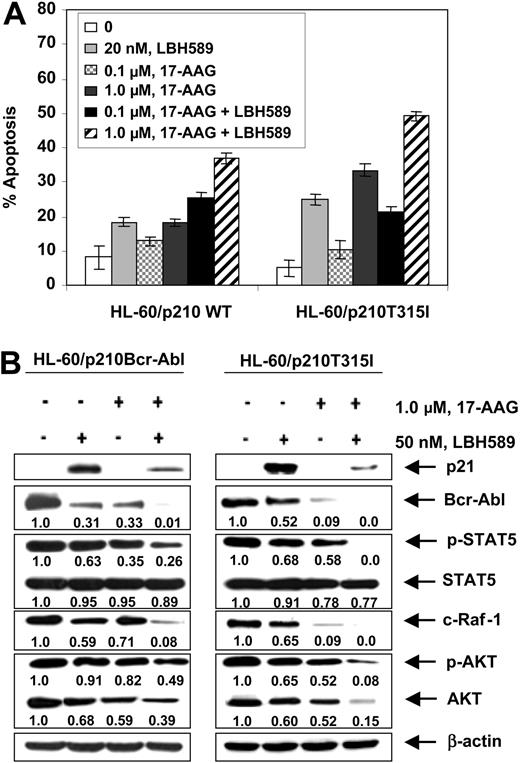
Next, we investigated whether treatment with LBH589 would also attenuate the levels of a mutant Bcr-Abl containing a point mutation in the kinase domain. For this we chose the point mutant Bcr-AblT315I, which has been shown to confer high level of resistance to imatinib in CML-BC cells.34 This mutation contains the single nucleotide C to T change that results in a threonine to isoleucine substitution at position 315 of Bcr-Abl. We engineered a T315I mutation into the wild type (wt) p210 bcr-abl and transfected the wt and the mutant construct into the human AML HL-60 cells through the pSTAR expression vector.35 Subclones of the cells were isolated that, as previously reported, demonstrated expression of either the wt (HL-60/p210 wt) or T315I mutation-containing Bcr-Abl (HL-60/p210 T315I).28 Consistent with this, while exposure to IM induced apoptosis of HL-60/p210 wt cells in a dose-dependent manner, HL-60/p210 T315I cells were completely resistant to IM-induced apoptosis.28 In contrast, treatment with LBH589 or 17-AAG for 24 hours induced significantly more apoptosis of HL-60/p210 T315I than HL-60/p210 cells (P < .05; Figure 5A), which was also associated with attenuation of the T315I mutation-containing Bcr-Abl and wt Bcr-Abl levels in HL-60/p210 T315I and HL-60/p210 cells, respectively (Figure 5B). In both transfectants, exposure to LBH589 or 17-AAG also attenuated the levels of p-STAT5, c-Raf, p-AKT, and AKT, but not of STAT5 (Figure 5B). In contrast, only exposure to LBH581 induced p21 levels. Similar to treatment with either agent alone, cotreatment with LBH589 and 17-AAG also resulted in significantly more apoptosis of HL60/p210T315I than HL-60/p210cells (Figure 5A), which was associated with more down-regulation of the levels of p-STAT5, c-Raf, p-AKT, and AKT, but not of STAT5 in HL60/p210T315I cells (Figure 5B).
Cotreatment with LBH589 and 17-AAG induces more apoptosis of HL-60 cells with the wild-type p210Bcr-Abl or mutant p210T315I Bcr-Abl expressing leukemia cells. (A) HL-60/p210Bcr-Abl and HL-60/p210T315I cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. (B) Following treatment with 50 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p21, Bcr-Abl, p-STAT5, STAT5, c-Raf-1, p-AKT, and AKT were performed in the cell lysates from wt p210Bcr-Abl or its mutant HL60/p210T315I expressing leukemia cells. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and 17-AAG induces more apoptosis of HL-60 cells with the wild-type p210Bcr-Abl or mutant p210T315I Bcr-Abl expressing leukemia cells. (A) HL-60/p210Bcr-Abl and HL-60/p210T315I cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 48 hours. Following this, the percentage of annexin-V-stained apoptotic cells was determined by flow cytometry. (B) Following treatment with 50 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p21, Bcr-Abl, p-STAT5, STAT5, c-Raf-1, p-AKT, and AKT were performed in the cell lysates from wt p210Bcr-Abl or its mutant HL60/p210T315I expressing leukemia cells. The levels of β-actin served as the loading control.
LBH589 and 17-AAG inhibit chaperone association of hsp90 with Bcr-Abl and FLT-3, promoting their polyubiquitylation and proteasomal degradation
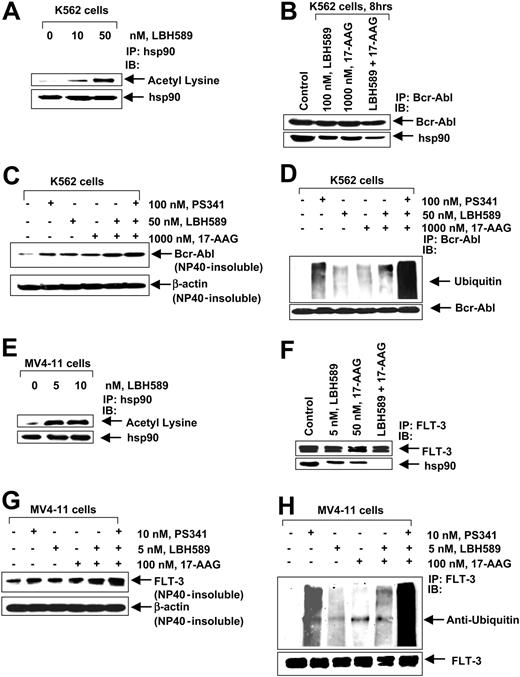
We next determined the effect of the treatment with LBH589 on the chaperone association of Bcr-Abl (in K562) and FLT-3 (in MV4-11) with hsp90. Treatment of K562 cells with 10 nM or 50 nM and of MV4-11 cells with 5 nM or 10 nM of LBH589 for 8 hours caused marked acetylation of hsp90, detected by immunoprecipitation of hsp90 followed by immunoblotting with an antibody that recognized antiacetylated lysine residues (Figure 6A,E). Cotreatment with LBH589 and 17-AAG, more than treatment with either agent alone, reduced the chaperone association of Bcr-Abl (K562 cells) or FLT-3 (in MV4-11 cells) to hsp90, detected as reduced levels of hsp90 that could be coimmunoprecipitated with Bcr-Abl or FLT-3 in LBH589- and/or 17-AAG-treated versus control cells (Figure 6B,F). The chaperone association of c-Raf and AKT was also reduced more following treatment of K562 and MV4-11 cells with LBH589 and 17-AAG than either drug alone (data not shown). Previous reports have shown that inhibition of the chaperone association with hsp90 induces the client proteins Bcr-Abl and FLT-3 to accumulate in the detergent-insoluble cellular fraction, as well as directs them for polyubiquitination and proteasomal degradation.19,28 Figure 6C demonstrates that cotreatment with LBH589 and 17-AAG more than either agent alone increased Bcr-Abl accumulation in the detergent-insoluble fraction, which was further enhanced by cotreatment with the proteasome inhibitor PS341 and the combination of LBH589 and 17-AAG. A similar observation was made with respect to FLT-3 in MV4-11 cells, following treatment with LBH589 and/or 17-AAG and cotreatment with the proteasome inhibitor PS341 (Figure 6G). Consistent with this, cotreatment with LBH589 and 17-AAG more than either agent alone increased the polyubiquitylation of proteins in the immunoprecipitates of Bcr-Abl and FLT-3 in K562 and MV4-11 cells, respectively, which was enhanced by cotreatment with PS341 and the combination of LBH589 and 17-AAG (Figure 6D,H).
LBH589 acetylates and inhibits hsp90 chaperone function, promoting polyubiquitylation and proteasomal degradation of Bcr-Abl and FLT-3. (A) K562 cells were exposed to the indicated concentrations of LBH589 for 8 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or antiacetylated lysine antibody. (B) K562 cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 8 hours. After this, immunoprecipitates containing Bcr-Abl from the cell lysates were immunoblotted with anti-Abl or anti-hsp90 antibodies. (C) K562 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone, for 8 hours. The detergent (NP-40)-insoluble fraction was immunoblotted with Bcr-Abl antibody. The levels of β-actin served as the loading control. (D) K562 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. After this, immunoprecipitates of Bcr-Abl from the cell lysates were immunoblotted with anti-ubiquitin or anti-Bcr-Abl antibodies. (E) MV4-11 cells were exposed to the indicated concentrations of LBH589 for 8 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or antiacetylated lysine antibody. (F) MV4-11 cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 8 hours. After this, immunoprecipitates of FLT-3 from the cell lysates were immunoblotted with anti-FLT-3 or anti-hsp90 antibodies. (G) MV4-11 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. The detergent (NP-40)-insoluble fraction was immunoblotted with anti-FLT-3 antibody. The levels of β-actin served as the loading control. (H) MV4-11 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. After this, immunoprecipitates of FLT-3 from the cell lysates were immunoblotted with anti-ubiquitin or anti-FLT-3 antibodies.
LBH589 acetylates and inhibits hsp90 chaperone function, promoting polyubiquitylation and proteasomal degradation of Bcr-Abl and FLT-3. (A) K562 cells were exposed to the indicated concentrations of LBH589 for 8 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or antiacetylated lysine antibody. (B) K562 cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 8 hours. After this, immunoprecipitates containing Bcr-Abl from the cell lysates were immunoblotted with anti-Abl or anti-hsp90 antibodies. (C) K562 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone, for 8 hours. The detergent (NP-40)-insoluble fraction was immunoblotted with Bcr-Abl antibody. The levels of β-actin served as the loading control. (D) K562 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. After this, immunoprecipitates of Bcr-Abl from the cell lysates were immunoblotted with anti-ubiquitin or anti-Bcr-Abl antibodies. (E) MV4-11 cells were exposed to the indicated concentrations of LBH589 for 8 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or antiacetylated lysine antibody. (F) MV4-11 cells were treated with the indicated concentrations of LBH589 and/or 17-AAG for 8 hours. After this, immunoprecipitates of FLT-3 from the cell lysates were immunoblotted with anti-FLT-3 or anti-hsp90 antibodies. (G) MV4-11 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. The detergent (NP-40)-insoluble fraction was immunoblotted with anti-FLT-3 antibody. The levels of β-actin served as the loading control. (H) MV4-11 cells were either cotreated with the indicated concentrations of LBH589, 17-AAG, and PS341, and/or with the agents alone for 8 hours. After this, immunoprecipitates of FLT-3 from the cell lysates were immunoblotted with anti-ubiquitin or anti-FLT-3 antibodies.
Cotreatment with 17-AAG and LBH589 causes more attenuation of Bcr-Abl and FLT-3 and loss of viability of primary CML-BC and AML cells with FLT-3 mutation than either agent alone
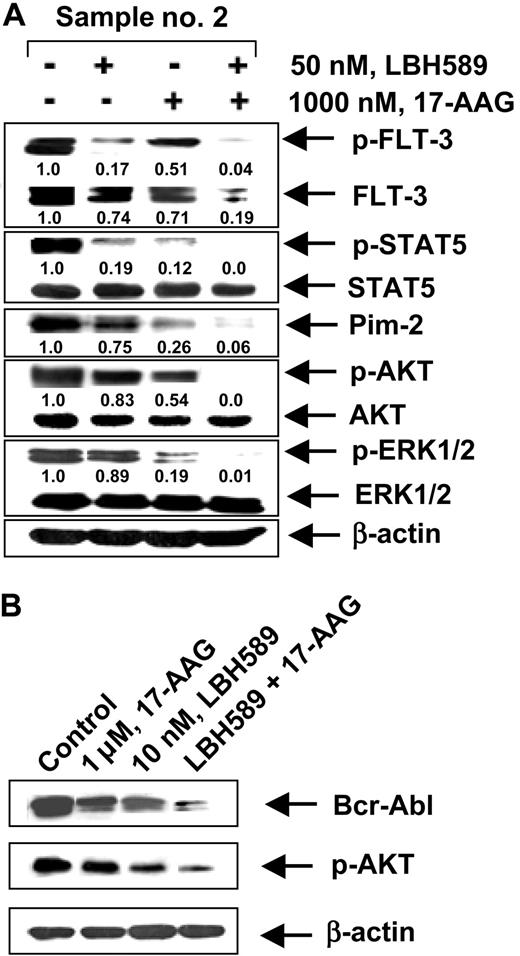
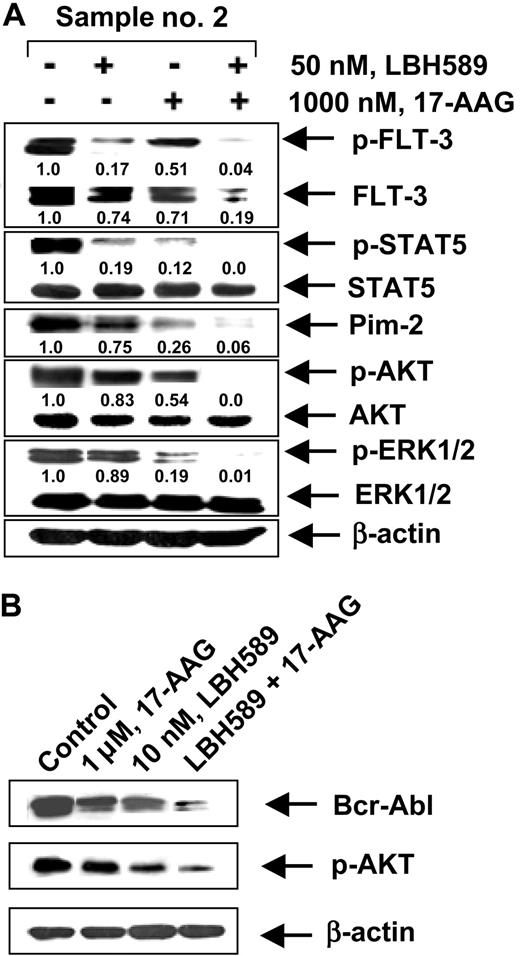
We next determined whether the combination of 17-AAG and LBH589 versus either agent alone would also have superior activity against primary CML-BC and AML cells isolated from the peripheral blood and/or bone marrow samples from 5 patients who had relapsed with IM-refractory CML-BC and 4 patients with AML with FLT-3 mutations. Among the samples of AML cells, sample no. 1 contained a point mutation D835Y in FLT-3, whereas samples no. 2, no. 3, and no. 4 each contained an internal tandem duplication of 30 to 51 base pairs of FLT-3 in the exon 14 or 15 (data not shown). The mutational status of Bcr-Abl in the IM-refractory primary CML-BC cells had not been determined. Table 1 indicates that treatment of CML-BC or AML cells with either 17-AAG or LBH589 in a dose-dependent manner for 48 hours increased the percentage of nonviable cells. Additionally, the table demonstrates that cotreatment with 17-AAG (250 nM or 1000 nM) and LBH589 (10 nM or 50 nM) resulted in a markedly higher percentage of nonviable cells than treatment with either agent alone (values represent mean of 2 experiments performed in duplicate). We also determined the loss of cell viability of CD34+ normal human bone marrow progenitor cells following exposure to LBH589 and/or 17-AAG. Importantly, the combined treatment with 50 nM LBH589 and 1.0 μM of 17-AAG for 48 hours induced the loss of cell viability (determined by trypan blue and propidium iodide uptake) in 25.3% of the CD34+ normal bone marrow progenitor cells (mean of experiments performed in duplicate on 2 samples), which was significantly lower (P < .05) than what was observed in identically treated AML or CML-BC cells (Table 1). Treatment with 50 nM LBH589 or 1.0 μM of 17-AAG alone for 48 hours also induced markedly less loss of cell viability than in AML and CML-BC cells (data not shown). Following treatment with LBH589 and/or 17-AAG, Western blot analyses of the total cell lysates of the AML sample no. 2 (Figure 7A) showed a dose-dependent, 17-AAG-mediated attenuation of the levels of the FLT-3, p-FLT-3, p-STAT5, p-AKT, and AKT levels, without significant alteration in STAT5, p-ERK1/2, and ERK1/2 (not shown). Additionally, cotreatment with LBH589 and 17-AAG for 24 hours resulted in more attenuation of Bcr-Abl and p-AKT in a representative CML-BC sample than treatment with either agent alone (Figure 7B). These data corroborate the results of the studies in the cultured leukemia cells.
Effect of 17-AAG and/or LBH589 on pro-growth and pro-survival signaling proteins. Cotreatment with LBH589 and/or 17-AAG attenuates Bcr-Abl in primary CML-BC (B) and p-FLT-3 and FLT-3 in primary AML cells (A). Following treatment with 50 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p-FLT-3, FLT-3, p-STAT5, STAT5, Pim-2, p-AKT, AKT, p-ERK1/2, and ERK 1/2 were performed on the cell lysates from sample no. 2. The levels of β-actin served as the loading control.
Effect of 17-AAG and/or LBH589 on pro-growth and pro-survival signaling proteins. Cotreatment with LBH589 and/or 17-AAG attenuates Bcr-Abl in primary CML-BC (B) and p-FLT-3 and FLT-3 in primary AML cells (A). Following treatment with 50 nM of LBH589 and/or 1000 nM 17-AAG for 24 hours, Western blot analyses of p-FLT-3, FLT-3, p-STAT5, STAT5, Pim-2, p-AKT, AKT, p-ERK1/2, and ERK 1/2 were performed on the cell lysates from sample no. 2. The levels of β-actin served as the loading control.
Discussion
In this report, we demonstrate for the first time that exposure to the novel cinnamic acid hydroxamate HDI, LBH589, in a dose-dependent manner, induced histone H3 and H4 acetylation and increased p21 levels, but down-regulated the levels of Bcr-Abl or mutant FLT-3 in human leukemia cells. This was associated with marked attenuation of the downstream, pro-growth and pro-survival signaling molecules, including p-AKT, p-ERK1/2, and p-STAT5, as well as with induction of apoptosis of K562 and MV4-11 cells. As has been demonstrated with the other HA-HDIs,28,36 treatment with LBH589 also induced the acetylation of hsp90 and inhibition of its chaperone activity for Bcr-Abl and mutant FLT-3. This resulted in polyubiquitylation and proteasomal degradation of the hsp90 client proteins Bcr-Abl, mutant FLT-3, AKT, and c-Raf. Treatment with LBH589 alone may have depleted the pro-survival and pro-growth activity of AKT and c-Raf not only by attenuating the levels of the upstream oncoprotein kinases Bcr-Abl and mutant FLT-3, but also by directly promoting the attenuation of downstream AKT and c-Raf levels through enhancement of their polyubiquitylation and proteasomal degradation. This `longitudinal' 2-step inhibition of the signaling initiated by Bcr-Abl or mutant FLT-3 may be responsible for the growth inhibitory and apoptotic activity of LBH589 against leukemia cells. This also occurs following cotreatment with inhibitors of Bcr-Abl and AKT, or of Bcr-Abl and mammalian target of rapamycin (mTOR) kinase.37,38 LBH589-mediated inhibition of p-STAT5 correlated with the inhibition of its DNA binding activity and depletion of its target gene products Bcl-xL, Pim-2, and c-Myc.5,19 A recent report had shown that a deacetylase activity may be required for the induction of STAT5 target genes, and treatment with HA-HDIs abrogated the induction of STAT5 target genes by preventing the recruitment of the basal transcription machinery and blocking the transcription initiation.39 Present findings with LBH589 are consistent with this report.
In the present studies, combined treatment with LBH589 and 17-AAG exerted synergistic apoptotic effects in K562 and MV4-11 cells. While cotreatment with 17-AAG did not enhance LBH589-mediated induction of p21, it resulted in greater decline of Bcr-Abl, p-AKT, AKT, and p-STAT5 levels. In both cell types, a greater decline in the p-AKT versus AKT level was observed following treatment with the combination of 17-AAG and LBH589. This may be because the combination has more dramatic inhibitory effect on the activity of the serine/threonine PP1 phosphatase, which has been shown to down-regulate AKT phosphorylation and activity.36 Additionally, consistent with the superior inhibitory effects of the combination on p-STAT5 levels and the DNA binding activity of STAT5, cotreatment with LBH589 and 17-AAG also caused greater attenuation of Pim-2, c-Myc, and Bcl-xL levels in K562 and MV4-11 cells. Of these, the serine-threonine kinase Pim-2 is especially important, since it has been shown to confer long-term apoptosis resistance, maintaining mitochondrial potential, survival, and cell size.40 This is due to the ability of Pim-2 to promote rapamycin-resistant phosphorylation of the protein translation repressor 4EBP1 and phosphorylation of the BH3 domain containing pro-death protein BAD.40 Collectively, the more dramatic inhibitory effects of the combination of LBH589 and 17-AAG on several pro-growth and pro-survival signaling molecules may be one mechanism responsible for the synergistic apoptotic effects in K562 and MV4-11 cells. Another reason may be that combined treatment with LBH589 and 17-AAG could lead to more profound inhibition and disruption of the chaperone association of hsp90 with Bcr-Abl and mutant FLT-3, directing these client proteins to increased polyubiquitylation and proteasomal degradation. This is supported by our findings demonstrating that cotreatment with LBH589 and 17-AAG, versus treatment with either agent alone, results in increased polyubiquitylation and accumulation of Bcr-Abl and mutant FLT-3 in the detergent-insoluble fraction K562 in MV4-11 cells, respectively.
Mutations in the kinase domain of bcr-abl represent the main mechanism of resistance to IM in the advanced phases of CML.41 Approximately 25 different point mutations have been described in the kinase domain of Bcr-Abl. Those mutations that interfere directly with the binding of IM to Bcr-Abl (eg, T315I) are the most resistant to inhibition by IM.41-44 BMS354 825 is among the several dual Abl/Src kinase inhibitors that have been developed, which are able to inhibit Bcr-Abl with mutations in its P loop and activation loop but not T315I.44 In contrast, treatment with HA-HDIs or 17-AAG has been shown to inhibit IM-resistant Bcr-Abl with mutations including Bcr-AblT315I, and induce apoptosis of IM-resistant CML-BC cells.28,31,45 In the present studies, we demonstrate that the combination of LBH589 and 17-AAG is more potent than either agent alone against human leukemia cells expressing Bcr-AblT315I. Furthermore, the combination is even more active in attenuating Bcr-AblT315I versus wild-type Bcr-Abl, and in inducing more apoptosis of HL-60/Bcr-AblT315I versus HL-60/p210wt Bcr-Abl cells. Although the mechanism is not definitively established, the combination may be more effective in attenuating Bcr-AblT315I levels because, as a client protein of hsp90, Bcr-AblT315I may be more dependent on the chaperone function of hsp90 than the wild-type Bcr-Abl to maintain a mature, stable, and functional conformation. Consequently, Bcr-AblT315I may be more susceptible to LBH589- and 17-AAG-induced polyubiquitylation and proteasomal degradation. A similar greater dependence on hsp90 function and susceptibility for 17-AAG-mediated proteasomal degradation has also been demonstrated for mutant versus wild-type FLT-3 and p53.29,46 Recently, 17-AAG has been shown to have greater affinity for binding to hsp90 from cancer versus normal host cells, which underscores the potential selectivity of this agent in exerting its effect against leukemia versus normal host cells.47 Importantly, since treatment with 17-AAG also leads to attenuation of the levels of Src, as well as the other client proteins AKT and c-Raf,21,43,48 17-AAG is able to abrogate the levels and activity of multiple signaling molecules downstream of Bcr-Abl that confer survival advantage and resistance to apoptosis.
Albeit preliminary, data are also presented that indicate that the combination of LBH589 and 17-AAG attenuates Bcr-Abl and mutant FLT-3 more than treatment with either agent in the primary CML-BC and AML cells, respectively. This is associated with greater loss of survival of the primary leukemia cells following treatment with the combination than with either agent alone. Further studies are needed to characterize the mutational status of Bcr-Abl in the IM-resistant primary CML-BC cells, and to determine whether the combination of LBH589 and 17-AAG is able to attenuate the levels and downstream activity of Bcr-AblT315I, and other IM-resistant Bcr-Abl mutants, as well as induce cell death of the IM-resistant primary CML-BC cells. Bcr-AblT315I expressing IM-resistant CML cells have also been shown to be refractory to novel dual Abl/Src and other Bcr-Abl kinase inhibitors, and may represent a pan-kinase inhibitor-refractory clone of CML-BC cells. Therefore, the growth inhibitory and apoptotic effects of the combination of LBH589 and 17-AAG against this clone of primary CML-BC cells is a significant finding. It supports the rationale for the future in vivo studies of this combination in IM-resistant CML with mutations in Bcr-Abl, especially since treatment with the combination exerts relatively less in vitro cytotoxic effects against CD34+ normal bone marrow progenitor cells.
Prepublished online as Blood First Edition Paper, October 28, 2004; DOI 10.1182/blood-2004-09-3413.
Supported in part by National Institutes of Health (NIH) grant CA95188.
P.A. is employed by and has declared a financial interest in Novartis Pharmaceutical Inc, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.