Abstract
Besides its role as an essential regulator of physiologic and pathologic angiogenesis, vascular endothelial growth factor (VEGF) triggers growth, survival, and migration of leukemia and multiple myeloma cells; plays a pivotal role in hematopoiesis; inhibits maturation of dendritic cells; and increases osteoclastic bone-resorbing activity as well as osteoclast chemotaxis. Dysregulation of VEGF expression and signaling pathways therefore plays an important role in the pathogenesis and clinical features of hematologic malignancies, in particular multiple myeloma. Direct and indirect targeting of VEGF and its receptors therefore may provide a potent novel therapeutic approach to overcome resistance to therapies and thereby improve patient outcome.
Introduction
VEGF
VEGF isoforms. The original peptide growth factor vascular endothelial growth factor (VEGF), first described as vascular permeability factor (VPF) and now denoted VEGF-A, was identified in the 1980s.1-5 The heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene,6,7 as well as the high homology of VEGF across species, reflects the pivotal importance of this protein.8,9 Beside the prototype member VEGF-A, there is now a VEGF family of structurally related dimeric glycoproteins belonging to the platelet-derived growth factor (PDGF) superfamily of growth factors. The VEGF family includes VEGF-B,10 VEGF-C,11 VEGF-D,12 VEGF-E, and placenta growth factor (PlGF).13,14 The active forms of VEGF are synthesized either as homodimers (40-45 kDa) or as heterodimers with other VEGF family members, such as PlGF. VEGF-A, located at chromosome 6p21.3, is encoded by 8 exons separated by 7 introns. Four principal isoforms of VEGF-A are generated by alternative splicing15,16 : VEGF121, the predominant isoform VEGF165, VEGF189, and VEGF206.4 These forms differ primarily in their bioavailability, which is conferred by heparin and heparan-sulfate binding domains encoded by exons 6 and 7. VEGF189 and VEGF206 contain additional stretches of basic residues, resulting in their nearly complete retention in the extracellular matrix (ECM). VEGF206 binds most strongly to heparin. VEGF165 lacks exon 6 and is secreted but also remains bound to the cell surface and the extracellular matrix via its heparin-binding sites. VEGF121, which lacks both exon 6 and exon 7, fails to bind heparin and is therefore a freely diffusible protein. The VEGF121, VEGF165, and VEGF189 forms are abundant and usually produced simultaneously. VEGF121 and VEGF165 isoforms induce mitogenic and permeability-enhancing activity on endothelial cells, whereas the other longer isoforms trigger only permeability-enhancing activity.15,17,18 Additionally, plasmin-induced cleavage of both VEGF165 and VEGF189 releases a bioactive carboxy-terminal domain111-165 of VEGF.19 Analysis of the crystal structure of VEGF-A shows an antiparallel homodimer, which is covalently linked by 2 disulfide bridges.20
VEGF expression. VEGF165, VEGF189, and VEGF206 levels are increased in several human malignancies including breast,21 lung,22 brain,23 pancreatic,24 ovarian,25 kidney, and bladder carcinomas.26 Less frequently expressed are the VEGF-A splice forms VEGF145, VEGF183, VEGF162, and VEGF165b.27 VEGF145, for example, is expressed by cells derived from reproductive organs, the skin, and kidney28-31 ; it is also expressed by both human multiple myeloma (MM) cells32 and Kaposi sarcoma (KS)–associated herpesvirus or human herpesvirus-8 (KSHV or HHV-8)–associated primary effusion lymphomas (PELs).33
Hypoxia is a key regulator of VEGF expression via the hypoxia-inducible factor-1 (HIF-1)/von Hippel-Lindau tumor suppressor gene (VHL) pathway,34,35 and VEGF is therefore most highly expressed adjacent to necrotic areas.36,37 Besides hypoxia, growth factors and cytokines including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), tumor necrosis factor α (TNFα), tumor growth factor α and β1 (TNFα, TGFβ1), keratinocyte growth factor, insulin-like growth factor-1 (IGF-1), interleukin-1β (IL-1β), and IL-638 up-regulate transcription of VEGF mRNA.39,40 VEGF expression is also induced by UVB and H2O241 ; mutant p53 (via PKC)42 and mutant Ras oncogenes43,44 ; and proangiogenic oncogenes including Src, c-Myc, Fos, and Bcl-2.45
VEGF receptors and signal transduction
VEGF receptors. VEGF-A, -B, -C, -D, and PlGF bind with different affinities to 3 related receptor tyrosine kinases: VEGF receptor-1 (VEGFR-1; fms-like tyrosine kinase-1; Flt-1), VEGFR-2 (kinase domain region, KDR; homolog to murine fetal liver kinase-1, Flk-1), and VEGFR-3. VEGF-A mediates its activity mainly via 2 receptor tyrosine kinases (RTKs): the high-affinity receptor VEGFR-1 (KD 10-20pM) located at chromosome 13q12-1346 and VEGFR-2 (KD 75-125pM) located at chromosome 4q11-1247-51 (Figure 1A). Both receptors have a crucial role in the development of the vascular system evidenced by embryonic lethality upon their disruption.52,53 These VEGF-RTKs are single-pass transmembrane receptors with 7 immunoglobulin-like loops in the extracellular domain and a cytoplasmic tyrosine-kinase domain, separated by an intervening, noncatalytic, 70-aa residue sequence. Both are modified after translation by N-linked glycosylation and phosphorylation on serine, threonine, and tyrosine. Using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), VEGFR-1 migrates at a molecular mass of 180 kDa, and VEGFR-2 at 230 kDa. Hypoxia directly up-regulates VEGFR-1 gene expression via an HIF-1α binding site within the VEGFR-1 promoter. In contrast to VEGFR-1, VEGFR-2 lacks this binding site.54 The VEGF-A aa residues Arg82, Lys84, and His85 are required for binding to VEGFR-2, whereas the VEGF-A aa residues Asp63, Glu64, and Glu67 are required for binding to VEGFR-155 (Figure 1B).
Interactions of VEGF family members with their receptors. (A) Growth factors and receptors of the VEGF family. Interactions of the VEGF family members VEGF-A, -B, -C, -D, -E, PlGF-1, PlGF-2 and the human immunodeficiency (HIV) Tat protein with the receptor tyrosine kinases VEGFR-1, VEGFR-2, and VEGFR-3; the accessory isoform specific receptors neuropilin-1 and -2 (NPR-1, -2); and heparansulfate proteoglycane (HSP) are shown. The receptors are displayed with their major structural motifs. VEGF-A splicing forms are in boxes. The extracellular domain of VEGFR-1 also is expressed as a soluble protein. (B) VEGFR-1 and -2. VEGF-A mediates its activity mainly via 2 receptor tyrosine kinases, VEGFR-1 and -2. VEGFR-1 and -2 are single-pass transmembrane receptors with 7 immunoglobulin (Ig)–like loops in the extracellular domain and a cytoplasmic tyrosine-kinase domain, separated by an intervening, noncatalytic, 70-aa residue sequence. Two VEGF monomers linked together by disulfide bonds induce receptor dimerization, thereby triggering kinase activation of both the receptor itself and several cytoplasmic signal transduction molecules.
Interactions of VEGF family members with their receptors. (A) Growth factors and receptors of the VEGF family. Interactions of the VEGF family members VEGF-A, -B, -C, -D, -E, PlGF-1, PlGF-2 and the human immunodeficiency (HIV) Tat protein with the receptor tyrosine kinases VEGFR-1, VEGFR-2, and VEGFR-3; the accessory isoform specific receptors neuropilin-1 and -2 (NPR-1, -2); and heparansulfate proteoglycane (HSP) are shown. The receptors are displayed with their major structural motifs. VEGF-A splicing forms are in boxes. The extracellular domain of VEGFR-1 also is expressed as a soluble protein. (B) VEGFR-1 and -2. VEGF-A mediates its activity mainly via 2 receptor tyrosine kinases, VEGFR-1 and -2. VEGFR-1 and -2 are single-pass transmembrane receptors with 7 immunoglobulin (Ig)–like loops in the extracellular domain and a cytoplasmic tyrosine-kinase domain, separated by an intervening, noncatalytic, 70-aa residue sequence. Two VEGF monomers linked together by disulfide bonds induce receptor dimerization, thereby triggering kinase activation of both the receptor itself and several cytoplasmic signal transduction molecules.
VEGFR-1 and VEGFR-2 are expressed in all adult vascular endothelial cells with the exception of vascular endothelial cells in the brain.56 Besides endothelial cells, VEGFR-1 is expressed on hematopoietic stem cells39 and monocytes57,58 ; human trophoblast, choriocarcinoma cells,59 renal mesangial cells,60 and vascular smooth muscle cells61 ; and MM and leukemic cells.62,63 VEGFR-2 also is expressed on circulating endothelial progenitor cells (CEPs),64-66 pancreatic duct cells,67 retinal progenitor cells,68 and megakaryocytes.69 Co-expression of VEGFR-1 and VEGFR-2 is found on normal human testicular tissue70 and in the myometrium. Significantly increased levels of both VEGFR-1 and -2 also are present on cancers of kidney, bladder,71 ovaries,25 and brain.23
The role of VEGFR-2 in endothelial cells has been extensively studied: VEGFR-2 mediates developmental angiogenesis and hematopoiesis by triggering vascular endothelial cell proliferation, migration, differentiation, and survival, as well as by inducing vascular permeability and blood island formation.53 The functional role of VEGFR-1 is complex and dependent on both the developmental stage and cell type.72-74 Although the role of VEGFR-1 signaling cascades in endothelial cells is not fully delineated, VEGFR-1 signaling is required in hematopoiesis,75,76 monocyte migration,57,58,77 and paracrine release of growth factors.78,79 In addition, a recent report links VEGFR-1 signaling with production of MMP-9 in lung endothelial cells, thereby facilitating lung metastasis.80 Blockade of VEGFR-1 attenuates blood vessel formation in the setting of cancer, ischemic retinopathy, and rheumatoid arthritis; importantly, blocking VEGFR-1 abrogates inflammatory processes such as atherosclerosis and rheumatoid arthritis, whereas inhibiting VEGFR-2 does not.78
VEGF-mediated signal transduction in endothelial cells. VEGF monomers linked by disulfide bonds induce receptor dimerization, thereby triggering kinase activation of both the receptor itself and several cytoplasmic signal transduction molecules including PLC-γ,81-84 VEGFR-associated protein (VRAP),85 Ras GTPase activating protein (Ras GAP),86 FAK,83,87,88 Sck,89,90 Src family of tyrosine kinases,91,92 Grb2,82,93 PI3-kinase/Akt,83,94-96 PKC,97-100 Raf-1,99,101 MEK/ERK,83,99,102-104 p38MAPK,88,105 Nck,86,94 Crk,82 Shc,93 and STAT.106 VEGFR kinase activity is additionally regulated by naturally occurring soluble forms of VEGFR-1 and -2; coreceptors including cadherins, integrin αvβ3, neuropilin-1 and -2107-110 ; polysaccharides heparin and heparan sulfate17,111 ; phosphatases SHP-1 and -293,94 and HCPTPA112 ; and small guanosine triphosphate (GTPase) RhoA.113
VEGF functions
Angiogenesis and tumor progression. Blood vessels are required for tumor growth and progression for provision of vital oxygen and nutrients within the diffusion limit for oxygen (100-200 μm). Normally, primary embryonic vasculature is assembled from endothelial precursors (vasculogenesis) followed by angiogenesis, the expansion of this primitive network by sprouting, bridge-formation, and intussusception (insertion of interstitial tissue columns into the lumen of preexisting vessels). In addition to sprouting and co-option114 of neighboring preexisting vessels, tumor-derived angiogenic factors like VEGF promote formation of the endothelial lining of tumor vessels (vasculogenesis) by recruitment of highly proliferative circulating endothelial precursors (CEPs, angioblasts) from the bone marrow, hematopoietic stem cells (HSCs), progenitor cells, monocytes, and macrophages.115 In addition, tumor cells (ie, melanoma cells) can act as endothelial cells and form functional avascular blood conduits or mosaic blood vessels, which are lined partially by tumor cells and vessel walls.116-120
CEPs, but not circulating endothelial cells sloughed from the vessel wall, are highly proliferative and contribute to tumor neoangiogenesis.115,121 HSCs and CEPs likely originate from a common precursor, the hemangioblast. Typically, CEPs express VEGFR-2, c-KIT, CD133, and CD146,64-66 whereas HSCs express VEGFR-1, Sca-1, and c-KIT; the lineage-specific differentiation of these HSCs into erythroid, myeloid, megakaryocytic, and lymphoid cells is dependent on the availability of specific cytokines including IL-3, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and thrombopoietin (TPO). Subsequently, hematopoietic progenitors and terminally differentiated precursor cells produce and secrete factors including VEGF, FGFs, brain-derived nerve growth-factor (BDNF), and angiopoietin; together with ECM proteins like fibronectin and collagen, these factors promote differentiation of CEPs, thereby contributing to new vessel formation. In addition, direct cellular contact with stromal cells also regulates the expansion of undifferentiated CEPs.115 Importantly, VEGFR-1–expressing hematopoietic cells and CEPs colocalize to cooperate in the formation of functional tumor vessels.122,123
Angiogenesis is tightly regulated by proangiogenic and antiangiogenic molecules. In tumorigenesis, this balance is derailed,124 thereby triggering tumor growth, invasion, and metastasis.125,126 Pro- and antiangiogenic molecules arise from cancer cells, stromal cells, endothelial cells, the ECM, and blood.127 Importantly, the relative contribution of these molecules is dependent on the tumor type and site, and their expression changes with tumor growth, regression, and relapse. The “angiogenic switch”128 is triggered by oncogene-mediated tumor expression of angiogenic proteins including VEGF, FGF, PDGF, EGF, lysophosphatic acid (LPA), and angiopoetin, as well as by metabolic stress, mechanical stress, genetic mutations, and the immune response.124,125,129-133 The imbalance of angiogenic regulators (ie, VEGF and angiopoietin) accounts for an abnormal structure of tumor vessels, which in turn results in chaotic, variable blood flow and vessel leakiness, thereby lowering drug delivery and selecting more malignant tumor cells.134-137
In the healthy adult, only 0.01% of endothelial cells undergo division,124 whereas up to 25% of endothelial cells divide in tumor vessels.138 Also, recent studies indicate that the gene expression pattern of normal endothelial cells differs from endothelial cells in tumors,139 for instance, 79 differentially expressed gene products were found in endothelial cells from colon cancer compared to normal endothelial cells.140 In addition, as tumor cells grow, a wider array of angiogenic molecules can be produced; therefore, if one proangiogenic molecule is blocked, tumors may use another molecule. A cocktail of antiangiogenic therapies may therefore be required to effectively prevent angiogenesis124 (Figure 2).
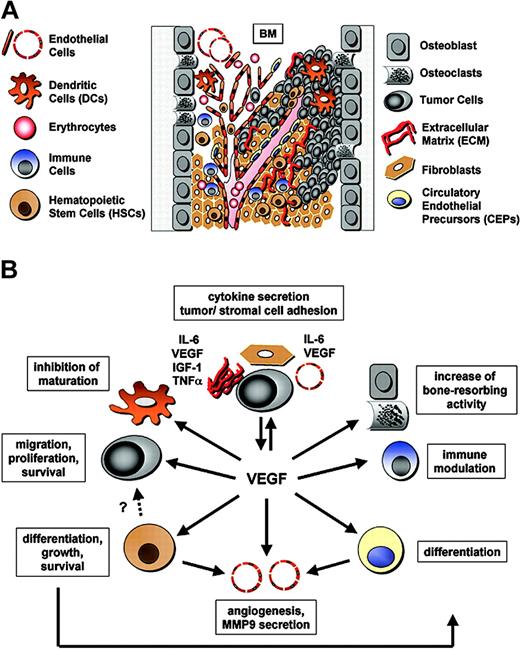
The pathophysiologic functions of VEGF within the BM microenvironment. (A) Components of the bone marrow microenvironment and tumor angiogenesis. The BM microenvironment is a heterogeneous population of cells that are in tight association with the extracellular matrix (ECM) (fibronectin, laminin, collagen): immune cells (including NK cells, T lymphocytes, and monocytes) and dendritic cells (DCs); erythrocytes (Erys); hematopoietic stem cells (HSCs); progenitor and precursor cells; bone-marrow–derived circulating endothelial precursors (CEPs); endothelial cells (ECs); fibroblasts; osteoblasts and osteoclasts; and the tumor cells. Pro- and antiangiogenic molecules secreted by both the stromal and tumor cells contribute to tumor growth and progression. Tumor-derived angiogenic factors like VEGF promote tumor angiogenesis. Tumor vessels grow by co-option, sprouting, and intussusception, as well as by recruitment of CEPs and HSCs. Moreover, some tumor cells act as endothelial cells and form avascular conduits or mosaic blood vessels. (B) Additional biologic functions of VEGF. Besides angiogenesis, VEGF regulates hematopoiesis by mediating HSC survival and repopulation via both an autocrine loop and by inducing differentiation of multiple hematopoietic lineages. Moreover, VEGF inhibits maturation of DCs, increases bone-resorbing activity, modulates immune responses (eg, enhances NK cell adhesion to tumor endothelium), triggers CEP differentiation, and mediates monocyte and CEP recruitment to the vasculature. In the context of cancer, VEGF is an important growth, migration, and survival factor in Kaposi sarcoma, leukemia, and MM. These highly integrated processes involve paracrine, autocrine, and juxtacrine secretion of multiple growth factors, cytokines, and chemokines including IL-6, VEGF, IGF-1, and TNFα; and release of active metalloproteinases (MMPs, such as MMP9); as well as direct tumor–stromal cell and tumor cell–ECM contact.
The pathophysiologic functions of VEGF within the BM microenvironment. (A) Components of the bone marrow microenvironment and tumor angiogenesis. The BM microenvironment is a heterogeneous population of cells that are in tight association with the extracellular matrix (ECM) (fibronectin, laminin, collagen): immune cells (including NK cells, T lymphocytes, and monocytes) and dendritic cells (DCs); erythrocytes (Erys); hematopoietic stem cells (HSCs); progenitor and precursor cells; bone-marrow–derived circulating endothelial precursors (CEPs); endothelial cells (ECs); fibroblasts; osteoblasts and osteoclasts; and the tumor cells. Pro- and antiangiogenic molecules secreted by both the stromal and tumor cells contribute to tumor growth and progression. Tumor-derived angiogenic factors like VEGF promote tumor angiogenesis. Tumor vessels grow by co-option, sprouting, and intussusception, as well as by recruitment of CEPs and HSCs. Moreover, some tumor cells act as endothelial cells and form avascular conduits or mosaic blood vessels. (B) Additional biologic functions of VEGF. Besides angiogenesis, VEGF regulates hematopoiesis by mediating HSC survival and repopulation via both an autocrine loop and by inducing differentiation of multiple hematopoietic lineages. Moreover, VEGF inhibits maturation of DCs, increases bone-resorbing activity, modulates immune responses (eg, enhances NK cell adhesion to tumor endothelium), triggers CEP differentiation, and mediates monocyte and CEP recruitment to the vasculature. In the context of cancer, VEGF is an important growth, migration, and survival factor in Kaposi sarcoma, leukemia, and MM. These highly integrated processes involve paracrine, autocrine, and juxtacrine secretion of multiple growth factors, cytokines, and chemokines including IL-6, VEGF, IGF-1, and TNFα; and release of active metalloproteinases (MMPs, such as MMP9); as well as direct tumor–stromal cell and tumor cell–ECM contact.
Additional biologic functions of VEGF
Besides its role as an essential regulator of physiologic endothelial cell growth, permeability, and migration in vitro and in vivo,1-5,141,142 VEGF is a pivotal factor in hematopoiesis, which specifically affects the differentiation of multiple hematopoietic lineages.6,7,53,76 Importantly, VEGF mediates HSC survival and repopulation via an autocrine loop,76 whereas angiogenesis is regulated via a paracrine VEGF loop. In addition, VEGF inhibits maturation of dendritic cells through inhibition of nuclear factor κB activation143 ; increases both osteoclastic bone-resorbing activity144 and osteoclast chemotaxis145 ; induces migration, parathyroid hormone (PTH)–dependent cAMP accumulation, and alkaline phosphatase in osteoblasts146 ; enhances natural killer (NK) cell adhesion to tumor endothelium147 ; recruits monocyte57 and endothelial cell progenitors122 to the vasculature; stimulates surfactant production by alveolar type II cells148 ; and mediates a direct neuroprotective effect on motor neurons in vitro.149 In the context of cancer, VEGF is an important growth, migration, and survival factor in KS150-155 ; leukemia, and MM63,156 (Figure 2).
The pathophysiologic role of VEGF in MM and other hematologic malignancies
Angiogenesis and microvessel density (MVD) in MM and other hematologic malignancies
In solid tumors, VEGF has an important role in the induction of neovascularization, thereby promoting tumor growth and metastatic potential. However, it was not until 1993 that a role for VEGF and other angiogenic molecules also was defined in leukemias and other hematologic malignancies. First, VEGF was isolated from the HL-60 myeloid leukemia cell line,4 and bFGF was shown to be expressed by bone marrow (BM) stromal and peripheral blood cells.157 Later, increased vascularity was observed in the lymph nodes of B-cell non-Hodgkin lymphoma158 and B-cell chronic lymphocytic leukemia (B-CLL)159-161 ; as well as in BM specimens from patients with childhood acute lymphoid leukemia (ALL),162 acute myeloid leukemia (AML),163 chronic myelocytic leukemia (CML),164 myelodysplastic syndromes (MDS),165 and idiopathic myelofibrosis.166
In MM, increased MVD in patient BM specimens correlates with disease progression and poor prognosis.167-171 Specifically, the extent of angiogenesis is directly correlated with the plasma-cell labeling index and inversely correlated with patient survival. The prognostic significance of angiogenesis in MM was further demonstrated by an Eastern Cooperative Oncology Group (ECOG) study of 75 newly diagnosed patients with MM. Overall survival was significantly longer in patients with low-grade (53 months) than with high-grade (24 months) or intermediate-grade (48 months) angiogenesis (P = .018).172 In addition, BM MVD at the time of initial diagnosis is an important prognostic factor for median overall survival and median progression-free survival in patients undergoing autologous transplantation as frontline therapy for MM. High-grade angiogenesis has similar prognostic impact in solitary bone plasmacytoma.173
Although increased MVD in the BM is associated with initial tumor burden and relapse in MM, it may not be a good indicator of therapeutic response174,175 when tumor cytotoxicity exceeds the disappearance of capillaries. Therefore, a decrease in MVD during treatment indicates the effectiveness of the agent: for example, MVD decreases significantly in MM patients responding to thalidomide, but not in nonresponders.176 However, the absence of such a decrease in MVD does not necessarily indicate lack of response.177 As a substitute for MVD, potential surrogate markers include levels of VEGF or circulating BM-derived endothelial cells.178
Importantly, a recent study of B-cell lymphomas found identical primary and secondary genetic aberrations simultaneously in endothelial cells and tumor cells, suggesting a close relationship between the genetic events in these 2 cell types. The authors suggest 4 different mechanisms that could explain this finding: (1) both cell types are derived from a common malignant precursor cell; (2) the endothelial cell carrying the genetic alteration of the lymphoma cell has arisen from a cell that was already committed to the lymphoid lineage; (3) lymphoma cell–endothelial cell fusion has occurred; or (4) apoptotic bodies from tumor cells have been taken up by endothelial cells.179
In MM, endothelial cells of the BM (MMECs) represent a heterogeneous cell population forming tortuous, uneven vessels with profuse branching, and shunts.167 When compared to healthy quiescent human umbilical vein endothelial cells (HUVECs), MMECs demonstrate (1) enhanced expression of specific antigens including Tie2/Tek, CD105/endoglin, VEGFR-2, bFGFR-2, CD133 (AC133), aquaporin 1, and CD61 (β3 integrin); (2) enhanced capillarogenic activity; and (3) secretion of growth and invasive factors for plasma cells including bFGF, VEGF, MMP-2, and MMP-9. These findings therefore indicate that MMEC facilitates tumor cell growth, invasion, and dissemination; and conversely, raises the possibility of myeloma-induced endothelial-cell growth in the bone marrow.180 The frequency of myeloma-specific genetic aberrations in microvascular endothelial cells remains to be investigated.
VEGF and VEGF receptors in MM and other hematologic malignancies
VEGF and VEGF receptor expression was first reported in acute and chronic leukemias and myelodysplastic syndromes160,181-185 as well as in cell lines derived from hematopoietic malignancies including MM.159 Most studies found VEGFR-1 to be more commonly expressed than VEGFR-2. In MM, VEGFR-1 is widely expressed on both MM cell lines and patient MM cells, confirmed both by reverse-transcriptase polymerase chain reaction (RT-PCR) analyses and immunoprecipitation.32,159,186-188 The role for paracrine and juxtacrine VEGF-mediated tumor cell growth and survival is well established. In addition, co-expression of both VEGF and VEGF receptors in leukemia, lymphoma, and MM, coupled with its direct effects on tumor cell survival, migration, and proliferation, confirms the pivotal role for autocrine VEGF loops in the pathogenesis of these malignancies. Only recently have signaling pathways mediating these effects in hematologic malignancies been delineated.32,183,189-191
Importantly VEGFR-1, but not VEGFR-2, is associated with inhibition of hematopoietic stem cell cycling, differentiation, and hematopoietic recovery in adults.76 The potential role of VEGF and VEGFR-1 in development of the MM cell clone in particular, and in leukemogenesis in general, remains to be investigated.
The functional role of VEGF in the pathogenesis of hematologic malignancies
VEGF and the tumor microenvironment. The BM microenvironment is a heterogeneous population of cells including hematopoietic stem cells; endothelial cells; stromal cells including fibroblasts, macrophages, T lymphocytes; and cells involved in bone homeostasis such as chondroclasts, osteoclasts, and osteoblasts (Figure 2). Differentiation, maintenance, and expansion of tumor cells, for example, MM cells, within the BM microenvironment is a highly coordinated process involving multiple growth factors, cytokines, and chemokines secreted by tumor cells (autocrine loop); stromal cells (paracrine loop); nonhematopoietic organs (eg, kidney and liver); and direct tumor cell–stromal cell contact (juxtacrine loop).
The function of VEGF and its receptors is one component of regulatory processes contributing to the pathogenesis of hematologic malignancies, including MM. VEGF is present in the BM microenvironment of patients with MM and associated with neovascularization at sites of MM cell infiltration.192 The secreted isoforms VEGF121, VEGF145, and VEGF165, as well as ECM- and surface-bound VEGF189 and VEGF206, are produced by MM cell lines, as well as by patient MM and plasma cell leukemia (PCL) cells.32
“Public” (or “external”) autocrine loops, where growth factors (eg, VEGF) are secreted by tumor cells and then activate receptors on both tumor cells and other cells, have been described in several hematologic malignancies, including MM. “Private” (or “internal”) autocrine loops, where the factor activates autonomous cell growth via an intracellular receptor without being secreted,193 have been described in AML cells and in murine experimental models (eg, for IL-3, GM-CSF, v-sis/c-sis/PDGF). In contrast to the “public” autocrine loop, cell proliferation mediated by a “private” VEGF autocrine loop is not cell-density dependent, and neutralizing antibodies do not prevent continued cell growth or differentiation.193-199 Importantly, this independence of factor secretion contrasts the regulatory role of VEGF during hematopoiesis versus angiogenesis.
Extracellular matrix (ECM) proteins laminin, microfibrillar collagen type VI, and fibronectin are strong adhesive components for tumor cells, and their adhesion to laminin and fibronectin is β1-integrin (CD29) mediated.200 β1-integrins expressed on MM cells, specifically α-integrins VLA-4 (α4β1) and VLA-5 (α5β1)201,202 mediate adhesion to endothelial cells and bone marrow stromal cells (BMSCs), thereby conferring protection against drug-induced apoptosis both by cell-cell contact and by triggering NFκB-dependent transcription and secretion of IL-6, a major MM growth, survival, and drug resistance factor.203,204 Importantly, IL-6 secreted by BMSCs enhances the production and secretion of VEGF by MM cells, thereby augmenting MM cell growth and survival159,186,205 ; conversely, binding of MM cells to BMSCs enhances IL-6, IGF-1, and VEGF secretion in BMSCs.186,206,207 Specifically, IGF-1 induces HIF-1α, which triggers VEGF expression208 ; consequently, inhibition of IGFR-1 activity markedly decreases VEGF secretion in MM/BMSC cocultures.206 Other mechanisms regulating VEGF expression in MM cells include c-maf and CD40. c-maf–driven expression of integrin β7 enhances adhesion to BM stroma and thereby increases VEGF production,209 and CD40 activation induces p53-dependent VEGF secretion.210
In CML, VEGF is expressed at high levels in the bone marrow and the peripheral blood; moreover, the CML-associated oncogene Bcr-Abl induces VEGF- and HIF1α-gene expression via a PI3K-mTOR–dependent pathway211 (Figure 3).
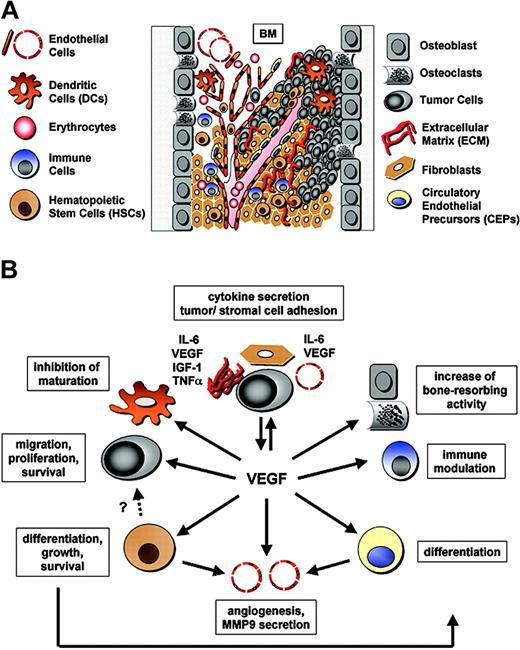
Regulation of VEGF expression. VEGF-A levels are increased in several hematologic malignancies, including MM. Factors modulating VEGF secretion include (A) secretion of IL-6 or VEGF by both BMSCs and tumor cells (paracrine/autocrine loop); (B) hypoxia and the presence of mutant oncogenes (ie, mutant Ras [mutRas] or Bcr-Abl, which up-regulate VEGF expression via HIF-1α protein); (C) secretion of IGF-1, which up-regulates VEGF production and secretion in tumor cells (paracrine loop); (D) c-maf–driven expression of tumor integrin β7; (E) tumor cell expression of ICAM1 and LFA1 modulating adhesion to ECM and BMSCs, thereby increasing VEGF production and secretion; and (F) CD40 activation, which induces p53-dependent VEGF secretion. Furthermore, VEGFR-1 expression is regulated, for instance, by IGF-1 via HIF-1α (C).
Regulation of VEGF expression. VEGF-A levels are increased in several hematologic malignancies, including MM. Factors modulating VEGF secretion include (A) secretion of IL-6 or VEGF by both BMSCs and tumor cells (paracrine/autocrine loop); (B) hypoxia and the presence of mutant oncogenes (ie, mutant Ras [mutRas] or Bcr-Abl, which up-regulate VEGF expression via HIF-1α protein); (C) secretion of IGF-1, which up-regulates VEGF production and secretion in tumor cells (paracrine loop); (D) c-maf–driven expression of tumor integrin β7; (E) tumor cell expression of ICAM1 and LFA1 modulating adhesion to ECM and BMSCs, thereby increasing VEGF production and secretion; and (F) CD40 activation, which induces p53-dependent VEGF secretion. Furthermore, VEGFR-1 expression is regulated, for instance, by IGF-1 via HIF-1α (C).
What is the functional role of VEGF in tumorigenesis of hematologic malignancies in general, and of MM in particular? In addition to stimulating angiogenesis, our studies show that VEGF directly stimulates MM cell migration on fibronectin, proliferation, and survival via autocrine and paracrine loops. Importantly, the range of VEGF target cells within the BM compartment of MM may be even broader since VEGF dramatically affects the differentiation of multiple hematopoietic lineages in vivo; increases the production of B cells and generation of myeloid cells143,212 ; (3) regulates HSC survival by an internal autocrine loop mechanism76 ; increases both osteoclastic bone-resorbing activity144 and osteoclast chemotaxis145 ; and inhibits maturation of dendritic cells.143 For example, we have shown that addition of anti-VEGF antibodies to MM patients' BM sera abrogates its inhibitory effect on dendritic cell maturation.213 Therefore, besides angiogenesis, dysregulation of VEGF plays an important role in MM pathogenesis and clinical features, including lytic lesions of the bone and immune deficiency. Inhibition of VEGF in MM may therefore target not only endothelial cells and MM cells, but also a broad array of cells contributing to MM pathogenesis.
VEGF signal transduction. In MM, tumor cell growth, survival, and migration are necessary for homing of MM cells to the BM, their expansion within the BM microenvironment, and their egress into the peripheral blood. Binding of exogenous VEGF165 to MM cells triggers VEGFR-1 tyrosine phosphorylation. Subsequently, several downstream signaling pathways are activated: (1) a PI3-kinase/protein kinase Cα (PKCα)–dependent cascade mediating MM cell migration on fibronectin, evidenced by using the PKC inhibitor bisindolylmaleimide I and LY294002; (2) a MEK-extracellular signal-regulated protein kinase (ERK) pathway mediating MM cell proliferation, evidenced by use of anti-VEGF antibody and PD09805932 ; and (3) a pathway mediating MM cell survival via up-regulation of Mcl-1 and survivin in a dose- and time-dependent manner214 (Figure 4).
VEGF signal transduction in MM cells. VEGF mediates MM cell proliferation via MEK-1/ERK signaling and MM cell survival via up-regulation of survivin and Mcl-1. VEGF-induced MM cell migration on fibronectin is dependent on the localization of VEGFR-1 within caveolae, followed by Src tyrosine kinase family–dependent phosphorylation of caveolin-1, PI3-kinase, and PKCα.
VEGF signal transduction in MM cells. VEGF mediates MM cell proliferation via MEK-1/ERK signaling and MM cell survival via up-regulation of survivin and Mcl-1. VEGF-induced MM cell migration on fibronectin is dependent on the localization of VEGFR-1 within caveolae, followed by Src tyrosine kinase family–dependent phosphorylation of caveolin-1, PI3-kinase, and PKCα.
As in MM, autocrine VEGF stimulation of VEGFR-2 triggers leukemic cell proliferation and migration, thereby inducing a more invasive tumor phenotype.183 Moreover, VEGF induces the expression of heat shock protein 90 (Hsp90) and its binding to Bcl-2 and Apaf-1, thereby increasing leukemic cell resistance to serum deprivation-induced apoptosis.190
In addition to the known paracrine/external autocrine/juxtacrine loops, an internal autocrine loop of VEGF similar to AML may contribute to growth factor and cell density–independent proliferation of MM cells. Internalization and functional intracellular trafficking of VEGFR-1 may be mediated by lipid rafts in general and caveolae in particular. Functionally, caveolae, vesicular flask-shaped invaginations of the plasma membrane, which are composed of caveolins, cholesterol, and sphingolipids, have been implicated in transmembrane transport and signal transduction.215,216 Specifically, we recently demonstrated that caveolae and caveolin-1 are present in MM cells and are required for IL-6- and IGF-1–triggered Akt-1–mediated survival of MM cells.217 Ongoing studies are exploring whether caveolae mediate VEGFR-1 trafficking in MM cells, thereby mediating both “private” and “public” autocrine MM cell proliferation and survival; as well as development of the tumor cell clone.
Novel therapeutic approaches targeting VEGF and the VEGF receptors in MM and other malignancies
Therapeutic approaches to target VEGF
Several direct and indirect VEGF and VEGF receptor inhibitor strategies are under clinical investigation for treatment of solid tumors and hematologic malignancies (http://www.cancer.gov/clinicaltrials/developments/anti-angio-table). Approaches to disrupt the VEGF/VEGF receptor signaling pathways range from small molecule ATP competitive VEGF receptor inhibitors to biologic agents like soluble receptors, anti-VEGF and anti-VEGF receptor antibodies, small molecule inhibitors, and VEGF transcription inhibitors. The earliest study showing that VEGF blockade by a monoclonal antibody suppressed angiogenesis and tumor growth in vivo was in a glioblastoma cell line.218 The antitumor effect of VEGF inactivation in vivo was more recently shown in a murine insulinoma model.219
Anti-VEGF antibody (bevacizumab; Avastin). The most successful approach to date to therapeutically target VEGF is the use of a humanized monoclonal antibody against VEGF, bevacizumab (Avastin),220 which was United States Food and Drug Administration (USFDA) approved for use as a first-line therapy for metastatic colorectal cancer in February 2004; specifically, bevacizumab in combination with intravenous 5-FU–based chemotherapy is a new treatment option.221 Furthermore, bevacizumab also significantly prolonged time to progression of disease in patients with metastatic renal-cell cancer in a clinical phase 2 trial.222,223 Effects of bevacizumab also were seen in combination with chemotherapy in non–small-cell lung cancer (NSCLC),224,225 pancreatic226 and breast carcinoma227 ; with interferon-α in both melanoma and metastatic renal cell carcinoma228 ; with radiotherapy in rectal cancer; and with thalidomide in metastatic renal-cell carcinoma. Interestingly, bevacicumab reduces the number of CEPs and increases the fraction of tumor endothelial cells with pericyte coverage, thereby reflecting the dropout of immature endothelial cells and potentially providing a novel biomarker.229 Ongoing studies in hematologic malignancies are evaluating the efficacy of bevacizumab in patients with relapsed or refractory MM (with or without thalidomide), blastic phase chronic myelogenous leukemia (CML-BP), myelodysplastic syndrome (MDS), and relapsed aggressive non-Hodgkin lymphoma (NHL).
VEGF-trap. Another approach is to target the VEGFR. Flt1-3 IgG, a Fc fusion with the first 3 Ig-like domains of VEGFR-1, inhibits tumor growth in a murine rhabdomyosarcoma xenograft model.230 A hybrid Fc construct in which domain 2 of VEGFR-1 is joined to domain 3 of the VEGFR-2 (VEGF-trap)231 causes regression of co-opted vessels in a model of neuroblastoma.232 Importantly, ongoing clinical studies are evaluating the efficacy of the VEGF trap in patients with incurable relapsed or refractory solid tumors or NHL.
PTK787/ZK222584. PTK787/ZK222584 is an orally available tyrosine kinase inhibitor (Novartis Pharmaceuticals) that binds to the ATP-binding sites of VEGF receptors.233,234 In MM, we have reported that PTK787/ZK222584 acts directly on MM cells to inhibit VEGF-induced MM cell growth and migration and inhibits paracrine IL-6–mediated MM cell growth in the BM milieu.188 PTK787/ZK222584 is now under evaluation in a phase 3 trial for colorectal cancer; a phase 1 trial together with imatinib mesylate (Gleevec) for AML, AMM, CML-BP; a phase 2 trial for primary or secondary MDS; and a phase 1 trial for MM.
Indazolylpyrimidine pan-VEGFR inhibitors. The indazolylpyrimidine GW654652 is a small molecule tyrosine kinase inhibitor that inhibits all 3 VEGF receptors with similar potency. Preclinical data demonstrates that GW654652 is a potent inhibitor of both VEGF- and bFGF-mediated angiogenesis, as well as VEGF-induced vascular permeability in vivo. In addition, daily oral dosing with GW654652 inhibits the growth of human tumor head, neck, colon, melanoma, and prostate cancer xenografts in vivo.235 Moreover, GW654652 has a favorable pharmacokinetic profile in murine and canine studies, suggesting its potential clinical application.236 In MM, the indazolylpyrimidine class of pan-VEGF receptor inhibitors acts both directly on tumor cells and in the BM microenvironment to overcome drug resistance. Specifically, GW654652 inhibits VEGF-triggered migrational activity and proliferation of MM cell lines, including those sensitive and resistant to conventional therapy, in a dose-dependent fashion. Furthermore, GW654652 blocks both VEGF-induced VEGFR-1 phosphorylation and downstream activation of AKT-1 and MAPK signaling pathways. Importantly, GW654652 also acts in the BM microenvironment, since it blocks HUVEC proliferation and inhibits both IL-6 and VEGF secretion, as well as proliferation of MM cells induced by MM cell binding to BM stromal cells. Importantly, inhibition of MM cell growth, survival, and migration is not reversed by removal of GW654652 after treatment; in contrast, its effects on HUVEC survival and proliferation are partially reversible after drug removal. The higher sensitivity of MM cells may be due to lower VEGF receptor expression levels in MM cells than in HUVECs. Alternatively, these data may indicate that long-term exposure of MM cells to GW654652 leads to stable destruction of VEGFR-1 on MM cells. These results therefore suggest potential use of this drug class to target both tumor cells and their microenvironment.237 A phase 1 clinical trial in MM is planned at our institution.
Other VEGF inhibitors. Significant efficacy of RTK inhibitors including molecule SU5416 (Sugen),238-240 SU11248 (Sugen),241 AG013676 and CP-547632 (Pfizer),242 ZD6474 (AstraZeneca),243 and BAY 43-9006 (Bayer/Onyx)244,245 has been demonstrated in both preclinical models and clinical trials of solid tumors and hematologic malignancies including AML, MDS, and MM.
Therapeutic approaches directly targeting endothelial cells
Thalidomide/IMiDs. Based upon its antiangiogenic activity,246 Thal was used empirically to treat patients with refractory relapsed MM and achieved responses in one third of cases.174 Subsequently, a series of immunomodulatory drugs (IMiDs) has been developed.247 Importantly, both Thal and the more potent IMiDs can overcome the growth and survival advantage conferred by the BM milieu, including down-regulating VEGF.248,249 Proliferation and capillarogenesis of MMECs also are significantly inhibited by thalidomide.180 Moreover the IMiDs costimulate T cells, enhance antitumor immunity mediated by IFNγ and IL-2, and augment NK cell cytotoxicity.250 These studies provided the basis for the use of IMiD CC-5013 (Revimid, now REVLIMID) (Celgene) in a phase 1 dose-escalation trial in patients with relapsed and refractory MM, which demonstrated either response or stabilization of disease in 79% cases.251 Two clinical phase 2 trials have confirmed these data, achieving complete responses with favorable side effect profiles, and 2 clinical phase 3 trials comparing Revlimid to dexamethasone/Revlimid treatment of relapsed MM are now completely enrolled.
A phase 1/2 study in patients with advanced MM using IMiD CC-4047 (Actimid) (Celgene) showed antimyeloma activity and an acceptable safety profile.252 In addition to MM, clinical studies are ongoing in MDS, large B-cell lymphoma, CLL, and small lymphocytic lymphomas.247
2-ME-2. 2-Methoxyestradiol (2-ME-2), a natural metabolite of estradiol, is a potent antitumor and antiangiogenic agent in leukemic cells.253-258 However, the mechanisms mediating its biologic effects remain unclear. Our recent studies show that 2ME2 also inhibits growth and induces apoptosis in MM cell lines and patient cells. Importantly, VEGF secretion induced by adhesion of MM cells to BMSCs is inhibited by 2ME2. Conversely, 2ME2 inhibits MM cell growth, prolongs survival, and decreases angiogenesis in a murine model.259 Clinical phase 1 studies are under way in both solid tumors and in MM.
Therapeutic approach inhibiting endothelial-specific integrin/survival signaling
The cyclic pentapeptide EMD 121974 (cilengitide) mediates its antiangiogenic activity via selective inhibition of integrins ανβ3 and ανβ5.263-266 Clinical trials are ongoing in AML and lymhomas.
Additional approaches directly or indirectly targeting VEGF
Lysophosphatidic acid acyltransferase-β (LPAAT) inhibitors. Lysophosphatidic acid (LPA), an intracellular lipid mediator with growth factor–like activities, stimulates a specific G protein–coupled receptor present in numerous cell types, thereby triggering a multitude of biologic responses.267 LPA stimulates VEGF expression and secretion in ovarian cancer cells, thereby increasing tumor angiogenesis and subsequent tumor growth and metastasis.268 Conversely, we have recently demonstrated that LPA acyltransferase (LPAAT) inhibitors (Cell Therapeutics, Seattle, WA) have antitumor activity in MM, at least in part due to their inhibitory effect on VEGF expression.269
Bortezomib. Bortezomib (previously denoted PS341) is a novel proteasome inhibitor recently approved by the USFDA for therapy of patients with progressive MM after previous treatment.270 It induces apoptosis in drug-resistant MM cells and inhibits binding of MM cells in the BM microenvironment, as well as production and secretion of cytokines that mediate MM cell growth and survival. IL-6–triggered phosphorylation of ERK, but not of STAT3, is blocked by bortezomib.271 Moreover, bortezomib mediates anti-MM activity by triggering phosphorylation of both p53 protein and JNK, cleavage of DNA-PKcs and ATM,272 and caspase-dependent down-regulation of gp130.273 The antiangiogenic effect of bortezomib274,275 is another potential mechanism of its anti-MM activity.276 Moreover, our recent studies show that bortezomib down-regulates caveolin-1 expression and inhibits caveolin-1 tyrosine phosphorylation, which are required for VEGF-mediated MM cell migration on fibronectin; and blocks VEGF-induced tyrosine phosphorylation of caveolin-1 in HUVECs, thereby inhibiting ERK-dependent endothelial cell proliferation.
Combination therapy/metronomic chemotherapy
The production of pro- and antiapoptotic molecules changes during the course of conventional therapy for cancer. Specifically, prior studies show that surgery and chemotherapy, in contrast to irradiation, may even enhance tumor angiogenesis by stimulating production of VEGF and other endothelial-cell survival and growth factors in tumor cells.278 High local VEGF concentrations in the BM microenvironment of MM patients suppress the antiproliferative effects of several chemotherapeutics, thereby promoting multidrug resistance.279,280 Therefore, combining chemotherapies and irradiation with drugs that block VEGF signaling may enhance antitumor efficacy, for example, by “normalizing” tumor vasculature and thereby improving oxygenation and delivery of chemotherapies to tumor cells.281 Enhanced antitumor activity of conventional chemotherapy282-284 and irradiation285,286 regimens has been achieved when combined with antiangiogenic drugs. Novel therapeutic strategies of metronomic chemotherapy287,288 use frequent uninterrupted administration of conventional chemotherapeutics in doses significantly below the maximum tolerated dose (MTD) for prolonged periods,289 thereby both reducing toxic side effects and improving antitumor effects.290-292 Another advantage of metronomic chemotherapy is the possibility that it may be combined with antiangiogenic drugs like bevacizumab (Avastin).221
Conclusions and future therapeutic perspectives
The complexity of VEGF actions is determined by a multitude of target cells. Besides its role as an essential regulator of physiologic and pathologic angiogenesis, VEGF is now known to trigger growth, survival, and migration of leukemia and MM cells via paracrine and autocrine pathways; to inhibit maturation of dendritic cells and to increase bone-resorbing activity. Moreover, VEGF also is associated with HSC differentiation and hematopoietic recovery in adults. VEGF levels and increased vascularity are correlated with clinical outcome in hematologic malignancies including leukemias, lymphomas, and MM. In addition, effects of VEGF on target cells other than endothelial cells may contribute to the clinical manifestations of leukemias, lymphomas, and MM. Direct and indirect targeting of VEGF and its receptors are therefore promising novel therapeutic approaches to improve patient outcome.
When tumor cells grow, a wide array of angiogenic molecules may be produced. This at least in part, may be the reason why single-agent therapy inhibiting VEGF is only effective initially and that a cocktail of antiangiogenic therapies is required to effectively prevent tumor angiogenesis. Enhancement of antitumor efficacy can also be achieved by combining drugs that block VEGF signaling with chemotherapies or irradiation, thereby “normalizing” and sensitizing tumor vasculature and improving oxygenation and delivery of chemotherapies to tumor cells and endothelial cells. In addition, VEGF actions and the array of its target cells may vary, depending on the stage of the malignancy and the tumor type. Ongoing studies are addressing these dynamic interactions and translating them into therapeutic strategies. Furthermore, since VEGF plays a pivotal role in HSC differentiation, the potential role of VEGF and VEGFR-1 in clonal leukemogenic development also is under investigation. A close collaboration between basic researchers and clinicians will be required to both enhance our understanding of the pathophysiologic role of VEGF in hematologic malignancies and derive related targeted clinical trials to eventually improve patient outcome.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-07-2909.
Supported by a Multiple Myeloma Research Foundation (MMRF) Senior Research Grant Award (K.P.); National Institutes of Health Grants IP50 CA100707, PO-1 78378, and RO-1 CA 50945; and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The authors thank all members of the Jerome Lipper Multiple Myeloma Center for helpful discussions.


![Figure 3. Regulation of VEGF expression. VEGF-A levels are increased in several hematologic malignancies, including MM. Factors modulating VEGF secretion include (A) secretion of IL-6 or VEGF by both BMSCs and tumor cells (paracrine/autocrine loop); (B) hypoxia and the presence of mutant oncogenes (ie, mutant Ras [mutRas] or Bcr-Abl, which up-regulate VEGF expression via HIF-1α protein); (C) secretion of IGF-1, which up-regulates VEGF production and secretion in tumor cells (paracrine loop); (D) c-maf–driven expression of tumor integrin β7; (E) tumor cell expression of ICAM1 and LFA1 modulating adhesion to ECM and BMSCs, thereby increasing VEGF production and secretion; and (F) CD40 activation, which induces p53-dependent VEGF secretion. Furthermore, VEGFR-1 expression is regulated, for instance, by IGF-1 via HIF-1α (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-07-2909/6/m_zh80040574430003.jpeg?Expires=1768048477&Signature=tnhjga16yEYsUszeAmYVSa~DYFfFQ2h9ZnJpn36dIpBysZG7sxPDhcAeX2SpEMJnXjhvCr6zb1PjwoUsjpIVkEolBrq4rQVJ0Kvbw0iR8Q~abqX7YV04FNyXfL-LDPO6y898HoJYRqbo5sB3aDFSKNzbio3QbT~J~HDdYj5VDgbbdKWhmIJ99aUVAYqevwb2uvNEWGgSgq97BrF5h8acFMttv8gDeKHASYvI56zByNbueHF0Zf4-PdiiDuV9~imoibVenv9hWJMw4p1HiSE6sQrQsEIJLL6bXB1ovEuc~e~Um4AnC6b456iVLu4vAw9jQC4gvKtIZ4iYUc1-brfGkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 3. Regulation of VEGF expression. VEGF-A levels are increased in several hematologic malignancies, including MM. Factors modulating VEGF secretion include (A) secretion of IL-6 or VEGF by both BMSCs and tumor cells (paracrine/autocrine loop); (B) hypoxia and the presence of mutant oncogenes (ie, mutant Ras [mutRas] or Bcr-Abl, which up-regulate VEGF expression via HIF-1α protein); (C) secretion of IGF-1, which up-regulates VEGF production and secretion in tumor cells (paracrine loop); (D) c-maf–driven expression of tumor integrin β7; (E) tumor cell expression of ICAM1 and LFA1 modulating adhesion to ECM and BMSCs, thereby increasing VEGF production and secretion; and (F) CD40 activation, which induces p53-dependent VEGF secretion. Furthermore, VEGFR-1 expression is regulated, for instance, by IGF-1 via HIF-1α (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-07-2909/6/m_zh80040574430003.jpeg?Expires=1768358165&Signature=rVcyKhpAy-tmACUdblwNbbrwXVsqAwVShX4GH33o2spNkOYGQRWK2qHZelnXwMGyH04uiSlyRlX5Td-7t-WSaYwyYJ5j~bX2aRceWTeOkDSx3wVmHZ1kcAgYFJob31ZoYxm4VgALQItHU0HhGUJSHT0I6CwTUQ31VpL6hpHh~voNESUiMZ2N9i2dg-c9Otq8Gi2AZAknXNKhSc5ZqXZj9hoBIs6Ret0Csp28gAl46hy-mtnjOwq97aKC~rpAr-SOD4KQaEFXPb8-73uv2x3~9WYwyDFk4X8WSglq1s1ciaMPZ~r~LCVDqj4UfLI5Fi4oLhzTFVItdTleJrhRC6H6Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
