Comment on Billiau et al, page 1648
Cytokine levels are broadly deranged in patients with hemophagocytic syndromes. Untangling the “Who, What, Where, When, and Why” of these abnormalities will be essential to understanding this fatal disorder.
The profound elevation of multiple serum cytokine levels, including interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and IL-10, has long intrigued investigators studying patients with hemophagocytic syndromes. While it is clear that T cells and macrophages are involved in this disease process, the sequence of events and the source(s) of the these cytokines remain unclear. While investigators have previously found that circulating CD8+ T cells in these patients are secreting IFN-γ and that monocytes from these patients can secrete IL-6 and TNF-α in vitro,1 these observations have not been repeated in a diverse patient population. In this issue of Blood, Billiau and colleagues have added to our understanding of hemophagocytic syndromes by staining tissue samples for the presence of each of these cytokines. They examined a series of liver biopsy specimens from a diverse group of patients with what is variably termed hemophagocytic lymphohistiocytosis, hemophagocytic syndrome, or macrophage activation syndrome. Despite the diversity of this group, they consistently found that CD8+ T cells prominently contained IFN-γ and that macrophages contained IL-6 and TNF-α in situ. These findings are notable for 2 reasons. First, they confirm the idea that despite a variety of clinical and etiologic features, patients with hemophagocytic syndromes have similar disease pathophysiology. Second, this study directly demonstrates which cell type is producing each cytokine.FIG1
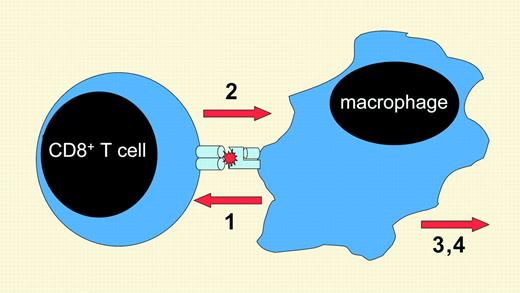
A theorized sequence of events in the development of hemophagocytic syndromes. In patients with hemophagocytic syndromes, antigen presentation (1) stimulates CD8+ T cells to secrete IFN-γ (2), which activates macrophages to produce multiple, toxic inflammatory mediators (3) and causes hemophagocytosis (4).
A theorized sequence of events in the development of hemophagocytic syndromes. In patients with hemophagocytic syndromes, antigen presentation (1) stimulates CD8+ T cells to secrete IFN-γ (2), which activates macrophages to produce multiple, toxic inflammatory mediators (3) and causes hemophagocytosis (4).
While the observations of Billiau et al do not establish causality, they are consistent with the findings of a murine model of hemophagocytic lymphohistiocytosis, which clearly demonstrated that IFN-γ was essential for disease development.2 The findings of this model and the current paper by Billiau and colleagues are consistent with the theory that hemophagocytic syndromes develop when CD8+ T cells secrete excessive amounts of IFN-γ and thereby drive macrophages to toxic levels of activation. How the T-cell response spirals out of control and precisely how IFN-γ is linked to the clinical phenotype of hemophagocytic syndromes are not yet known. Future investigators will need to establish a clear, causal sequence of events that leads to the unfortunate and fatal immune activation seen in the hemophagocytic syndromes. ▪