Abstract
When adopting basic principles learned in mice to clinical application in humans, it is often difficult to distinguish whether a “translation” fails because of an invalid target in the human disease or because the therapeutic agents are not optimal for the human target. It is, therefore, desirable to develop preclinical models to optimize therapies for human targets using in vivo settings. Although anti–mouse CTLA-4 antibodies are known to enhance immune responses in vivo, their effect on T-cell activation in vitro ranges from enhancement to inhibition. Here we use the hu-PBL-SCID mouse model of Epstein-Barr virus (EBV)–associated lymphoma development to screen a panel of anti–human CTLA-4 monoclonal antibodies (mAbs) for their effect on human lymphocytes in an in vivo “humanized” environment. We report significant heterogeneity of anti–human CTLA-4 mAbs in enhancing the expansion of human T cells in mice, and this heterogeneity cannot be attributed to immunoglobulin isotypes or affinity for CTLA-4. These data validate the development of additional screening tools, such as the one described, to further characterize functional activity of antihuman antibodies before proceeding with clinical translation to human studies.
Introduction
A major challenge in translating basic principles learned in mice to clinical application in humans is that diseases and therapeutics are changed at the same time. This is particularly relevant for cancer immunotherapy involving monoclonal antibodies (mAbs) that are species specific. Monoclonal antibodies directed against costimulatory molecules are a promising treatment modality that can bypass the requirement for, or can enhance endogenous costimulation by, antigen-presenting cells. Although a number of mAbs have shown significant effect in murine systems, the translation of these treatments has been less successful because of several factors. One critical factor is that antihuman mAbs may not have the same functional properties their murine counterparts have. Individual antibodies may bind to different epitopes of the same costimulatory molecule with varying binding affinities and mechanisms of action (agonist or antagonist). Another key issue is the lack of adequate in vivo models with which to prescreen large numbers of candidate antibodies to determine the most effective molecules for clinical translation.
The costimulatory molecule cytotoxic T-lymphocyte antigen 4 (CTLA-4) has been widely investigated as a target of mAb therapy to boost antitumor immunity. The initial study, performed by Leach et al,1 showed that anti–CTLA-4 mAb could stimulate the rejection or could reduce the growth of colon carcinoma and fibrosarcoma in mice. Significantly, this mAb was able to reduce the growth of established tumors and to protect against a second tumor challenge. Subsequent studies extended the use of anti–CTLA-4 mAb to the treatment of prostate cancer,2-4 melanoma,5-7 ovarian carcinoma,8 and mammary carcinoma.9 A number of these groups combined anti–CTLA-4 mAb with a granulocyte macrophage–colony-stimulating factor (GM-CSF)–producing tumor cell vaccine5,6,9 or with the depletion of regulatory T cells7 to enhance the elimination of tumors normally resistant to an antitumor response. These studies showed the requirement of CD8+, but not CD4+, T cells in the protective response.5-7 Conflicting evidence exists as to whether anti–CTLA-4 mAb is capable of reversing T-cell tolerance to tumor antigen.10,11 More recently, anti–human CTLA-4 mAb has been tested in phase 1 clinical trials in patients with cancer, showing mixed results. In one study, patients with metastatic melanoma or ovarian carcinoma showed evidence of tumor necrosis and T-cell infiltration into tumors, but with no significant clinical benefit.12 Another study involving patients with metastatic melanoma demonstrated significant clinical activity, with objective tumor regression in 3 of 14 patients.13 However, 6 of 14 patients in this same study developed grade 3/4 autoimmune toxicities.
This variability of responses in different settings suggests that more extensive preclinical screening of different mAb clones may prove beneficial in selecting clones that induce more potent antitumor immunity while minimizing autoimmune adverse effects. Preclinical screening of anti–human CTLA-4 mAbs is fraught with difficulty because in vitro immunologic correlates are sometimes of little value, as demonstrated by experience with anti–mouse CTLA-4 mAb. The same anti–mouse CTLA-4 antibodies that induced potent antitumor immunity in the above-mentioned studies had variable effects on T cells in vitro. Anti–CTLA-4 mAb enhanced T-cell proliferation in response to alloantigen but suppressed T-cell proliferation in response to costimulation by anti-CD28.14,15 In addition, CTLA-4 engagement with antibody could promote or inhibit the proliferation of different subsets of T cells in the same culture.16
This complication can be overcome by studying human T-cell responses in a rodent model. A widely used model is the hu-SCID mouse.17 One approach involves the establishment of human thymus or stem cells in these mice,17,18 which then produce human T cells. This model has been extensively used for studies of HIV infection19 and development of human T cells.17 At the same time, less demanding chimeras have been produced involving SCID mice engrafted with human peripheral blood leukocytes (PBLs)20 or antigen-specific T cells.21 In the area of cancer immunotherapy, hu-SCID mice have been used to measure the effector function of tumor-specific T cells21 and cloned tumor-specific T-cell lines.22,23
Despite numerous attempts, varying success has been reported using hu-SCID mice to study the immune response of human T cells. A notable exception, reported by Caballido et al,24 demonstrated that grafting fetal human bone, thymus, skin, and lymph nodes into SCID mice allows the induction of immune responses of human lymphocytes. The requirement of multiple surgeries, however, makes this an impractical model to prescreen immune modulators for activation of T cells. Interestingly, peptide-loaded dendritic cells were found to be capable of priming CD4 T cells in hu-SCID mice.25 We have recently reported the boosting of Epstein-Barr virus (EBV)–specific cytotoxic T lymphocyte (CTL) responses in hu-PBL-SCID mice using a combination of interleukin-2 (IL-2) and GM-CSF.26 Remarkably, up to 20% of the T cells recovered from spleens of the engrafted mice can be specific for a single EBV viral peptide. Administration of these cytokines also leads to a significant CD8 T cell–dependent protection from EBV-associated lymphoproliferative disease. This makes it possible to study human CTL responses in mice, at least for antigens to which T cells have been primed. Here we report that in a similar hu-PBL-SCID model, 5 different clones of anti–human CTLA-4 mAbs promoted the enhancement of T-cell responses, with significant variability in efficacy between clones. The functional heterogeneity suggests that this model can be used to screen for therapeutic antibodies targeting the CTLA-4 molecule.
Materials and methods
Experimental animals
BALB/c mice were purchased from Charles River Laboratories under contract with the National Cancer Institute. CB.17 SCID mice and BALB/c RAG-2(–/–) mice were purchased from Taconic (Germantown, NY). All mice were maintained in the University Laboratory Animal Research Facility at The Ohio State University under specific pathogen-free conditions.
Monoclonal antibody production
BALB/c mice were immunized 2 times with a fusion protein consisting of the extracellular domain of the human CTLA-4 protein and the Fc fragment of human immunoglobulin G1 (IgG1) (huCTLA-4Ig). Spleen cells were harvested from immunized mice and were fused with myeloma cell line XAg8.653 using polyethylene glycol (molecular weight [MW] 1000) (Sigma, St Louis, MO). Hybridomas were selected in HAT media and were further cultured in HT media. Culture supernatant was screened for the presence of anti–human CTLA-4 mAb by enzyme-linked immunosorbent assay (ELISA). Clones producing mAbs that bound to human CTLA-4Ig fusion protein, but not mouse CD28Ig fusion protein, were rescreened by ELISA and were further subcloned and expanded. Large-scale antibody production of selected clones was achieved by purifying mAbs from culture media using a protein G column or by intraperitoneal injection of 5 × 106 hybridoma cells into BALB/c RAG-2(–/–) mice to produce ascites. Isotyping of mAbs was performed using a kit purchased from BD PharMingen (San Diego, CA).
Binding kinetics and affinity of monoclonal antibodies
Experiments were performed by Biacore (Piscataway, NJ) through a contract service. Human CTLA-4Ig fusion protein was immobilized on a Biacore sensor chip using primary amine covalent chemistry. Briefly, N-hydroxysuccinimide (NHS) esters were introduced on the chip surface by modification of the carboxymethyl groups on the chip surface with a mixture of N-hydroxysuccinimide and N-ethyl-N′-(3-dimethylaminopropyl)–carbodiimide hydrochloride (EDC) for 7 minutes. Human CTLA-4Ig was diluted in 10 mM sodium acetate (pH 5.5) at a concentration of 2.5 μg/mL and was injected over the activated surface for approximately 1 to 3 minutes. The surface was then blocked for 7 minutes with ethanolamine to remove any remaining esters. An NHS/EDC-activated and ethanolamine-blocked surface was used as the reference surface. Anti–human CTLA-4 mAbs were injected at various concentrations in duplicate over the protein and reference surface for 3 minutes, followed by 10 minutes of dissociation time using an automated method. The running buffer was HBS-EP (0.01 HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 0.15 M NaCl, 3 mM EDTA [ethylenediaminetetraacetic acid], 0.005% surfactant P20), pH 7.4, and the detection temperature was 25°C.
Engraftment of human peripheral blood leukocytes
PBLs were obtained from healthy donors who gave informed consent under an Ohio State University institutional review board (IRB)–approved protocol (2003A0041) for leukapheresis performed by The Ohio State University Hospitals apheresis unit in accordance with the tenets of the Declaration of Helsinki.
Selected donors were EBV-seropositive and hepatitis B– and HIV-seronegative. These PBLs were previously shown to generate EBV lymphoproliferative disorder in more than 90% of engrafted hu-PBL-SCID mice. PBLs were separated from other cell types using a Ficoll gradient. PBLs (50 × 106) were injected intraperitoneally in 0.5 mL phosphate-buffered saline (PBS) into CB.17 SCID mice.
Monoclonal antibody and cytokine treatment
Mice were given intraperitoneal injections of 100 μg anti–IL2Rβ (TMβ1) mAb to deplete murine NK cells on the day preceding or the day of engraftment. In the experiments analyzing T-cell expansion and latent membrane protein 1 (LMP-1) expression, this initial treatment was followed by 2 additional treatments of 100 μg of TMβ1 mAb every other day. Mice received intraperitoneal injections of 300 μg purified anti–human CTLA-4 mAb or 100 μL ascites containing anti–human CTLA-4 mAb, or they received 100 to 300 μg control mouse IgG (Sigma) on days 1, 5, 9, and 13 after PBL engraftment. Mice also received intraperitoneal injections of 3 μg human GM-CSF every other day for 3 weeks. In the experiment assessing interferon-γ (IFN-γ) production, mice received a single dose of 100 μg TMβ1 mAb, followed by 300 μg purified anti–human CTLA-4 mAb or control mouse IgG and 3 μg human GM-CSF on days 1, 5, and 9.
Flow cytometry
All antibodies used for staining of cell surface and intracellular proteins—such as CD3, CD4, CD8, CD45, LMP-1, and IFN-γ—were purchased from BD PharMingen (San Diego, CA). Intracellular staining for LMP-1 and IFN-γ was performed using a Cytofix/CytoPerm kit (BD PharMingen). Samples were analyzed on a Becton Dickinson FACScalibur flow cytometer.
IFN-γ production assay
Hu-PBL-SCID spleen cells were stimulated with an autologous EBV+ lymphoblastoid cell line or an allogeneic EBV– Burkitt lymphoma cell line at a 4:1 ratio for 6 hours in the presence of GolgiStop (BD PharMingen). After stimulation, cells were washed and stained for extracellular CD45, CD8, and CD4, followed by intracellular staining with IFN-γ or isotype IgG1.
Survival experiment
CB.17 SCID mice were engrafted with 50 × 106 PBLs and were treated with 100 μg TMβ1 mAb on the same day, followed by 100 μL ascites containing anti–human CTLA-4 mAb or 100 μg mouse IgG and 3 μg human GM-CSF on days 1, 5, 9, and 13. Mice were monitored for signs of illness and were killed when moribund. Necropsy was performed to determine the presence of lymphoproliferative disorder or graft-versus-host disease.
Statistical analysis
Statistical significance and P values for T-cell expansion experiments were determined using 1-way analysis of variance (ANOVA) with Tukey procedure for multiple comparisons. P for difference in LMP-1 expression was determined by 2-sample t test. For the survival curve, mean survival time and standard error of the mean (SEM) survival time were calculated for each group using the Kaplan-Meier estimate. Survival times of the groups were compared using the log rank test.27
Results
Generation of a panel of mouse anti–human CTLA-4 monoclonal antibodies
BALB/c mice were immunized twice with human CTLA-4Ig fusion protein, consisting of the extracellular domain of human CTLA-4 and the Fc fragment of human IgG1. Spleen cells from these mice were fused with the myeloma cell line XAg8.653. After several fusions, we generated a panel of more than 20 hybridomas producing significant amounts of mAb against the human CTLA-4 molecule. Five of these clones were selected for experimentation after significant binding to human CTLA-4 was demonstrated using ELISA. All 5 antibodies were determined to be of the IgG1κ isotype, which facilitated direct comparison of any immunologic response that might have been mediated by these antibodies. The affinities of each antibody for human CTLA-4Ig fusion protein were measured using a Biacore instrument. As shown in Table 1, the KD of the antibodies ranged from 0.72 nM to 10.9 nM.
Anti–human CTLA-4 mAb promotes a profound expansion of T cells in a hu-PBL-SCID mouse model
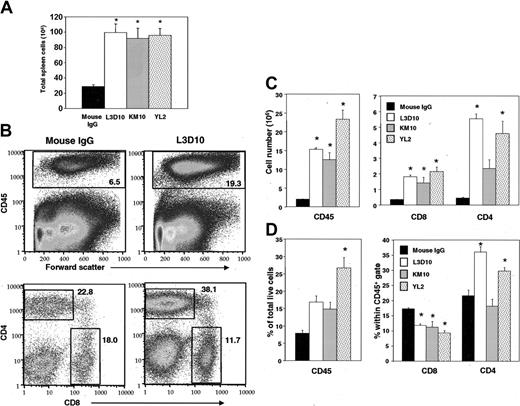
To test whether our anti–human CTLA-4 mAb had any biologic activity in vivo, we used the hu-PBL-SCID mouse model. This model provides a unique setting in which we can observe the interaction of a functional human immune system with EBV-generated lymphoproliferative disease.20 SCID mice were engrafted with human PBLs and were treated with different clones of anti–human CTLA-4 mAbs plus human GM-CSF to promote the generation and maturation of antigen-presenting cells.28 As shown in Figure 1A, at 12 days after the injection of human PBLs, all 3 anti–human CTLA-4 antibodies increased the total number of splenocytes by more than 3-fold compared with control mice (P < .002). In addition, a selective expansion of human leukocytes, as marked by the expression of human CD45, was observed among all antibody-treated mice (P < .003) (Figure 1B-D [B, top panel; C-D, left panels). The total number of CD8 T cells was increased in all antibody-treated groups (P < .03), whereas the total number of CD4 T cells was increased in L3D10- and YL2-treated groups (P < .0003) (Figure 1C, right panel). However, at this time point, the antibodies differed in their ability to selectively expand human T-cell subsets. First, in mice that received L3D10 (P < .0003) and YL2 (P < .02), the proportion of CD4 T cells expanded significantly compared with those treated with control IgG. In contrast, KM10 did not cause any preferential expansion (P = .52) (Figure 1B, D [B, lower panels]). The proportion of CD8 T cells among human leukocytes decreased significantly (P < .009) even as the total numbers increased (Figure 1C-D). A comparison between Figure 1A and 1C revealed that the numbers of mouse cells were also significantly increased, perhaps in response to cytokines induced by anti–CTLA-4 antibodies.
Anti–human CTLA-4 mAb promotes the engraftment of PBLs and the expansion of human T cells within 12 days. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on days 0, 2, and 4, followed by 300 μg anti–human CTLA-4 mAb or mouse IgG on days 1, 5, and 9 and by 3 μg human GM-CSF every other day. At 12 days after engraftment, mice were killed and spleens were harvested for staining. (A) Total cellularity within spleens. (B) Representative fluorescence-activated cell sorter (FACS) plot showing expanded percentages of CD45+, CD4+, and CD8+ cells. CD4+ and CD8+ were gated from among CD45+ cells. (C) Total cell numbers of CD45+ (left panel) and CD4+ and CD8+ cells (right panel). (D) Percentages of CD45+, CD4+, and CD8+ cells within live cell gate. All panels are representative of 4 to 5 mice per treatment group. Bars represent mean ± SEM. P values were generated using 1-way ANOVA with Tukey procedure for multiple comparisons. Asterisks indicate a difference from the control mouse IgG treatment with a significance of P < .05.
Anti–human CTLA-4 mAb promotes the engraftment of PBLs and the expansion of human T cells within 12 days. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on days 0, 2, and 4, followed by 300 μg anti–human CTLA-4 mAb or mouse IgG on days 1, 5, and 9 and by 3 μg human GM-CSF every other day. At 12 days after engraftment, mice were killed and spleens were harvested for staining. (A) Total cellularity within spleens. (B) Representative fluorescence-activated cell sorter (FACS) plot showing expanded percentages of CD45+, CD4+, and CD8+ cells. CD4+ and CD8+ were gated from among CD45+ cells. (C) Total cell numbers of CD45+ (left panel) and CD4+ and CD8+ cells (right panel). (D) Percentages of CD45+, CD4+, and CD8+ cells within live cell gate. All panels are representative of 4 to 5 mice per treatment group. Bars represent mean ± SEM. P values were generated using 1-way ANOVA with Tukey procedure for multiple comparisons. Asterisks indicate a difference from the control mouse IgG treatment with a significance of P < .05.
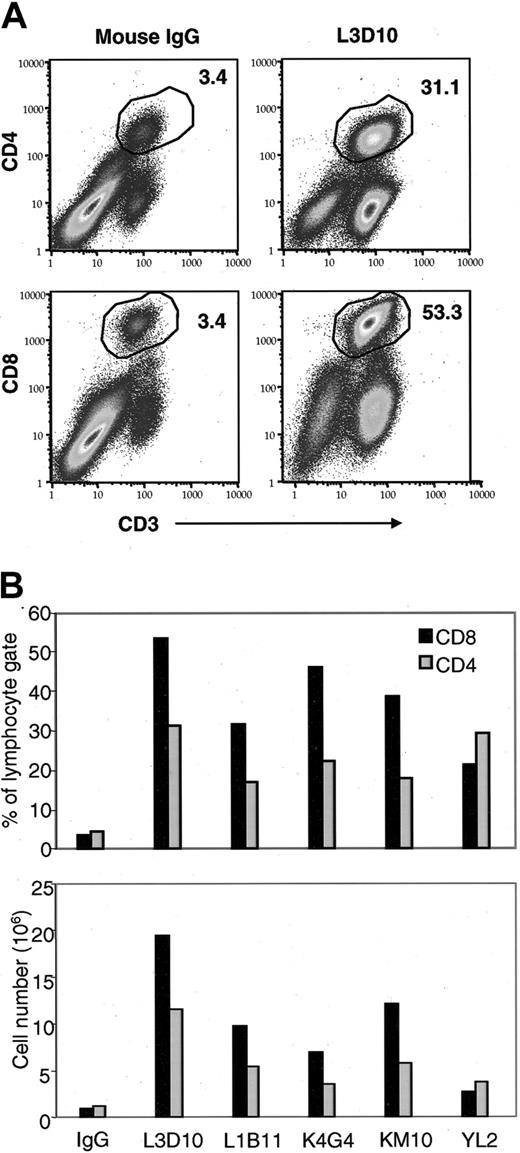
At the third week after reconstitution, we analyzed all 5 anti–CTLA-4 antibodies for their effect on the number of human CD4 and CD8 T cells in the spleen. An example is given in Figure 2A, and the comparison of the different antibodies is presented in Figure 2B. As shown in Figure 2A, L3D10 caused a more than 10-fold expansion of CD4 and CD8 T cells. Interestingly, the 5 clones of anti–human CTLA-4 mAb displayed differential effects not only on the amount of T-cell expansion but also on the relative effect on CD4 versus CD8 T-cell subsets. Most clones of anti–human CTLA-4 mAb showed a preferential expansion of CD8 T cells at this time, but one clone of mAb showed a slightly preferential increase in CD4 T cells. These data clearly demonstrate that our anti–human CTLA-4 mAb promoted the expansion of human T cells and increased the engraftment of total human PBLs in the hu-PBL-SCID mouse model. Surprisingly, the extent of T-cell expansion did not correlate with the binding affinities of the antibodies to human CTLA-4 (Table 1). The 2 antibodies with highest affinities, KM10 and YL2, did not induce the greatest T-cell expansion and even varied from one another in their ability to induce T-cell expansion despite similar Kd values. Given that anti–human CTLA-4 mAb clone L3D10 showed the greatest effect in expanding human T cells, this clone was chosen for further characterization.
Anti–human CTLA-4 mAb promotes the engraftment of PBLs and the expansion of human T cells at 24 days. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on days –1, 1, and 3, 100 μL ascites containing anti–human CTLA-4 mAb or 100 μg mouse IgG on days 1, 5, 9, and 13, and 3 μg human GM-CSF every other day. At 24 days after engraftment, mice were killed and spleens were harvested and pooled for staining. (A) Representative dot plot showing expansion of CD8 and CD4 T cells with anti–human CTLA-4 mAb clone L3D10 treatment. (B) Variable expansion of CD8 and CD4 T cells with treatment by different clones of anti–human CTLA-4 mAb. Bars represent cells from pooled spleens from 2 to 3 mice per treatment group.
Anti–human CTLA-4 mAb promotes the engraftment of PBLs and the expansion of human T cells at 24 days. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on days –1, 1, and 3, 100 μL ascites containing anti–human CTLA-4 mAb or 100 μg mouse IgG on days 1, 5, 9, and 13, and 3 μg human GM-CSF every other day. At 24 days after engraftment, mice were killed and spleens were harvested and pooled for staining. (A) Representative dot plot showing expansion of CD8 and CD4 T cells with anti–human CTLA-4 mAb clone L3D10 treatment. (B) Variable expansion of CD8 and CD4 T cells with treatment by different clones of anti–human CTLA-4 mAb. Bars represent cells from pooled spleens from 2 to 3 mice per treatment group.
Anti–human CTLA-4 mAb decreases EBV-mediated transformation of human cells
When PBLs from EBV-seropositive donors were used in the hu-PBL-SCID mouse model, transformation of PBLs by EBV promoted the development of lymphoproliferative disease. LMP-1 is an EBV oncoprotein involved in the immortalization of B cells leading to this transformation,29-33 and LMP-1 has been shown to be a potential target of T-cell responses.34,35 Hence, the number of cells expressing LMP-1 can be taken as a reflection of the number of cells that were transformed by EBV and could undergo oncogenesis. One way to determine whether the expansion of T cells mediated by anti–human CTLA-4 mAb treatment has any therapeutic effect is to examine the level of LMP-1 expressed within engrafted cells. To test this, SCID mice were engrafted with PBLs and were treated with anti–human CTLA-4 mAb L3D10 or mouse IgG. Mice were killed 22 days after engraftment, and spleen cells were analyzed. Similar to the experiment shown in Figure 2, we observed a substantial expansion of CD8 and CD4 T-cell subsets (data not shown). As shown in Figure 3A-B, the percentage of LMP-1+ cells was 3- to 4-fold lower in mice treated with L3D10 (P = .0015). A similar magnitude of reduction was observed in the total number of LMP-1+ cells in the spleens. However, large intragroup variation reduced the P value to .0854. These results suggest that the percentage of EBV-infected cells can be reduced as a result of anti–CTLA-4 mAb treatment. Interestingly, although essentially all CD19+ cells expressed LMP-1, most of the LMP-1+ cells lacked the CD19 marker. The origin of these LMP-1+CD19– cells is unclear at this stage.
Anti–human CTLA-4 mAb L3D10 decreases percentages of engrafted cells expressing EBV LMP-1. Mice were treated as described in the legend to Figure 2 except that TMβ1 mAb was given on days 0, 2, and 4. Spleens were harvested at day 22 after engraftment and were stained for intracellular LMP-1 or isotype IgG2a. (A) Representative FACS plot of LMP-1 staining, depicting cells from the lymphocyte gate. Data shown are LMP-1 and CD19 profiles of gated human CD45+ cells. (B) Summary graph showing percentages (top) and numbers (bottom) of LMP-1–staining cells for 3 to 4 individual mice per treatment group. Data are representative of 2 independent experiments. P values were generated using a 2-sample t test.
Anti–human CTLA-4 mAb L3D10 decreases percentages of engrafted cells expressing EBV LMP-1. Mice were treated as described in the legend to Figure 2 except that TMβ1 mAb was given on days 0, 2, and 4. Spleens were harvested at day 22 after engraftment and were stained for intracellular LMP-1 or isotype IgG2a. (A) Representative FACS plot of LMP-1 staining, depicting cells from the lymphocyte gate. Data shown are LMP-1 and CD19 profiles of gated human CD45+ cells. (B) Summary graph showing percentages (top) and numbers (bottom) of LMP-1–staining cells for 3 to 4 individual mice per treatment group. Data are representative of 2 independent experiments. P values were generated using a 2-sample t test.
Anti–human CTLA-4 mAb promotes the expansion of LCL-reactive CD8 T cells
To test whether antigen-specific T cells were induced in our model, we stimulated spleen cells harvested from hu-PBL-SCID mice with an autologous EBV-positive lymphoblastoid cell line (LCL) or allogeneic EBV-negative Burkitt lymphoma for 6 hours in vitro and evaluated IFN-γ production by CD8 T cells. The LCL was generated from a tumor harvested from a hu-PBL-SCID mouse previously engrafted with PBLs from the same donor. To verify the expression level of EBV protein, we stained these stimulator cell types for intracellular LMP-1 expression. As shown in Figure 4A, almost all the LCL cells expressed high levels of intracellular LMP-1, whereas the Burkitt lymphoma cells had minimal or no LMP-1 expression. After 6 hours of stimulation with these cells, we observed nearly a 3-fold increase in the percentage of IFN-γ–producing CD8 T cells in L3D10-treated mice compared with control mice (Figure 4B). This indicates that anti–human CTLA-4 mAbs can promote the preferential expansion of antigen-specific CD8 T-cell responses and can promote the overall expansion of T cells.
Anti–human CTLA-4 mAb L3D10 promotes preferential expansion of lymphoblastoid cell line–reactive CD8 T cells. (A) LMP-1 expression by an autologous EBV-positive lymphoblastoid cell line (LCL), and an allogeneic EBV-negative Burkitt lymphoma cell line used as stimulators for IFN-γ production by hu-PBL-SCID spleen cells. (B) CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on the same day, followed by 300 μg anti–human CTLA-4 mAb L3D10 or mouse IgG and 3 μg human GM-CSF on days 1, 5, and 9. Spleen cells were harvested at day 29 after engraftment and were stimulated for 6 hours with autologous LCL or allogeneic Burkitt lymphoma cells as a control. Samples were then stained for IFN-γ–producing CD8 T cells. L3D10-treated mice showed an almost 3-fold increase in percentages of IFN-γ–producing CD8 T cells with LCL stimulation compared with control mice. Neither treatment group showed reactivity to Burkitt lymphoma. FACS plots represent pooled spleens from 9 mouse IgG-treated and 5 L3D10-treated mice. Plots shown are within the CD45+CD8+ gate.
Anti–human CTLA-4 mAb L3D10 promotes preferential expansion of lymphoblastoid cell line–reactive CD8 T cells. (A) LMP-1 expression by an autologous EBV-positive lymphoblastoid cell line (LCL), and an allogeneic EBV-negative Burkitt lymphoma cell line used as stimulators for IFN-γ production by hu-PBL-SCID spleen cells. (B) CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb on the same day, followed by 300 μg anti–human CTLA-4 mAb L3D10 or mouse IgG and 3 μg human GM-CSF on days 1, 5, and 9. Spleen cells were harvested at day 29 after engraftment and were stimulated for 6 hours with autologous LCL or allogeneic Burkitt lymphoma cells as a control. Samples were then stained for IFN-γ–producing CD8 T cells. L3D10-treated mice showed an almost 3-fold increase in percentages of IFN-γ–producing CD8 T cells with LCL stimulation compared with control mice. Neither treatment group showed reactivity to Burkitt lymphoma. FACS plots represent pooled spleens from 9 mouse IgG-treated and 5 L3D10-treated mice. Plots shown are within the CD45+CD8+ gate.
Anti–human CTLA-4 mAb L3D10 delays the development of lymphoproliferative disease in hu-PBL-SCID mice
We performed a long-term survival experiment in which engrafted mice were treated with anti–human CTLA-4 mAbs and human GM-CSF for 2 weeks after engraftment and then were observed for signs of illness. Figure 5 shows the survival curve of mice that received control IgG or 1 of 5 different anti–CTLA-4 antibodies. Based on our previous experience that no lymphoproliferative disease can be observed within 1 month of engraftment, we excluded from the final analysis 2 mice that died before 30 days. L3D10 mediated an almost 2-fold extension in the mean survival time of engrafted mice compared with control mice (100.3 ± 17.5 days with L3D10 vs 53.0 ± 6.2 days with mouse IgG), which was statistically significant (P = .0195). In addition, KM10G11 also had a statistically significant impact (P = .045) on survival. However, though it appears that other anti–CTLA-4 antibodies also extended the lifespan of recipient mice somewhat, pairwise comparisons demonstrate no statistical difference between these antibodies and control IgG.
Some anti–human CTLA-4 mAbs prolong survival and delay onset of lymphoproliferative disorder in hu-PBL-SCID mice. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb the same day, followed by 100 μL ascites containing anti–human CTLA-4 mAbs or 100 μg mouse IgG and 3 μg human GM-CSF on days 1, 5, and 9, and 13 after engraftment. Mice were monitored for signs of illness and were killed when moribund. One L3D10-treated mouse with early death on day 15 and 1 KM10G11-treated mouse with early death on day 13 were excluded from the survival analysis based on our experience that no lymphoma-related death is possible at this point. Survival times of the antibody-treated groups were compared with those of the control IgG-treated group using the log rank test. Probability values were as follows: mouse IgG versus L3D10, P = .0195; mouse IgG versus L1B11, P = .481; mouse IgG versus K4G4, P = .323; mouse IgG versus KM10G11, P = .045; mouse IgG versus YL2, P = .324.
Some anti–human CTLA-4 mAbs prolong survival and delay onset of lymphoproliferative disorder in hu-PBL-SCID mice. CB.17 SCID mice were engrafted with 50 × 106 human PBLs and were treated with 100 μg TMβ1 mAb the same day, followed by 100 μL ascites containing anti–human CTLA-4 mAbs or 100 μg mouse IgG and 3 μg human GM-CSF on days 1, 5, and 9, and 13 after engraftment. Mice were monitored for signs of illness and were killed when moribund. One L3D10-treated mouse with early death on day 15 and 1 KM10G11-treated mouse with early death on day 13 were excluded from the survival analysis based on our experience that no lymphoma-related death is possible at this point. Survival times of the antibody-treated groups were compared with those of the control IgG-treated group using the log rank test. Probability values were as follows: mouse IgG versus L3D10, P = .0195; mouse IgG versus L1B11, P = .481; mouse IgG versus K4G4, P = .323; mouse IgG versus KM10G11, P = .045; mouse IgG versus YL2, P = .324.
Discussion
The manipulation of costimulatory pathways using mAbs has proven to be a highly effective strategy for boosting antitumor immunity in mice. Unfortunately, few of these achievements have been successfully translated to the treatment of patients with cancer. Since the first use of mAbs targeting CTLA-4 to treat mouse tumors,1 CTLA-4 has shown great promise for clinical translation. Two recent human trials using mAbs to target the human CTLA-4 molecule showed promising effects12,13 but also highlighted several difficulties in translating results observed in murine models to human patients. For example, in one study, the anti–human CTLA-4 mAb showed no toxicities but also showed minimal clinical benefit in patients with metastatic melanoma and ovarian cancer.12 Another study with the same mAb showed several dramatic clinical responses that were accompanied by serious autoimmune toxicities.13 Although these studies validate the potential of anti–human CTLA-4 mAb as a therapy for patients with cancer, further preclinical testing of this mAb is appropriate to address these issues.36
Here we describe the use of a hu-PBL-SCID mouse model to obtain a more thorough preclinical screening of anti–human CTLA-4 mAb to identify the most efficacious clones from a panel of mAbs. The hu-PBL-SCID mouse model was first described by Mosier et al20 as a method to reconstitute a functional human immune system in SCID mice by intraperitoneal injection of human peripheral blood leukocytes. This report described the long-term engraftment of all cellular components of the human immune system and also observed the spontaneous development of human B-cell lymphomas when PBLs from EBV-seropositive donors were used. These lymphomas were subsequently characterized as similar to the large cell lymphomas observed in patients with immunosuppression transplant,37 a condition also known as posttransplant lymphoproliferative disorder (PTLD). Since the initial reports, numerous groups have used this model to test various aspects of immune function and lymphomagenesis and, in the process, have discovered a number of limitations of this model, including xenograft-versus-host disease (XGVHD), variations in PBL engraftment, and leakiness of the SCID phenotype.38-42 Despite these caveats, the hu-PBL-SCID model remains one of the few mouse models with which to assess spontaneous human tumor development and the resultant antitumor immune response. More recently, evidence has accumulated that the control of EBV-lymphoproliferative disorder is mediated by CD8 cytotoxic T lymphocytes in patients with PTLD43,44 and in hu-PBL-SCID mice.26,45 With the identification of EBV latent and lytic antigens, it has been demonstrated that specific CD8 T-cell responses to these EBV antigens can be detected in seropositive patients34,46,47 and in hu-PBL-SCID mice.26 Correlation of CD8 T-cell responses to protection against EBV-lymphoproliferative disorder in hu-PBL-SCID mice makes this model valuable for the study of anti–human CTLA-4 mAbs.
In this study, we have clearly demonstrated the ability of anti–human CTLA-4 mAbs to mediate the dramatic expansion of CD8 and CD4 T-cell populations. In conjunction, mAb promotes the overall engraftment or survival of human PBLs in the SCID mouse. Interestingly, each clone of anti–human CTLA-4 mAb has varying ability to promote T-cell expansion and PBL engraftment. Given that all antibodies were of the same isotype, these variations cannot be attributed to isotype difference. In addition, the affinity of the antibodies used does not adequately explain the functional heterogeneity. For instance, 2 pairs of antibodies with essentially identical affinity showed different function in vivo. This lack of in vitro and in vivo correlation warrants the use of more stringent preclinical screening regimens such as the one described here to select mAb clones that can elicit the most dramatic T-cell response in vivo.
It has been reported that human T cells engrafted in SCID mice represent an anergic phenotype and that once anergy is broken, most reactivity of CD4 T cells is directed against mouse antigens.48 It is possible that the lack of T-cell expansion in our control mice resulted from an anergic state of the T cells and that treatment with anti–human CTLA-4 mAbs was sufficient to reverse this anergy and to permit T-cell expansion. This has important implications for a tumor setting in which T cells might be tolerized to tumor antigen, as been demonstrated in at least one mouse model.11
Not only did anti–human CTLA-4 mAbs promote overall T-cell expansion in vivo, several different parameters suggested that robust T-cell expansion with anti–human CTLA-4 mAb treatment had therapeutic value. First, we observed a significant decrease in the percentage of cells that expressed intracellular LMP-1. As an EBV oncoprotein critical for the generation of lymphoma, LMP-1 may be viewed as a surrogate marker for the potential formation of tumors within mice. Reduced levels at an early time point, before lymphoproliferative disease normally appears, reveal the impact of mAb treatment in reducing the oncogenic source of tumor.
Second, we observed the preferential expansion of antigen-specific CD8 T cells with anti–human CTLA-4 mAb treatment. This enhanced expansion was elicited in mice treated with a more limited GM-CSF regimen than that used in experiments showing overall T-cell expansion and LMP-1 reduction. Interestingly, mice treated with L3D10 under the limited GM-CSF regimen did not show overall expansion of T cells compared with control mice, despite their preferential increase in antigen-specific CD8 T cells. Additional experiments using frozen spleen cells from mice treated with L3D10 and the more extensive GM-CSF protocol (every other day) showed enhanced overall T-cell expansion but not antigen-specific expansion when compared with control mice. It is difficult to directly compare the use of fresh and frozen cells for in vitro stimulation because of the decreased viability and increased background staining associated with thawed cells, but perhaps the interaction of anti–CTLA-4 mAbs and GM-CSF is more complicated than predicted in the hu-PBL-SCID mouse model.
Third, in a longer-term experiment, we observed a prolongation of survival with anti–human CTLA-4 mAb treatment, providing another piece of evidence that mAb can promote antitumor immune responses. This result must be viewed with caution because this experiment and other attempts to reproduce the finding were complicated by the development of severe illness, most likely XGVHD, which sometimes caused death before lymphoma formation in a substantial fraction of the mice. However, in mice that escaped XGVHD, the trend toward prolonged survival is intriguing. Taken together, our data show an important role for anti–human CTLA-4 mAb in the expansion of human T cells and the promotion of immunity against a spontaneous, virally induced tumor. Furthermore, variability in the efficacy of different clones of mAb warrants the use of novel models, such as this one, to provide more thorough preclinical screening of candidate mAbs for clinical translation.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-07-2561.
Supported by the National Cancer Institute (grants R01CA69091, R01CA58033, R41CA93107, and P01CA9542-01). K.F.M. and S.R. are in The Ohio State University Medical Scientist Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.