Abstract
A total of 21 patients with myelofibrosis with myeloid metaplasia (MMM), with a median age of 54 years (range, 27-68 years), were prepared with a reduced-intensity conditioning (RIC) regimen. The patients received an allogeneic marrow (n = 3) or peripheral blood stem-cell (n = 18) transplant from HLA-matched related (n = 18) or unrelated (n = 2), or 1 Ag-mismatched related (n = 1), donors. RIC regimens included fludarabine/total body irradiation 200 cGy (n = 5) or 450 cGy (n = 1), fludarabine/melphalan (n = 7), thiotepa/cyclophosphamide (n = 7), and thiotepa/fludarabine (n = 1). At the time of transplantation, all of the patients were at intermediate (n = 13) or high (n = 8) risk, according to the Dupriez classification. Of the patients, 19 had grade III or IV marrow fibrosis. All of the patients achieved full engraftment but one. Posttransplantation chimerism analysis showed more than 95% donor cells in 18 patients, while 2 patients achieved complete donor chimerism after donor leukocyte infusion (DLI). Acute graft-versus-host disease (GVHD) grades II to IV was observed in 7 patients, grades III to IV in 2, and extensive chronic GVHD in 8 of 18 evaluable patients. There were 3 patients who died from acute GVHD, infection, and relapse. There are 18 patients alive 12 to 122 months (median, 31 months) after transplantation, and 17 are in remission (1 after a second transplantation). The use of RIC regimens in allogeneic stem cell transplantation results in prolonged survival in intermediate/high-risk MMM patients.
Introduction
Myelofibrosis with myeloid metaplasia (MMM) is a chronic clonal myeloproliferative disorder characterized by blood cytopenias, megakaryocytic hyperplasia, dysplastic myelopoiesis, reactive marrow fibrosis, and extramedullary hematopoiesis.1,2 Among the other chronic myeloproliferative diseases, MMM has the worst prognosis, with a median survival of 3.5 to 5.5 years. Many prognostic factors, such as leukocytosis or leukopenia, circulating blast cells and/or immature granulocytic precursors, anemia, thrombocytopenia, and cytogenetic abnormalities, have been previously reported to predict the outcome of this disease.3-6 A prognostic scoring system was developed by Dupriez et al,5 in which the presence of leukocytosis with more than 30 × 109/L white cells or leukopenia with less than 4 × 109 white cells/L, or anemia (hemoglobin [Hgb], < 100 g/L [10 g/dL]), was used to identify 3 groups of patients with different prognoses, ranging from a median survival of 93 months for score 0, to 26 and 13 months for scores 1 and 2, respectively.
Allogeneic hematopoietic stem-cell transplantation is the only curative treatment for MMM,7 since conventional chemotherapy and/or splenectomy are palliative and do not seem to affect survival.8-12 Nevertheless, the use of fully myeloablative conditioning regimens has been shown to be associated with a high transplantation-related mortality (TRM) rate,13-16 especially in advanced and elderly patients. In particular, patients older than 45 years were previously reported to have a significantly worse probability of survival compared with younger patients.17 More favorable results were initially reported in 4 cases of myelofibrosis patients receiving an allogeneic peripheral blood stem cell (PBSC) transplant from HLA-matched related donors, with a reduced-intensity conditioning (RIC) regimen.18
Within the clinical centers of the Philadelphia-negative Myeloproliferative Disorder-Research Consortium (MPD-RC) the data on 21 MMM patients, with Dupriez score higher than 0 who underwent allotransplantation using RIC regimens, were collected. The results are consistent with previous reports demonstrating that allogeneic hematopoietic stem cell (HSC) transplantations induce a high rate of complete responses in MMM. Moreover, the use of RIC regimens may constitute a preferable strategy in elderly patients due to a limited toxicity and a low rate of TRM.
Patients, materials, and methods
Patients
All of the patients with MMM in chronic phase, either idiopathic, or developing following polycythemia vera (PV) or essential thrombocythemia (ET), who were eligible for and received an allogeneic bone marrow (BM) or PBSC transplant with RIC regimen in the centers connected with the MPD-Research Consortium were analyzed without any selection in this retrospective study. All of the patients met the Italian diagnostic criteria for myelofibrosis.19 An informed consent was signed prior to transplantation, and the transplantations were performed according to each center's protocol. The protocol for each institution was approved by that institution's institutional review board (IRB) (University of Illinois at Chicago; Baylor College of Medicine, Houston; Johns Hopkins University, Baltimore; Rush University, Chicago; Mount Sinai School of Medicine, New York) or ethical committee (IRCCS Policlinico S. Matteo, Pavia, Italy; Ospedale S. Martino, Genova, Italy). Results of 4 patients included in this study were previously reported18 and are here updated with a longer follow-up. The patients' clinical characteristics are shown in Table 1. The median age was 54 years, and 18 of 21 patients were older than 45 years. Median hematologic values at the time of transplantation were as follows: white blood cell (WBC) count, 4.4 × 109/L (range, 0.1-30.4 × 109/L); Hgb level, 85 g/L [8.5 g/dL] (range, 72-114 g/L [7.2-11.4 g/dL]); and platelet count, 124 × 109/L (range, 6-341 × 109/L). Of the patients, 17 (85%) were either red blood cell (RBC) or platelet-transfusion dependent. All of the patients had a poor prognosis based upon a Dupriez score5 of 1 at the time of transplantation. Marrow fibrosis, as assessed by examination of a silver-stained bone marrow biopsy by the local institutional hemopathologist, was reported to be grades III to IV in 19 of 21 patients and grade II in the remaining 2. All of the patients were still in chronic phase and had less than 5% blast cells in the peripheral blood. The median time from diagnosis to transplantation was 11 months (range, 1.5-68 months). Of the patients, 8 received the transplant as front-line treatment, whereas 13 had been previously treated with hydroxyurea (HU, n = 6), and/or the following: interferon-α (n = 4), steroids (n = 4), danazol (n = 2), recombinant human erythropoietin (rhEPO, n = 2), and thalidomide (n = 1).
There were 3 patients who had been previously splenectomized, while 16 patients had a palpable spleen, which in 5 cases was more than 10 cm below the costal margin.
Donors
Of the patients, 3 received a BM graft from an HLA-matched related donor. There were 15 patients who received PBSC grafts from HLA-matched related donors; 1 from a 1-antigen-mismatched related donor; and 2, from a 6/6 HLA-matched unrelated donor (Table 2). In these cases, HLA matching was determined by high-resolution molecular typing for both class I and class II antigens. The PBSC donors received recombinant human granulocyte colony-stimulating factor (rhG-CSF) 10 μg/kg subcutaneously per day for 5 days and underwent PBSC collection by leukapheresis.
In selected cases, donors of patients who showed persistent mixed chimerism after transplantation, or relapse of the disease, after complete withdrawal of immunosuppression underwent one leukapheresis procedure in order to collect peripheral blood mononuclear cells. Donor leukocyte infusion (DLI) was then performed with cell products containing 0.5 to 2 × 108 T cells/kg.
Conditioning regimens
The patients were prepared with different RIC regimens, listed in Table 2. Most of them included fludarabine that was combined with melphalan (n = 7),18 or low-dose total body irradiation (TBI) (n = 6),20 or thiotepa (n = 1).21 In addition, 7 patients were conditioned with a regimen containing thiotepa and cyclophosphamide.22 Graft-versus-host disease (GVHD) prophylaxis included tacrolimus and methotrexate in 9 cases; cyclosporine-A and methotrexate in 7 cases; cyclosporine-A, methotrexate, and mycophenolate in 4 cases; and tacrolimus alone in 1 case. In addition, thymoglobulin (Genzyme, Cambridge, MA) was added to the preparative regimen in 3 patients, including the 2 receiving a graft from an unrelated donor, at a dose of 3 mg/kg (n = 1) or 15 mg/kg (n = 2). A single 30-mg dose of alemtuzumab (anti-CD52 monoclonal antibody; Berlex Laboratories, Richmond, CA) was used in one case.
Criteria for engraftment, response, and GVHD
Standard criteria for engraftment were applied, as previously reported.18 Posttransplantation donor-recipient chimerism was assessed by means of DNA microsatellite analysis as previously described.23 The degree of marrow fibrosis was graded as previously reported.24 Hematologic response was defined as complete if all peripheral blood counts had been normalized with or without the persistence of splenomegaly, as long as 100% donor chimerism was achieved. Acute GVHD and chronic GVHD (cGVHD) were graded according to standard criteria.25,26
Statistical analysis
Time to engraftment was compared between groups by means of the Wilcoxon nonparametric test, and P ≤ .05 was considered significant. Overall survival (OS) and event-free-survival (EFS) estimates were obtained using the method of Kaplan and Meyer.
Results
Engraftment
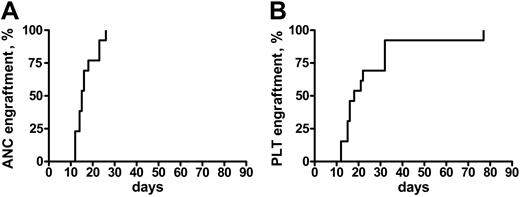
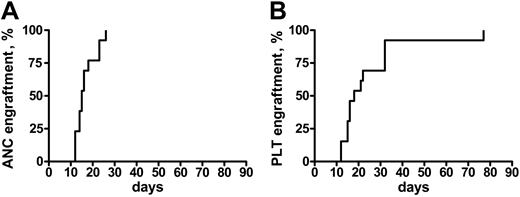
Engraftment was achieved in all of the patients but one, who did not recover a platelet count more than 20 × 109/L. In patients who engrafted, the median time to an absolute neutrophil count (ANC) more than 0.5 × 109/L and a platelet (Plt) count more than 20 × 109/L was 16 days (range, 12-26 days) and 18 days (range, 12-77 days), respectively (Figure 1). Median ANC recovery in patients who, prior to transplantation, had not been treated except for transfusion therapy occurred on day 13 (range, 12-18 days), and on day 16 (range, 14-26 days) in the others (P = .05). Platelet recovery also was more rapid in untreated patients (median, day 15 [range, 12-77 days] vs day 22 [range, 15-33 days]), but the difference was not significantly different. Because of the heterogeneity of the conditioning regimens, it is not possible to correlate the use of any of these regimens with the kinetics of engraftment.
Time to engraftment after RIC transplantation in MMM. Time to recovery of absolute neutrophil counts (ANC > 0.5) (A) and platelets (Plt > 20 K) (B) in 21 patients with MMM following a RIC allogeneic HSC transplantation.
Time to engraftment after RIC transplantation in MMM. Time to recovery of absolute neutrophil counts (ANC > 0.5) (A) and platelets (Plt > 20 K) (B) in 21 patients with MMM following a RIC allogeneic HSC transplantation.
On day 30 after transplantation, each of the patients had more than 1.5 × 109 ANC/L and 19 of 21 had more than 20 × 109 Plts/L. No episode of graft rejection was documented. Of the 2 patients who did not recover a platelet count more than 20 × 109/L at 30 days after transplantation, 1 had received an unrelated PBSC transplant and died from a fatal infection (see “Overall survival and event-free survival”). Multiple posttransplantation chimerism studies showed 100% stable chimerism in 18 patients, whereas 2 patients achieved 100% only after DLI.
GVHD
Of the patients, 7 (33%) developed acute GVHD grades II to IV and 2 (9%), grades III to IV. Neither of the 2 patients receiving an unrelated transplant had acute GVHD, likely because of partial T-cell depletion due to the administration of thymoglobulin. Among the 3 patients splenectomized prior to transplantation, only 1 had acute GVHD (aGVHD, grade IV).
Chronic GVHD occurred in 13 of 18 evaluable patients, with a cumulative incidence of 72%; however extensive cGVHD was observed in only 8 patients (44%).
Overall survival and event-free survival
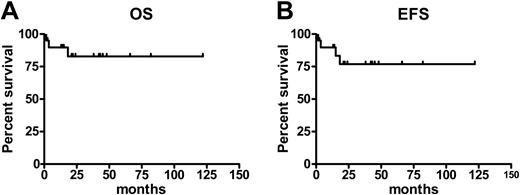
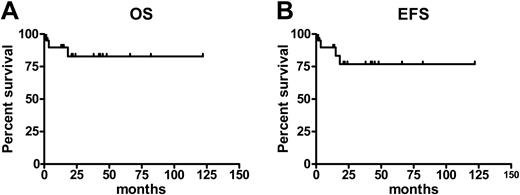
Currently, 18 patients (85%) are alive 12 to 122 months (median, 31 months) after transplantation (Figure 2, left); 3 patients have died. One patient who received a transplant from an HLA-matched sibling developed acute GVHD grade IV and cytomegalovirus (CMV) disease and died within 100 days after transplantation. Another patient, who received a transplant from an HLA-matched unrelated donor, died of bacterial pneumonia on day 110. A third patient relapsed 380 days after transplantation, received DLI, but died from progression of the disease on day 547 after transplantation. Of these 3 patients, 2 had a Dupriez score of 2 prior to transplantation. Of 15 patients who underwent transplantation with a Dupriez score of 1, 14 are alive; 6 of 8 patients with a Dupriez score of 2 are surviving with a median follow-up of 33 months (range, 13-82 months).
According to the criteria described, 17 patients (81%) are in remission. Relapse or progression of the disease was observed in 3 patients (14%) (Figure 2, right). One of these patients, who initially received also one dose of alemtuzumab in the conditioning regimen, relapsed on day 121 and achieved a complete remission after a second transplantation from the same donor, conditioned with standard busulfan and cyclophosphamide. Another patient achieved the remission after DLI. The third patient who relapsed is alive 15 months after transplantation and is transfusion independent.
A more than 80% reduction in the degree of splenomegaly occurred in the majority of the patients. One patient who had a persistently large splenomegaly and 70% donor cell chimerism at day 30 after transplantation underwent splenectomy and DLI, and is alive in remission 377 days after transplantation Following transplantation, marrow fibrosis was grades 0 to II in all of the evaluable patients after a follow-up of at least 12 months. In 4 cases, a serial analysis of the volume density of reticulin fibers in the bone marrow was performed at 1, 3, 6, and 12 months after transplantation, demonstrating the progressive resolution of marrow fibrosis.27
Discussion
This study demonstrates that the use of RIC regimens in allogeneic transplantation for MMM patients with poor prognosis (Dupriez score 1 or 2) results in a high rate of remission with low transplant-related mortality.
The prognosis of MMM patients has been previously related to multiple risk factors, such as age, cytogenetics, anemia, leukocytosis, leukopenia, clinical symptoms, circulating blasts, and myeloid precursors.2 However, anemia and leukopenia or leukocytosis have been identified as the most useful factors in order to predict survival of MMM, and based upon these factors Dupriez et al5 divided the patients into low-, intermediate-, and high-risk categories. Currently, no chemotherapeutic agent has been demonstrated to prolong the survival in this disease, although supportive care with transfusions or treatment with HU, interferon, danazol, or thalidomide may improve the symptoms and possibly improve the quality of life of these patients.1,8-11 Allogeneic bone marrow transplantation, however, is the only curative treatment that by means of the administration of radio/chemotherapy agents, or a graft-versus-tumor effect elicited by donor lymphocytes, is capable of reducing the tumor burden. Nevertheless, previous studies have shown that fully myeloablative preparative regimens, with or without total body irradiation, lead to a transplant-related mortality in 27% to 48% of the patients (Table 3).13,15-17 In particular, the age of patients has been shown to be a critical prognostic factor in this setting, as demonstrated in the report of Guardiola et al,17 where only 14% of patients older than 45 years at the time of transplantation survived after 5 years of follow-up. A greater percentage of older patients with myelofibrosis included in a recent Canadian study is also likely to account for greater TRM compared with other studies.14 Recently, Deeg et al15 suggested that the use of busulfan in the conditioning regimen, with doses adjusted to achieve targeted busulfan plasma levels, has a positive effect in the outcome of myelofibrosis patients compared with a regimen with TBI. It should be noted that, in the same study, the patients with a low Dupriez score (25/56) had an 80% overall survival rate at 3 years after transplantation, whereas patients with intermediate (17/56) or high risk (13/56) had approximately 45% and 25% probability of survival at 3 years, respectively. In addition, the grade of marrow fibrosis also correlated with hazard of mortality. Therefore, in the report by Deeg et al15 intermediate- and high-risk patients were likely to have an increased mortality due to both relapse and causes not related to relapse. In addition, it is not known whether the prolonged median time from diagnosis to transplantation (33 months) may also represent a risk factor for nonrelapse mortality.
Survival and event-free survival after RIC transplantation in MMM. Estimates of overall survival (OS) (A) and event-free survival (EFS) (B) for patients with myelofibrosis following a RIC allogeneic HSC transplantation.
Survival and event-free survival after RIC transplantation in MMM. Estimates of overall survival (OS) (A) and event-free survival (EFS) (B) for patients with myelofibrosis following a RIC allogeneic HSC transplantation.
By taking into consideration the advanced age of MMM patients at diagnosis (often > 60 years) and the substantial prolonged survival for those at low risk (93 months according to Dupriez5,6 classification and 177 months according to Cervantes classification), it is clear that allogeneic stem cell transplantation with a fully myeloablative conditioning is a valid therapeutic option for young patients and/or for those with poor prognosis. In order to reduce the transplant-related toxicity and mortality, 4 myelofibrosis patients initially underwent transplantation with a RIC regimen including fludarabine and melphalan,18 showing that all of them achieved a hematologic remission with limited toxicity, regression of marrow fibrosis, and no TRM. In this study, we analyzed the data of 21 patients (including the previous 4) with myelofibrosis in chronic phase, all of them at intermediate or high risk at the time of transplantation, who underwent a RIC allotransplantation in centers within the MPD-Research Consortium. Different RIC regimens (Table 1) were used, the majority of them included fludarabine. The patients fulfilled standard inclusion criteria for allotransplantation, with no major organ toxicity prior to transplantation. No further selection criteria were used. The policy of the participating institutions has been to recommend an allogeneic transplantation in all eligible patients; however, since stem cell transplantation is still experimental in MMM and possibly a very risky approach, a number of patients refused this option.
Compared with previous fully myeloablative transplant studies (Table 3), we observed an increased overall survival (85%) and event-free-survival (76%), despite a median patient age of 54 years and all patients with intermediate/high-risk disease. These encouraging findings using a RIC regimen suggest that myelofibrosis is highly responsive to donor T-cell alloantigen recognition eliciting a graft-versus-tumor effect, as also demonstrated by the antitumor effect observed after DLI,28,29 albeit immunogenic tumor-associated antigens have not been yet characterized. The role of in vivo T-cell modulation by thymoglobulin remains to be validated in a larger series of cases. In this study, 2 of the 3 patients who died had received thymoglobulin, but none of them relapsed. On the contrary, one patient who received one dose of alemtuzumab in the conditioning regimen relapsed 121 days after transplantation and then successfully underwent a second transplantation. The low TRM could be related to a better performance status of patients who underwent transplantation early after diagnosis, but is also likely a consequence of the conditioning regimen used, due to the more limited toxicity and acute GVHD than observed in previous studies. In particular, a low rate of acute GVHD could be possibly explained by a reduced toxicity caused by the regimens used, which would result in a decreased cytokine and antigen release from damaged tissues, and/or by the prevalence of HLA-matched related donors. The use of PBSCs, which is associated with a rapid hematopoietic recovery after RIC transplantations, may be associated with an increased rate of cGVHD.30 In this study, 18 of 21 patients received PBSCs, and cGVHD was observed in 72% of the cases, but was extensive in only 44%. Although the cGVHD might be associated with mortality after a longer follow-up, it might also contribute to the slow, but progressive, normalization of marrow cellularity, which has been observed after approximately one year.27 However, at this time it is not known whether PBSC transplantation results in a greater response rate compared with bone marrow transplantation, or may just limit the TRM by reducing the neutropenic phase after transplantation. Marrow fibrosis was grade 3 or 4 in 19 of 21 patients, and therefore does not seem to represent a negative prognostic factor. Splenectomy prior to transplantation was not necessary to insure hematologic recovery. At present, we do not recommend splenectomy since the surgery is likely to delay the transplantation, and the surgical procedure itself is associated with significant morbidity and mortality.
The results of this study strongly suggest that myelofibrosis patients with a Dupriez score of 1 or 2, and older than 45 years, have a better probability of long-term survival following an allotransplantation conditioned with reduced-intensity, rather than a fully myeloablative, regimen. Moreover, this retrospective analysis has prompted a new prospective study, within the MPD-Research Consortium, that will evaluate one single conditioning regimen, with fludarabine and melphalan, in allogeneic transplant from HLA-matched donors. In conclusion, since allogeneic stem cell transplantation is highly effective in MMM, various fully myeloablative and RIC regimens represent multiple steps toward developing the optimum treatment for this disease.
Appendix: members of the MPD-Research Consortium
R. Hoffman, D. Rondelli, V. Lindgren, H. Ni, M. Xu, and C. Ferrans, University of Illinois at Chicago; T. Barbui, A. Falanga, G. Finazzi, M. Introna, and A. Rambaldi, Ospedali Riuniti di Bergano, Italy; G. Barosi, M. Marchetti, and E. P. Alessandrino, IRCCS Policlinico S. Matteo, Pavia, Italy; S. Fruchtman, H. Gilbert, R. Weinberg, and J. Strauchen, Mt Sinai Hospital, New York; C. Kessler, Georgetown University Medicine Center; A. R. Migliaccio, M. Migliaccio, and R. Lorenzini, Instituto Superiore Sanita', Rome, Italy; H. Pahl, University Hospital, Freiburg, Germany; J. T. Prchal, U. Popat, K. Baker, M. P. Mimms, and D. Stockton, Baylor College of Medicine; J. Prchal, McGill University, Montreal, QC; R. Silver, New York Weill Medical College of Cornell University; J. Spivak, B. D. Smith, and D. R.Williams, Johns Hopkins University School of Medicine; R. Marchioli and G. Tognoni, Ist di Ricerche Farmacologiche Mario Negri, Italy; A. M. Vannucchi, University of Florence, Italy; H. Kantarjian and S. Verstovsek, M. D. Anderson Cancer Center; J. Goldberg, I. Belitskaya-Levy, J. Matthew, and Y. Shao, New York University School of Medicine.
Prepublished online as Blood First Edition Paper, January 25, 2005; DOI 10.1182/blood-2004-11-4299.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
A complete list of the members of the MPD-Research Consortium appears in “Appendix.”