Abstract
The CD28 family of receptors (CD28, cytotoxic T-lymphocyte–associated antigen 4 [CTLA-4], inducible costimulator [ICOS], program death-1 [PD-1], and B- and T-lymphocyte attenuator [BTLA]) plays a critical role in controlling the adaptive arm of the immune response. While considerable information is available regarding CD28 and CTLA-4, the function of the more recently discovered members of the CD28 family is less well understood. This review will highlight recent findings regarding the CD28 family with special emphasis on effects the CD28 family has on immunopathology, the discovery of costimulatory antibodies with superagonist function, and the status of clinical trials using various strategies to augment or block T-cell costimulation.
An introduction to the CD28 family of receptors
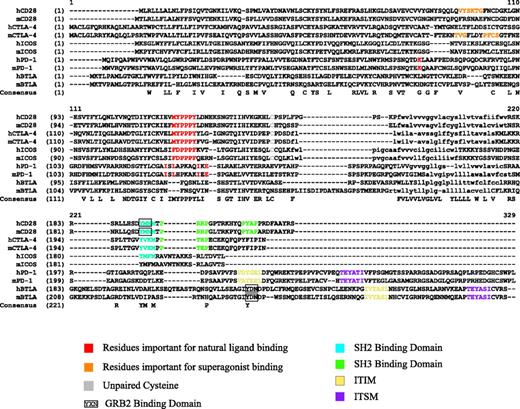
Members of the CD28 family have a single extracellular immunoglobulin variable-like (IgV) domain followed by a short cytoplasmic tail. CD28 and inducible costimulator (ICOS) were discovered by the functional effects their monoclonal antibodies (Abs) had on augmenting T-cell proliferation.1,2 Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4, CD152), program death-1 (PD-1), and B- and T-lymphocyte attenuator (BTLA) were discovered by screening for genes differentially expressed in cytotoxic T lymphocytes (CTLs) or in cells undergoing apoptosis, or overexpressed in T helper 1 (Th1) cells, respectively. In contrast to the CD28 family members that were discovered by their functional effects, most of the B7 family member ligands were discovered by homology searches. Phylogenetic and sequence analysis may provide insight into the evolutionary relationship of the CD28 family. CD28, ICOS, and CTLA-4 are clustered in close proximity on chromosome 2q33 and have an unpaired cysteine that allows them to homodimerize on the T-cell surface (Figure 1). The ligand binding sites on these 3 receptors surround a PPP motif and their cytoplasmic tails contain a central Src homology 2 (SH2) binding motif YXXM. In contrast, PD-1 and BTLA are located in distinct locations in the genome (2q37 and 3q13, respectively) and are more similar to each other than to the other members of the family (Figure 2). Recent studies demonstrate that PD-1 exists as a monomer on the cell surface due in part to the lack of an unpaired cysteine. It is likely that BTLA also exists as a monomer since it also lacks this unpaired cysteine residue.4 PD-1 and BTLA have much longer cytoplasmic tails than the other members of the CD28 family and are similarly structured with a membrane-proximal immunoreceptor tyrosine inhibitory motif (ITIM) followed by a membrane-distal tyrosine-based switch motif (ITSM). Interestingly, the human and murine homolog pairs of CD28, CTLA-4, and ICOS have diverged less from their predicted common ancestors than the corresponding homolog pairs of PD-1 and BTLA; the genetic distance calculated between the human and murine homologs of CD28, ICOS, and CTLA-4 ranges between 0.131 and 0.167, whereas the corresponding distance between PD-1 and BTLA homolog pairs ranges from 0.190 and 0.232 (Figure 2). This may indicate that CD28, CTLA-4, and ICOS are under stronger selective pressure than PD-1 and BTLA.
Alignment of CD28 family. The alignment is created using the Clustal W algorithm3 via Vector NTI (Invitrogen, Carlsbad, CA). Functional domains illustrated by indicated color; lowercase indicates transmembrane domain.
Alignment of CD28 family. The alignment is created using the Clustal W algorithm3 via Vector NTI (Invitrogen, Carlsbad, CA). Functional domains illustrated by indicated color; lowercase indicates transmembrane domain.
Guide tree analysis of CD28 family. Protein sequences of both the murine (m) and human (h) members of the CD28 family were analyzed via Vector NTI using Clustal W program.3
Guide tree analysis of CD28 family. Protein sequences of both the murine (m) and human (h) members of the CD28 family were analyzed via Vector NTI using Clustal W program.3
CD28 and CTLA-4: the original yin and yang of T-cell costimulation
CD28 is expressed constitutively on almost all human CD4 T cells and approximately 50% of CD8 T cells. Individuals with chronic infections or advanced age have significantly more CD28– T cells, suggesting that loss of CD28 correlates with immune senescence. Reintroduction of CD28 expression by either interleukin-12 (IL-12) signaling5 or retroviral-mediated gene transfer restores effector functions to these CD28– T cells,6 suggesting that restoration of CD28 expression may be an attractive strategy to rejuvenate T-cell function in these individuals. CD28 is not entirely lineage specific in expression, as it is also found on mouse stromal cells that support B-cell lymphopoiesis.7 Additionally, 90% of human extramedullary myelomas express CD28,8 suggesting that CD28 signaling plays a role in B-cell survival. Earlier studies concluded that CD28 was not expressed on normal human plasmablasts,9 whereas more recent studies indicate that CD28 is expressed on at least a subset of plasmablasts and plasma cells.10,11 Expression of CD28 on myeloma cells may correlate with disease progression.12,13 In contrast to the subset-specific expression of CD28 on human T cells, murine CD28 is expressed on all T cells and CD28 is not down-regulated with age or chronic disease.14 This difference in CD28 regulation may explain some differences observed between human and murine immune systems.15
CD28 is the best understood of the costimulatory molecules, and delineating the pathways by which it enhances T-cell activation will be central for the design of T-cell costimulatory therapeutics. The most discernable effects of CD28 ligation are observed when this signal is given in concert with T-cell receptor (TCR) stimulation. Ligation of CD3 and CD28 (CD3/28 costimulation) promotes increases in glucose metabolism, high levels of cytokine/chemokine expression including a unique ability to produce very high levels of IL-2, resistance to apoptosis, and long-term expansion of T cells (reviewed in June14 ; Frauwirth and Thompson16 ; Sharpe and Freeman17 ; and Frauwirth and Thompson18 ). Mutational analysis of the CD28 cytoplasmic tail indicates that induction of cytokines and control of cell survival (Bcl-xL induction) are regulated by distinct domains within the CD28 cytoplasmic tail. This indicates that multiple independent signals are initiated upon CD28 ligation.19-21 How these multiple CD28-generated signals interface with TCR-generated signals has not been clearly defined.22,23 Gene expression profiling revealed that CD28 costimulation modifies the gene regulation induced by TCR stimulation rather than turning on the expression of a separate subset of genes,24,25 suggesting that TCR- and CD28-generated signals merge. An often overlooked aspect of this expression profiling data is that CD28 costimulation also enhances the down-regulation of a large number of transcripts including the tumor suppressor genes BIN1, TOB1,26 TSC22, and KLF2, reinforcing the view that commitment to T-cell activation requires a highly choreographed up-regulation of factors that are crucial for T-cell activation and down-regulation of factors whose role is to keep T cells in G0.24,25,27 Given the modular nature of the CD28 cytoplasmic domain and the fact that multiple independent signals are generated from CD28 ligation, therapeutics may be devised to target one set of CD28-mediated events (eg, cytokine production) without interfering with others (eg, cell survival).
CTLA-4 expression is difficult to detect on resting T cells, yet ligation of CTLA-4 is able to block the earliest events in T-cell activation.28,29 Unlike other members of the CD28 family, CTLA-4 undergoes complex intracellular trafficking mediated by its binding to the clathrin-adaptor molecules AP-1 and AP-2.30,31 Upon T-cell activation, CTLA-4 traffics to the site of antigen-presenting cell (APC) and T-cell interaction.32,33 This observation, along with data showing that CTLA-4 has a higher affinity for CD80 and CD86 than CD2834 and elegant structural studies demonstrating that CTLA-4 engagement with CD80 induces a lattice structure that results in an alternating network of CTLA-4 and CD80 homodimers35 (which may limit the ability of CD80 to interact and cluster CD28), offers some explanation as to how limiting levels of CTLA-4 expression can be so effective in preventing immune responses. While there is general agreement that CTLA-4 is a potent inhibitor of T-cell activation, the mechanism by which this inhibition is achieved remains unclear. CTLA-4's higher affinity for CD80 and CD86 allows it to compete effectively with CD28 for these shared ligands. Mice expressing only the extracellular domain of CTLA-4 exhibited lymphadenopathy but were spared the massive lymphoproliferation that killed their CTLA-4–deficient littermates, indicating an inhibitory role for both ligand competition and intracellular signaling mechanisms in CTLA-4 function.36 There are 2 phosphatases, SH2 domain–containing protein tyrosine phosphatase (SHP-2)37 and protein phosphatase 2A (PP2A),38 that have been described to bind the CTLA-4 cytoplasmic tail. Establishment of a clear cause-and-effect relationship between phosphatase binding the CTLA-4 cytoplasmic tail and inhibition of T-cell activation is complicated by many factors: (1) CD28 also binds SHP-2 and PP2A; (2) the structural requirements within the CTLA-4 tail that enable binding of these phosphatases have not been clearly established; and (3) inactivation of these molecules through gene deletion or pharmacologic inhibitors is toxic to the T cells.22 CTLA-4 ligation can also disrupt lipid raft formation39,40 and induce the production of transforming growth factor β (TGF-β)41,42 (although others have found no role for TGF-β in CTLA-4–mediated T-cell suppression43 ).
The relationship between CTLA-4 genetic polymorphisms and susceptibility to autoimmune disease has been vigorously debated in the literature.44 A recent study examining cohorts of patients with Graves disease, autoimmune hypothyroidism, and type 1 diabetes found that a polymorphism leading to decreased levels of a secreted form of CTLA-4 was strongly linked with susceptibility to these autoimmune diseases,45 adding yet another mechanism by which CTLA-4 may control immune responses. The existence of alternatively spliced forms of the CD28 family and their ligands has been known for a long time,14,46,47 but only recently has functional importance been described to these alternative forms of the CD28 family.45,48 Along the same lines, levels of a soluble form of CD8649 in the serum of leukemia patients correlates negatively with survival,50 suggesting that soluble B7 family ligands can have immunomodulatory function. These studies suggest that alternatively spliced forms of both the CD28 and B7 family members do have functional significance, a finding that greatly increases the complexity of an already complicated system.
PD-1 and receptor X: another yin and yang relationship with shared ligands?
Program death-1 (PD-1) is an inhibitory member of the CD28 family that is expressed on T and B cells. The role of PD-1 in the immune system may be to maintain peripheral tolerance, and the evidence leading to this conclusion has been reviewed.17,51,52 There are 2 ligands for PD-1 that have been identified,53-56 a situation similar to CD28 family members CD28 and CTLA-4. PD-L1 (B7-H1) and PD-L2 (B7-DC) are B7 homologs and bind to PD-1 but not to other known members of the CD28 family. PD-1 contains 2 tyrosine molecules within its cytoplasmic tail. The most membrane-proximal tyrosine is located in the ITIM and the distal tyrosine is located in an immunoreceptor tyrosine switch motif (ITSM),57 a stretch of amino acids recently identified by virtue of its ability to bind to the small adaptor Src homology 2 domain containing molecule 1A (SH2D1A).58 The ITSM, originally defined in CD150, is known to recruit SHP-2 to the CD150 cytoplasmic tail. However, in the presence of the small adapter SH2D1A, the ITSM recruits SHIP to CD150, and hence, it is able to “switch” which molecules it recruits based on the presence or absence of SH2D1A. In contrast to the CD150 ITSM, the PD-1 ITSM appears to be unable to bind SH2D1A, suggesting that there is heterogeneity in the mechanism by which ITSMs signal.59 However, several lines of evidence point to the importance of the ITSM in mediating inhibitory PD-1 signals.60 Recently, we observed that PD-1 also recruits SHP-1 to the PD-1 cytoplasmic domain in primary human T cells,59 whereas others did not detect this association in leukemic cell lines.55 The functional significance of this finding is currently not known, but it remains a promising area to explore since SHP-1 is thought to play a more important role in inhibiting cell activation than SHP-2.61
It is likely that PD-1 regulates organ-specific T-cell homeostasis in the periphery, as PD-L1 (B7-H1)–deficient mice have a specific intrahepatic accumulation of T cells.62 However, controversy remains regarding the function of PD-1.51 Studies using antibodies specific for PD-1 and some studies that used the natural ligands PD-L1 and PD-L2 to engage PD-1 show that these ligands inhibit T-cell proliferation under suboptimal CD3 stimulation.54,55 In contrast, other studies have found that PD-L1 and PD-L2 can costimulate T cells.53,56 An interesting explanation for these discrepancies is the possibility that an additional receptor is able to interact with PD-L1 or PD-L2 ligands. Thus, there may be a receptor whose binding to PD-L1 and PD-L2 promotes T-cell activation and another that blocks T-cell activation (PD-1), a situation reminiscent of the CD80 and CD86 to CD28 and CTLA-4 relationship. Site-directed mutagenesis studies of PD-L1 demonstrate that there are mutations that enhance the ability of this ligand to repress or activate T-cell responses.63 Other studies show that B7-DC–mediated costimulation of PD-1–deficient T cells promotes antitumor responses.64 Taken together, these studies provide strong evidence for the existence of an additional receptor for PD-L1 and/or PD-L2 whose role appears to activate the immune response. Understanding the circumstances by which lymphocytes are influenced by these distinct PD-L1 and PD-L2 receptors will aid in the discovery of therapeutic agents to alter T-cell activation and provide the explanation for contradictory data.
ICOS costimulation: effector functions without expansion?
Inducible costimulator (ICOS) was the third member of the CD28 family to be discovered.1,65 ICOS expression, although readily detectable on resting T cells, develops comparable expression to CD28 only upon T-cell activation.66,67 In contrast to some other members of the CD28 family, ICOS and ICOS-L appear to be a monogamous pair, as mice who are deficient in ICOS-L expression have the same phenotype as ICOS-deficient mice.68 ICOS costimulation results in high levels of cytokine secretion (except IL-2 and IL-9),1,24 but ICOS-costimulated T cells are unable to expand long term and die of apoptosis after 3 to 5 cell divisions.66 Selective use of ICOS costimulation rather than CD28 costimulation in the periphery would confer effector functions to T cells without endowing them with the ability to further expand (lack of IL-2 production greatly diminishes the ability of ICOS-stimulated T cells to expand long term).66 This hypothesis is supported by the findings that ICOS expression is enhanced on effector T cells69 and ICOS costimulation enhanced the effector functions of T cells.70 Thus, ICOS-based costimulation may represent a mechanism to rapidly activate memory effector responses without resulting in further T-cell clonal expansion that may increase the risks of autoimmune disease.
Both ICOS- and CD28-deficient mice display similar defects in T-cell–dependent B-cell responses,71,72 suggesting that CD28 and ICOS play nonredundant roles in generating B-cell responses. There are several possibilities that explain the nonredundant roles ICOS and CD28 play in the immune system. One possibility is that there is a threshold of costimulation that is required to get an optimal T-cell response and that both ICOS and CD28 costimulation are required to provide this level of costimulation in vivo, and thus, loss of either costimulatory molecule leads to a defect in T-cell help. Another possibility is that CD28 and ICOS costimulation are essential at 2 distinct time points in the immune response. Based on the temporal expression patterns of CD28 and ICOS and their ligands, it would appear that CD28 costimulation is crucial for priming the immune response, while ICOS is more important for maintaining a T-cell response.73,74 For example, evidence suggests that ICOS is essential for Th2 and regulatory T-cell (Treg) function.68,75,76 Thus, whereas CD28 is more important in initiating the immune response, ICOS costimulation may be required to further stimulate the effector functions of T cells (cytokine production and CD40L expression) that are crucial to initiate and maintain the B-cell response.
BTLA: how many ways does the immune system need to suppress T-cell activation?
B- and T-lymphocyte attenuator (BTLA), the most recently recognized member of the CD28 family is expressed in activated T and B cells. T cells isolated from BTLA-deficient mice show enhanced proliferation upon TCR engagement.77 Interestingly, BTLA is preferentially expressed on CD4 Th1 cells,77 perhaps providing a means for specific control for this subset, while ICOS may be more dedicated for Th2 and Treg cells (Figure 3). Based on a comparison with PD-1– and CTLA-4–deficient animals, BTLA appears to be dispensable for keeping T cells in check, as no autoimmune disease or lymphadenopathy was reported in these animals. BTLA-deficient animals do have a higher susceptibility to myelin oligodendrocyte glycoprotein–induced experimental autoimmune encephalomyelitis (EAE),77 suggesting that BTLA does have nonredundant functions, perhaps limited to Th1 cells. BTLA also inhibits CTL maturation and proliferation.78
Increased CTLA-4 and ICOS expression in tumor-infiltrating CD4+CD25+ cells. Quantitative reverse-transcription–polymerase chain reaction for the indicated transcripts was done on CD4+CD25– and CD4+CD25+ cells isolated from the ascites of patients with ovarian cancer.
Increased CTLA-4 and ICOS expression in tumor-infiltrating CD4+CD25+ cells. Quantitative reverse-transcription–polymerase chain reaction for the indicated transcripts was done on CD4+CD25– and CD4+CD25+ cells isolated from the ascites of patients with ovarian cancer.
One possibility for the lack of an obvious phenotype in BTLA-null mice analogous to that seen in CTLA-4–deficient mice, is that BTLA and PD-1 (and perhaps other yet-to-be-defined CD28 family members) have overlapping functions. If PD-1 can substitute for BTLA and, to a slightly lesser extent vice versa, no dramatic phenotype would be observed in either deficient animal. If true, one would expect the double-deficient BTLA and PD-1 mouse to have a more severe phenotype in terms of either autoimmune disease or lymphocyte homeostasis. Whether PD-1 and BTLA use similar or distinct mechanisms to block T-cell activation is less clear. Both PD-1 and BTLA have related and relatively long cytoplasmic tails that consist of an ITIM followed by a distal ITSM. BTLA also has a membrane-proximal growth factor receptor-bound protein 2 (GRB2) binding site, but the functional importance of this motif is currently unknown as only mutations to the ITIM or ITSM blocked the association of SHP-1 and SHP-2 phosphatases to the BTLA cytoplasmic tail.79
Regulatory T cells and the CD28 family
Any therapeutic approach that attempts to exploit the ability of the CD28 family to regulate conventional T-cell responses must also consider the effects this therapy has on regulatory T-cell (Treg) function. Tregs have garnered a great deal of attention over the past few years due to their ability to potently suppress CD4 and CD8 T-cell effector responses.80 Like conventional T cells, Tregs need antigen stimulation to become activated and suppress an immune response, and these TCR-induced signals are also modified by the CD28 family. The first clue to the critical role the CD28 family plays in Treg function was the observation that prevention of CD28 ligation with CTLA-4–Ig exacerbated autoimmune disease in the nonobese diabetic (NOD) mouse.81 However, in this study it was not clear whether CD28 costimulation was directly required for Treg development; whether IL-2 produced by CD28 costimulation of conventional T cells is required to expand the Tregs, which are unable to produce IL-2; or whether CD28 costimulation is required for negative selection of autoreactive T cells.82,83 Recently, it was proposed that CD28 costimulation directly augmented Treg activation (and suppressive activities) by enhancing TCR-generated signals and indirectly enhancing Treg survival by inducing IL-2 production by conventional T cells.84 However, others have questioned the direct involvement of CD28 in Treg function and have noted that TCR and IL-2 signals are sufficient for Treg activation and survival.85 Either way, these observations offer new insight into the phenotype of the CD28-deficient mouse. While significant immune deficiencies were noted in these mice, they were able to mount effective immune response to most pathogens. These data must now be reinterpreted with the knowledge that CD28-deficient mice also have offsetting defects in Treg function. Thus, while these mice are lacking a potent costimulatory factor to stimulate effector T cells (CD28), they are also lacking one of the most effective mediators of self tolerance, Tregs, yielding a balanced deficit that results in the surprising preservation of antigen-mediated activation of T cells.
Emerging data indicate that Tregs have a distinct pattern of CD28 family expression when compared with conventional T cells. As opposed to conventional T cells, CTLA-4 expression is constitutively expressed on Tregs, however Tregs in a resting state have expression of CTLA-4 that is confined to intracellular location. In contrast, activated Tregs such as those isolated from human tumor specimens show a striking up-regulation of surface CTLA-4 expression and CTLA-4 and ICOS mRNA transcripts,86 while PD-L1 (B7-H1) and several cytokine transcripts are not overexpressed when compared with conventional CD4+CD25– cells (Figure 3). While there is a characteristic pattern of costimulatory receptor expression in Tregs, the functional significance of CD28 family expression on Tregs unclear. For example, while CD28 is expressed on Tregs at levels similar to conventional T cells, CD28 ligation fails to induce IL-2 secretion from Tregs, in striking contrast to conventional T cells. Further, whether CTLA-4 has distinct functions in Tregs remains unclear. The addition of CTLA-4 Ab disrupted the ability of Tregs to prevent intestinal inflammation,87 but it is not clear from these studies whether the Ab was preventing CTLA-4 signaling or was removing the regulatory T cells.
Evidence indicates that CTLA-4 maintains tolerance by several mechanisms, only some of which are dependent on Tregs.88,89 CTLA-4 engagement has been shown to induce TGF-β expression,41 which in turn induces forkhead box protein 3 expression,90 a master regulatory switch required for the development of Tregs. Thus, CTLA-4 may play a role in the development/differentiation of conventional T cells to Tregs. Another proposed role for CTLA-4 expression on Tregs is tryptophan catabolism. Treatment of mice with CTLA-4–Ig in an allograft islet transplant model induced tolerance to the graft by binding B7 on the surface of DCs, which in turn induced interferon-γ (IFNγ) production.91 IFNγ activates indoleamine 2,3-dioxygenase (IDO), which breaks down tryptophan, and the local deficiency of this essential amino acid suppresses T-cell activation. Tregs, by virtue of their constitutive high-level CTLA-4 expression, act in a similar manner as CTLA-4–Ig,92 suggesting that one mechanism by which Tregs suppress T-cell activation is inducing DCs to be tolergenic by limiting the availability of tryptophan that T cells need to expand to become effectors.
Much less is known about how ICOS, PD-1, and BTLA influence Treg cell development and effector functions. Agents that interfere with the ICOS/ICOS-L signaling pathway not only block the ability of Tregs to abrogate pulmonary inflammation and asthma, but also limit IL-10 production and the induction of T-cell tolerance.76 Recently, the blocking of ICOS/ICOS-L interactions was shown to prevent Tregs from producing IL-10 and suppressing T-cell responses.93,94 However, other studies using a murine colitis model demonstrated that lack of CD28 greatly impaired the function of Tregs despite the fact that ICOS blocking reagents had minimal effect, suggesting a limited role of ICOS costimulation in Treg function in this experimental model.95 The ability of ICOS costimulation to activate or repress T-cell responses correlates with the level of ICOS expressed on the T cell. T cells expressing low to moderate levels of ICOS promote the T-cell response by secreting cytokines such as IFN-γ, IL-4, and IL-6, whereas T cells that express high levels of ICOS secrete IL-10.69 Currently, it is unclear whether varied levels of ICOS costimulation produce unique signals that result in distinct cytokine profiles or whether high ICOS expression is a marker of Tregs (Figure 3). Treg inhibitory function is partially overcome when the PD-1 inhibitory pathway is blocked.96 It is likely that the various subsets of Tregs80 use distinct sets of CD28 family members and TNF family members, such as OX40 and GITR, as costimulatory signals.
Costimulatory superagonists
Much experimental evidence has indicated that CD28 signaling is conditional on TCR signals. However, a few studies show that multivalent cross-linking of CD28 can induce T-cell activation and proliferation.97-99 More recently, using microarray analysis, we and others found that conventional anti-CD28 antibody and CD86-Ig stimulation of resting T cells was sufficient to cause the up-regulation of a set of genes in the absence of TCR stimulation.24,25 The induction of “CD28-only” genes appears to occur through VAV/SH2-containing leukocyte protein 76 (SLP-76) signalling.100 These CD28-only genes include CD69, TNFα, and a number of transcription factors.24 In addition to direct activation following multivalent cross-linking of CD28, a subset of “superagonistic” CD28 antibodies binding distinct epitopes on CD28 directly stimulate T cells.101 Together, these data provide an interesting challenge to the 2-signal model of T-cell activation.
Recently, some understanding between the properties of these superagonist anti-CD28 Abs and conventional CD28 Abs has come to light.102 Conventional agonistic anti-CD28 monoclonal antibodies (mAbs) bind near the B7 binding site MYPPPY in the most membrane-distal region of the CD28 extracellular domain, whereas superagonistic CD28 Abs bind near the membrane-proximal C”D loop (Figure 1). In general, binding of the conventional Abs mimics the effects of B7 binding,103 and T-cell expansion and IL-2 production require TCR engagement and zeta-associated protein 70 (ZAP-70) activation in addition to B7 binding to CD28. In contrast, activation of primary T cells with superagonist Abs induced high levels of IL-2 production and T-cell proliferation in the absence of TCR engagement or phosphorylation of TCRζ, linker of activated T cells (LAT), or ZAP-70.104,105 Moreover, superagonist anti-CD28 Abs induced nuclear factor κB (NFκB) activity to a greater degree than conventional CD3/28 costimulation, whereas the opposite was true for nuclear factor of activated T cell (NFAT) binding activity, suggesting that distinct signaling pathways are activated by superagonist CD28 Abs and conventional CD28 Abs in combination with TCR engagement. Interestingly, upon TCR engagement the epitope recognized by the superagonist anti-CD28 Ab becomes more exposed and accessible.106 While the in vivo relevance to this finding remains questionable, the observation that superagonist CD28 mAbs have now been identified in rats107 and humans105 seems to indicate that this may be a conserved property of CD28 signaling.
Regardless of the physiologic significance of superagonist CD28 mAbs, they do have therapeutic potential. Administration of superagonist anti-CD28 mAb in rats with experimental autoimmune neuritis was able to prevent or reverse the induction of this T-cell–mediated autoimmune disease.108 The precise mechanism on how a polyclonal activator of T cells suppressed a T-cell–mediated autoimmune disease has not been clearly defined, but 2 models are likely. CD28 costimulation is known to alter the chemokine receptor expression of T cells and in particular down-regulate CC chemokine receptor 5 expression.109 This change in chemokine receptor expression patterns may direct the autoreactive T cells away from the target tissue in the periphery. A second and mutually nonexclusive possibility is that superagonist stimulation of T cells preferentially expands Tregs, and these cells may down-regulate autoreactive T-cell functions.
This recent information on CD28 conformational changes and the identification of partially agonistic and fully agonistic CD28 mAbs is reminiscent of CD2 biology. It is perhaps relevant that most Abs to CD2 are not directly mitogenic, however some CD2 Abs bind to a normally cryptic epitope of CD2 termed CD2R, and Abs to this epitope are fully mitogenic.110 Given the overall structural homology that exists between members of the CD28 family, it would not be surprising that Abs to the other CD28 family members could be isolated that function as superagonist reagents. In fact, a bispecific tandem single-chain variable region fragment ligand of CTLA-4 has recently been described that appears to have superagonist activity. Interestingly, this reagent activates rather than suppresses T-cell activation.111
Roles of costimulatory receptor dysfunction in immunopathology
Given the critical roles of costimulation in the regulation of T-cell tolerance and proliferation, it would be surprising if aberrant regulation of B7 and CD28 family members failed to elicit immunopathology. Indeed, increasing information suggests that costimulatory dysfunction can play a role in the initiation, progression, and pathogenesis of autoimmune diseases.112 In humans, homozygous ICOS deficiency leads to the common variable immunodeficiency syndrome, manifest by defects in B-cell differentiation, B-cell lymphopenia, and B-cell class switching.113 ICOS function in mice differs because ICOS-deficient mice have normal B-cell numbers and preserved secretion of IgM.72,114 In contrast to mice, it is interesting to note that immunodeficiencies consequent to CD28 deficiency have not been observed in humans, suggesting that clinical CD28 deficiency is lethal.
Multiple studies suggest that dysregulated expression of CTLA-4 leads to autoimmunity. Studies using positional genetics indicate that a soluble CTLA-4 isoform generated by alternative splicing115,116 is underexpressed in patients with Graves disease, autoimmune hypothyroidism, and type 1 diabetes.45 In the NOD mouse that is a model of type 1 diabetes, disease susceptibility is associated with a variation in CTLA-4 gene splicing leading to reduced production of a splice form encoding a molecule lacking the ligand-binding domain.45 Infusion of antagonistic CTLA-4 antibodies leads to autoimmunelike syndromes in cancer patients,117 suggesting that both congenital and acquired abnormalities of CTLA-4 function can lead to autoimmunity.
Loss of PD-1 expression in mice also leads to autoimmunity. The phenotype of PD-1–null mice is dependent on genetic background, as BALB/c mice develop a fatal dilated cardiomyopathy,118 while C57BL/6 mice develop arthritis and glomerulonephritis.119 Surprisingly, ectopic expression of PD-L1 (B7-H1) on pancreatic islets leads to type 1 diabetes in C57BL/6 mice,120 suggesting that dysregulation of either PD-1 or PD-L1 expression can lead to loss of tolerance. Many primary tumors express PD-L1, perhaps indicating that malignant cells have taken advantage of the ability of PD-1 to mediate tolerance as a means to escape the immune system.121 An intriguing finding is that an IgM monoclonal paraprotein was isolated from a patient with Waldenstrom macroglobulinemia that appears to bind to both human and mouse PD-L2 (B7-DC).122 The antibody is functionally active, and it may have therapeutic use123 and/or offer clues to the function of the PD-1/PD-L1 system in tumorigenesis or immunoevasion. Thus, therapies that disrupt PD-L1 and PD-1 interactions may promote the immune response against tumor antigens and aid in tumor eradication.
It is possible that disorders of costimulation could initiate or sustain malignant transformation of lymphocytes. Primary mediastinal large B-cell lymphoma (MLBCL) is a subtype of diffuse large B-cell lymphoma defined by a combination of clinical and pathologic features, including a preponderance of young women who present with large mediastinal masses that often have gains of the chromosome 9p24 region.124 Intriguing recent studies indicate that the best gene that discriminates MLBCL from other lymphomas is overexpression of PD-L2 (B7-DC), and that overexpression of PD-L2 is due to both genomic amplification as well as chromosomal gains of the 9p region.125 Thus, it will be interesting to learn if altered PD-L2 expression has a role in transformation or escape from immunosurveillance.
Costimulatory therapeutics: manipulation of costimulatory pathways
Although the precise mechanisms of CD28 family receptor function remain to be fully uncovered, attempts to pharmacologically regulate the pathway already appear promising in the clinic (Table 1). Results from early clinical trials show that CD28 blockade by CTLA-4–Ig has little apparent toxicity and exhibits substantial clinical activity in transplantation and autoimmune diseases.127,128 Given that this reagent is already in phase 3 trials for rheumatoid arthritis, it is likely that CTLA-4–Ig will become the first FDA-approved drug that targets costimulatory pathways. The main concern with CTLA-4–Ig is that the growth and maintenance of both conventional T cells and CD4+CD25+ regulatory T cells are dependent on CD28 stimulation,135 so that both immunosuppressive and enhanced autoreactive immune effects might be anticipated following CTLA-4–Ig therapy.
While it is generally recognized that most tumors present antigens that can be recognized by T cells, many tumors are poorly immunogenic because they do not express B7 family molecules. Tumors that lack costimulatory ligands may be ignored by the immune system or may be poor stimulators of immune effector cells. Therefore, the immunogenicity of tumors could be increased by augmentation of costimulatory signals. Several general approaches are being used to increase costimulation: (1) systemic administration of reagents that activate CD28 or limit CTLA-4 signaling; (2) genetic modification of tumor cells and viruses to express B7; and (3) adoptive transfer of CD28-stimulated T cells (Table 1). Initial phase 1 clinical trial results indicate that agonistic CTLA-4 monoclonal antibody therapy has considerable promise as an adjuvant for the immunotherapy of cancer.117,129 Upcoming clinical trials are awaited to determine the potential clinical utility of costimulation augmentation for tumor immunotherapy. The clinical development of costimulatory therapeutics promises to be exciting, however the ultimate clinical utility of these reagents remains dependent on a more complete understanding of the complex cell biology of the CD28 family.
Prepublished online as Blood First Edition Paper, September 7, 2004; DOI 10.1182/blood-2004-04-1596.
Supported in part by National Institutes of Health 5P50CA083638-05 SPORE in Ovarian Cancer (C.H.J.) and R01 AI057838 (J.L.R.).
One of the authors (C.H.J.) has declared a financial interest in a company whose potential product was studied in the present work.
We would like to thank all members of our laboratories for their contributions of experimental work, which made this review possible. We thank Dr Brian Brunk of the Penn Bioinformatics Core for help with the phylogenetic analysis; Dr Heidi Yeh and Dr Richard Carroll for Treg isolation; and Dr Robert Vonderheide and Inbal Braunstein for their constructive comments. We apologize to colleagues for our inability to cite all primary references, due to space constraints.