Abstract
We expressed 2 chimeras between human protein S (PS) and human prothrombin (FII) in which the prothrombin γ-carboxyglutamic acid (Gla) domain replaced the PS Gla domain in native PS (GlaFII-PS) or in PS deleted of the thrombin-sensitive region (TSR) (GlaFII-ΔTSR-PS). Neither PS/FII chimera had activated protein C (APC) cofactor activity in plasma clotting assays or purified systems, but both bound efficiently to phospholipids. This pointed to a direct involvement of the PS Gla domain in APC cofactor activity through molecular interaction with APC. Using computational methods, we identified 2 opposite faces of solvent-exposed residues on the PS Gla domain (designated faces 1 and 2) as potentially involved in this interaction. Their importance was supported by functional characterization of a PS mutant in which the face 1 and face 2 PS residues were reintroduced into GlaFII-PS, leading to significant APC cofactor activity, likely through restored interaction with APC. Furthermore, by characterizing PS mutants in which PS face 1 and PS face 2 were individually replaced by the corresponding prothrombin faces, we found that face 1 was necessary for efficient phospholipid binding but that face 2 residues were not strictly required for phospholipid binding and were involved in the interaction with APC.
Introduction
Protein S (PS) is a vitamin K–dependent protein involved in regulating the anticoagulant activity of activated protein C (APC). In purified systems, PS functions as a nonenzymatic cofactor for APC in proteolytic inactivation of activated factor V (FVa) and activated factor VIII (FVIIIa), 1 reflecting the ability of PS to interact with APC. However, these effects are relatively weak and are inconsistent with the major physiologic role of PS. Indeed, the importance of PS as a natural anticoagulant is clearly demonstrated by the occurrence of severe thrombotic complications in infants with homozygous hereditary PS deficiency and by a mild thrombotic tendency in heterozygous subjects.2 This suggests that additional components may determine the expression of the APC cofactor activity of PS in plasma. Thus, activated factor X (FXa) and activated factor IX (FIXa) have been shown to protect FVa and FVIIIa from inactivation by APC, and, in this system, PS is able to abolish these protective effects.3-5 Another determinant is FV, which acts in synergy with PS in FVIIIa inactivation by APC in purified systems.6 PS also demonstrates APC-independent anticoagulant activity by inhibiting prothrombinase and tenase activities, 7,8 and this may contribute to the overall anticoagulant activity of PS in plasma.
PS is a single-chain, 635–amino acid glycoprotein composed of an N-terminal domain rich in γ-carboxyglutamic acid (Gla domain; amino acids 1-45), a thrombin-sensitive region (TSR; amino acids 46-74), 4 epidermal growth factor (EGF)–like domains (amino acids 75-242), 9 and a large C-terminal sex hormone–binding globulin (SHBG)–like region (amino acids 243-635).10 The SHBG-like domain of PS binds tightly to C4b-binding protein (C4BP), a regulatory component of the complement system.11 Approximately 60% of PS is complexed with C4BP, and only the free 40% is able to function as a cofactor for APC.12
Although the molecular basis of the APC cofactor functions of PS are poorly understood, many efforts have been made to determine the importance of each PS domain in APC cofactor activity. Studies based on epitope mapping with monoclonal antibodies, 13,14 site-directed mutagenesis, 15-17 in vitro expression or chemical synthesis of isolated PS domains, 18-20 and functional characterization of thrombin- or FXa-cleaved PS21 support the involvement of the TSR and EGF1 domains in APC binding. Recently, the second EGF-like (EGF2) domain was also shown to play an important though elusive role in optimal expression of APC cofactor activity.22 The PS Gla domain is highly homologous to the Gla domain of other vitamin K–dependent clotting factors and binds to appropriate phospholipid membranes in the presence of Ca2+ ions.23 PS binding to activated platelets, 24 endothelial cells, 25 and platelet microparticles26 is a prerequisite for APC cofactor activity.27 In addition, naturally occurring mutations in the PS Gla domain are associated with qualitative (functional) defects in APC cofactor activity, probably because of impaired phospholipid binding.28
We previously prepared a recombinant analog of PS lacking the TSR (TSR-less) to investigate the role of the TSR.29 Because TSR-less failed to bind to negatively charged phospholipid vesicles or to activated platelets, 29 we attempted to restore the ability of TSR-less to bind to phospholipids by preparing a chimeric PS molecule in which the TSR was deleted and the Gla domain was replaced by the Gla domain of prothrombin (FII) (GlaFII-ΔTSR-PS). Because the chemically synthesized prothrombin Gla domain binds to phospholipid membranes with an affinity similar to that of wild-type prothrombin, 30 we anticipated that GlaFII-ΔTSR-PS would bind efficiently to phospholipid membranes. Another PS/FII chimera, GlaFII-PS, in which only the PS Gla domain is replaced by the prothrombin Gla domain, was also expressed to investigate the role of the PS Gla domain in APC cofactor activity. We present the first evidence that the PS Gla domain not only mediates PS anchorage to a phospholipid surface but also plays a direct role in APC cofactor activity through molecular interaction with APC. Two clusters of solvent-exposed amino acid residues located on 2 opposite faces of the PS Gla domain were also examined for their respective contributions to APC cofactor activity; the results further support a direct interaction of the PS Gla domain with APC.
Materials and methods
Materials
A polyclonal antibody directed against the C4BP α-chain and a monoclonal antibody (mAb) directed against the PS EGF1 domain (HPS54) were kindly provided by Dr B. Dahlbäck (Lund University, Malmö, Sweden). An antihuman PS mAb recognizing the SHBG-like region (20C11) was kindly supplied by bioMérieux (Marcy l'Etoile, France). Oligonucleotides were from Genset (Paris, France). PS-deficient plasma and factor X activator from Russell viper venom (RVV-X) were from Diagnostica Stago (Asnières, France). EcoRI and AflIII restriction endonucleases were from Gibco BRL (Life Technologies, Cergy-Pontoise, France). Synthetic 1,2-diacyl-sn-glycero-3-phosphocholine OLEOYL 18:1 (phosphatidylcholine), 1,2-diacyl-sn-glycero-3-[phospho-l-Serine] DIOLEOYL 18:1 (phosphatidylserine), and 1,2-diacyl-sn-glycero-3-phosphoethanolamine DIOLEOYL 18:1 (Δ9-cis) (phosphatidylethanolamine) were from Avanti Polar Lipids (Coger, Paris, France). Various pore-size polycarbonate membranes were from Poretics Corporation (Livermore, CA). Bovine FV/FVa, human FIXa, and human FXa were from Kordia (Leiden, The Netherlands). Human FVIII was from Baxter (Maurepas, France). Coatest APC Resistance Kit and the chromogenic substrates S2238 and S2765 were from Chromogenix (Milan, Italy). All other reagents were of the highest available grade.
Site-directed mutagenesis
Human PS cDNA was introduced into a eukaryotic expression vector, as previously described.31 The resultant plasmid, pCI-neo-WT-PS, together with the expression vector containing human prothrombin cDNA (pNUT-hII), 32 were used as templates for site-directed mutagenesis.
Expression plasmids containing cDNAs coding for GlaFII-ΔTSR-PS and GlaFII-PS were prepared by using the same strategy: in a first round of polymerase chain reaction (PCR), prothrombin Gla domain cDNA was amplified with the primer combination EcoFII+GlaΔ1 and EcoFII+Gla1 for GlaFII-ΔTSR-PS and GlaFII-PS, respectively, whereas PS cDNA was amplified with the primer combination GlaΔ2+Afl and Gla2+Afl for GlaFII-ΔTSR-PS and GlaFII-PS, respectively (primer sequences are listed in Table 1). For each PS/FII chimera, the first round of PCR generated 2 fragments that were used as templates for a second round of PCR, together with primers EcoFII and Afl, generating 2 final products designated GlaΔ and Gla for GlaFII-ΔTSR-PS and GlaFII-PS, respectively.
For the PS mutant F1FII-GlaPS-PS, amino acids 33, 35, 36, and 39 were replaced by the corresponding residues of prothrombin by amplification of the PS cDNA with the primer combinations EcoPS+F1FII-1 and F1FII-2+Afl (Table 2). This first round of PCR generated 2 fragments used as templates for a second round of PCR, together with primers EcoPS and Afl, generating a final product designated F1FII. For F2FII-GlaPS-PS, amino acids 34, 41, and 45 were first replaced by the corresponding residues of prothrombin through a strategy identical to that used for F1FII-GlaPS-PS: a first round of PCR amplifying the PS cDNA with the primer combinations EcoPS+F2FII-1 and F2FII-2+Afl (Table 2) generated 2 fragments that were used as templates for a second round of PCR, together with primers EcoPS and Afl. The generated intermediate product served as a template to replace the amino acids at positions 21, 23, 24, and 28 by the corresponding residues of prothrombin, with the primer combinations EcoPS+F2FII-3 and F2FII-4+Afl (Table 2). Thus, 2 fragments were generated that were used as templates in a last round of PCR, together with primers EcoPS and Afl, yielding a final product designated F2FII.
For the PS mutant F12PS-GlaFII-PS, amino acids 33, 34, 35, 36, 39, 41, 42, and 45 were first replaced by the corresponding residues of PS by using a first round of PCR amplifying the GlaFII-PS cDNA with the primer combinations EcoFII+F12PS-1 and F12PS-2+Afl (Table 2). Two fragments were generated and were used as templates for a second round of PCR, together with primers EcoFII and Afl, generating an intermediate product that served as a template with the primer combinations EcoFII+F12PS-3 and F12PS-4+Afl (Table 2) to replace the amino acids at positions 21, 23, 24, and 28 by the corresponding residues of PS. Two fragments were generated that were used as templates in a final round of PCR, together with primers EcoFII and Afl, yielding a final product designated F12PS.
The final products GlaΔ, Gla, F1FII,F2FII, and F12PS were digested with EcoRI and AflIII and were ligated into plasmid pCI-neo-WT-PS, from which the corresponding fragment had been removed. Escherichia coli strain DH5αF' was used for routine transformation and plasmid preparation. Mutations were confirmed by DNA sequence analysis.
Stable expression, purification, and characterization of recombinant PS mutants
Transfection of HEK-293 cells and clone selection were performed as previously described.31 The PS mutants were immunopurified as described, 33 except that the pseudoaffinity chromatography step was replaced by an ion-exchange chromatography step and by elution with an Na+ ion gradient. For each PS mutant, protein concentrations were determined by monitoring absorbance at 280 nm, using an ϵ(1%, 1 cm) value of 9.5 and a molecular mass of 78 000 Da. Wild-type recombinant PS (rPS) and the PS mutants were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in reducing conditions and were stained with Coomassie blue or were transferred for Western blot analysis, as described.29
C4BP-binding assay and conformational mapping in monoclonal antibody–binding assays
Apparent dissociation constants (Kd app) for PS-C4BP and PS-mAb interactions were determined as previously described.33
Preparation of phospholipid vesicles
Extruded phospholipid vesicles containing 60% phosphatidylcholine, 20% phosphatidylserine, and 20% phosphatidylethanolamine (PC/PS/PE vesicles) were prepared as previously described.34 Briefly, phospholipids were combined in the weight proportions indicated and were dissolved in chloroform; the solvent was then removed by evaporation at room temperature. Solvent-free phospholipids were resuspended in Tris-buffered saline (TBS) to a concentration of 1 mg total lipid per milliliter, vortexed for 15 minutes, and submitted to a series of extrusion cycles through polycarbonate membranes, as previously described.35 Two cycles with 400-nm pore size membranes, 2 cycles with 200-nm pore size membranes, 5 cycles with 100-nm pore size membranes, and 6 cycles with 50-nm pore size membranes were required to prepare vesicles with diameters of 100 nm, as determined by light scattering. These vesicles were used immediately or were stored at –20°C.
Sonicated phospholipid vesicles containing 80% phosphatidylcholine and 20% phosphatidylserine (PC/PS vesicles), for use in the FVa and FVIIIa inactivation assays, were prepared as previously described.36
Phospholipid vesicle–binding assay
Phospholipid-binding assays were performed as previously described.33 Briefly, increasing concentrations of wild-type rPS and PS mutants were incubated for 2 hours in plates coated with 80 μM PC/PS/PE vesicles, and bound protein was detected with a polyclonal peroxidase-conjugated anti-PS antibody. All incubation steps were run in the presence of 3 mM CaCl2.
Digestion by FXa
PS was digested by FXa, as previously described, 29 by the incubation of PS (2 μM) and FXa (23 nM) with 800 μM PC/PS/PE vesicles in TBS containing 3 mM CaCl2 and 0.2% polyethylene glycol (PEG) for 3 hours at 37°C. Each digest was tested by Western blot analysis with mAb 20C11 as primary antibody.
Clotting assays
FXa 1-stage clotting assay. Human protein C was purified from plasma and activated as previously described.29 All reagents were diluted in TBS containing 1 mg/mL gelatin and 5 mg/mL bovine serum albumin (BSA). PS-deficient plasma (40 μL) was incubated for 2 minutes at 37°C with 20 μL PC/PS/PE vesicles (final concentration, 10 μM), 20 μL of 0.1 μg/mL RVV-X, 20 μL APC (final concentration, 7.3 nM), and 20 μL PS at increasing concentrations. Clotting was initiated by adding 50 μL of 25 mM CaCl2. For each experimental point, we expressed the result as the following ratio: clotting time with APC + PS/clotting time with APC only.
APTT assays. Increasing concentrations of the PS mutants were added to PS-deficient plasma. Plasma aliquots (50 μL) were incubated for 180 seconds at 37°C with an activated partial thromboplastin time (APTT) reagent (50 μL) from the Coatest APC Resistance Kit (Chromogenix) before clotting was initiated with the addition of the APC/CaCl2 mixture (50 μL) from the APC resistance kit, as described by Giri et al.37
APC cofactor activity assays in purified systems
FVa inactivation assay. Human prothrombin was purified from plasma, as previously described.38 The capacity of PS to act as an APC cofactor in FVa inactivation was quantified by determining the rate of FXa-catalyzed prothrombin activation in reaction mixtures containing 2 nM bovine FV/FVa, 0.1 nM human FXa, 0.5 nM APC, 100 nM PS, 10 μM PC/PS vesicles, and 100 nM human prothrombin. Ninety microliters of prothrombinase mixture was first prepared by adding a mixture of FV/FVa (4 nM) to human FXa (0.2 nM) and 20 μM PC/PS vesicles in TBS containing 5 mM CaCl2 and 2 mg/mL BSA at 37°C. Thrombin generation was triggered by adding an equal volume of a reaction mixture containing 200 nM human prothrombin, 1 nM APC, 200 nM PS in TBS containing 5 mM CaCl2, and 2 mg/mL BSA at 37°C. At timed intervals, an aliquot was withdrawn, and the reaction was quenched by adding 100 mM EDTA (ethylenediaminetetraacetic acid). The amount of thrombin generated during the assay was then determined by adding 200 μM S2238 and measuring the initial rate of chromogenic substrate cleavage at 405 nm.
FVIIIa inactivation assay. A first mixture was prepared by adding 4 nM human factor IXa to 40 nM human FX, 10 μM PC/PS vesicles, 4 nM APC, and 100 nM PS in TBS containing 5 mM CaCl2, 2 mg/mL BSA, and 0.2% PEG at 37°C. Human FVIII (10 U) was activated for 2 minutes at 37°C by 2 nM thrombin in the presence of 20 μM PC/PS vesicles in TBS containing 5 mM CaCl2. The reaction was quenched by adding 10 μM hirudin, and 10 μL of FVIIIa was then added to 90 μL of the first mixture containing FIXa, FX, PS, and APC. At timed intervals, an aliquot was withdrawn, and the reaction was quenched by adding 100 mM EDTA. The amount of FXa generated during the assay was determined by adding 200 μM S2765 and measuring the initial cleavage rate of chromogenic substrate cleavage at 405 nm.
Protein modeling and structural analysis
A structural model of the human prothrombin Gla domain was constructed by using the x-ray structure of bovine prothrombin as template, 39 with Accelrys (San Diego, CA) and ICM (MolSoft, San Diego, CA) software packages running on a Silicon Graphics Fuel (Mountain View, CA) workstation or a Dell (Round Rock, TX) Linux personal computer. The model of the human PS Gla-TSR-EGF1 modules has been published elsewhere.13,40 The structures of the PS and prothrombin Gla domains were compared to identify differences in the solvent-exposed regions.
Results
Involvement of the PS Gla domain in APC cofactor activity
Two PS/FII chimeras in which the prothrombin Gla domain replaced the PS Gla domain in native PS (GlaFII-PS) or in PS deleted of the TSR (GlaFII-ΔTSR-PS) were constructed by site-directed mutagenesis (Figure 1). HEK-293 cells were transfected with expression plasmids containing cDNAs coding for wild-type rPS, GlaFII-ΔTSR-PS, or GlaFII-PS. Similar PS antigen levels were detected in the culture medium of clones transfected with each construct. Wild-type rPS and PS/FII chimeras migrated as a single band by SDS-PAGE in reducing conditions, with apparent molecular masses consistent with those expected (Figure 2). In Western blotting experiments, both PS/FII chimeras were recognized by an mAb directed against the PS SHBG-like domain (20C11) (results not shown). We then compared the affinity of each PS/FII chimera with that of wild-type rPS for immobilized C4BP in a solid-phase assay. Binding curves yielded Kd app values of 4.4 ± 0.7 nM for wild-type rPS, 11.4 ± 1.3 nM for GlaFII-ΔTSR-PS, and 2.6 ± 0.2 nM for GlaFII-PS (Table 3). These values, of the same order of magnitude, supported correct overall folding of the SHBG-like domain in both PS/FII chimeras.
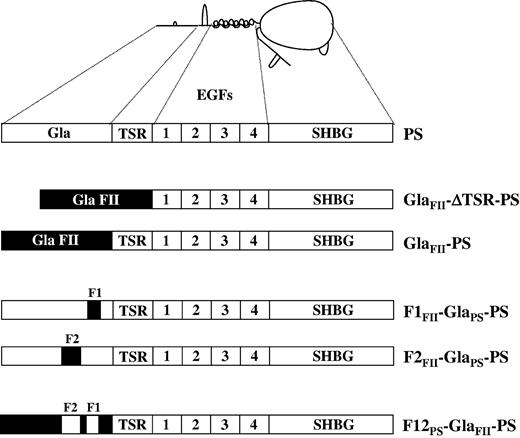
Schematic representation of the PS mutants used in the study. Human PS consists of an N-terminal Gla domain, followed by the TSR, 4 EGFs, and a large SHBG-like region. In each PS mutant, regions in black denote the prothrombin Gla domain. GlaFII-ΔTSR-PS is a PS molecule whose TSR has been deleted (residues 46-74, PS numbering) and whose Gla domain (residues 1-45, PS numbering) has been replaced by the prothrombin Gla domain (residues 1-45, prothrombin numbering). In the GlaFII-ΔTSR-PS chimera, the prothrombin Gla domain ends at Thr45, and the PS EGF1 domain starts at Ala75. GlaFII-PS is a PS molecule in which the only change is the replacement of the PS Gla domain with the prothrombin Gla domain. In GlaFII-PS, the prothrombin Gla domain ends at Thr45, and the PS TSR starts at Val46. In the PS mutant F1FII-GlaPS-PS, the amino acid residues 33, 35, 36, and 39 of PS Gla domain face 1 are replaced with the corresponding residues of prothrombin. In F1FII-GlaPS-PS, the amino acid residues 21, 23, 24, 28, 34, 41, and 45 of PS Gla domain face 2 are replaced with the corresponding residues of prothrombin. In F12PS-GlaFII-PS, the amino acids at position 21, 23, 24, 28, 33, 34, 35, 36, 39, 41, 42, and 45 in GlaFII-PS are replaced with the corresponding residues of PS.
Schematic representation of the PS mutants used in the study. Human PS consists of an N-terminal Gla domain, followed by the TSR, 4 EGFs, and a large SHBG-like region. In each PS mutant, regions in black denote the prothrombin Gla domain. GlaFII-ΔTSR-PS is a PS molecule whose TSR has been deleted (residues 46-74, PS numbering) and whose Gla domain (residues 1-45, PS numbering) has been replaced by the prothrombin Gla domain (residues 1-45, prothrombin numbering). In the GlaFII-ΔTSR-PS chimera, the prothrombin Gla domain ends at Thr45, and the PS EGF1 domain starts at Ala75. GlaFII-PS is a PS molecule in which the only change is the replacement of the PS Gla domain with the prothrombin Gla domain. In GlaFII-PS, the prothrombin Gla domain ends at Thr45, and the PS TSR starts at Val46. In the PS mutant F1FII-GlaPS-PS, the amino acid residues 33, 35, 36, and 39 of PS Gla domain face 1 are replaced with the corresponding residues of prothrombin. In F1FII-GlaPS-PS, the amino acid residues 21, 23, 24, 28, 34, 41, and 45 of PS Gla domain face 2 are replaced with the corresponding residues of prothrombin. In F12PS-GlaFII-PS, the amino acids at position 21, 23, 24, 28, 33, 34, 35, 36, 39, 41, 42, and 45 in GlaFII-PS are replaced with the corresponding residues of PS.
SDS-PAGE analysis of the PS mutants used in the study. Purified wild-type rPS and PS mutants (1 μg) were subjected to 10% SDS-PAGE in reducing conditions (5% β-mercaptoethanol) and were stained with Coomassie blue, as described in “Materials and methods.”
SDS-PAGE analysis of the PS mutants used in the study. Purified wild-type rPS and PS mutants (1 μg) were subjected to 10% SDS-PAGE in reducing conditions (5% β-mercaptoethanol) and were stained with Coomassie blue, as described in “Materials and methods.”
The PS TSR and EGF1 domains have been implicated in APC cofactor activity and interaction with APC.13-21 Given that the deleted or replaced domains (TSR and Gla) were adjacent, it is possible that the EGF1 domain might have been nonfunctional in the PS/FII chimeras, resulting in a loss of APC cofactor activity. However, this possibility was ruled out by the equal recognition of wild-type rPS and the PS/FII chimeras by a Ca2+- and conformation-dependent mAb (HPS54) directed against the PS EGF1 domain, which has been shown to inhibit APC cofactor activity14 (Table 3). Proper folding of the TSR in GlaFII-PS was supported by analyzing FXa cleavage at position 60. Indeed, on the basis of the structural model of the PS Gla-TSR-EGF1 modules and experimental results of PS cleavage by FXa, it has been deduced that Arg60 is solvent-exposed.13,40 In the presence of Ca2+ ions and phospholipids, wild-type rPS and GlaFII-PS were cleaved to the same extent by FXa (results not shown), supporting normal solvent accessibility of the structurally important amino acid residue Arg60 in GlaFII-PS. As expected, no cleavage was observed with the GlaFII-ΔTSR-PS chimera lacking the TSR.
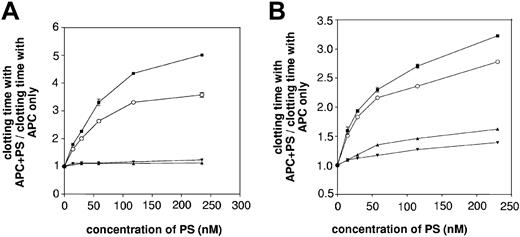
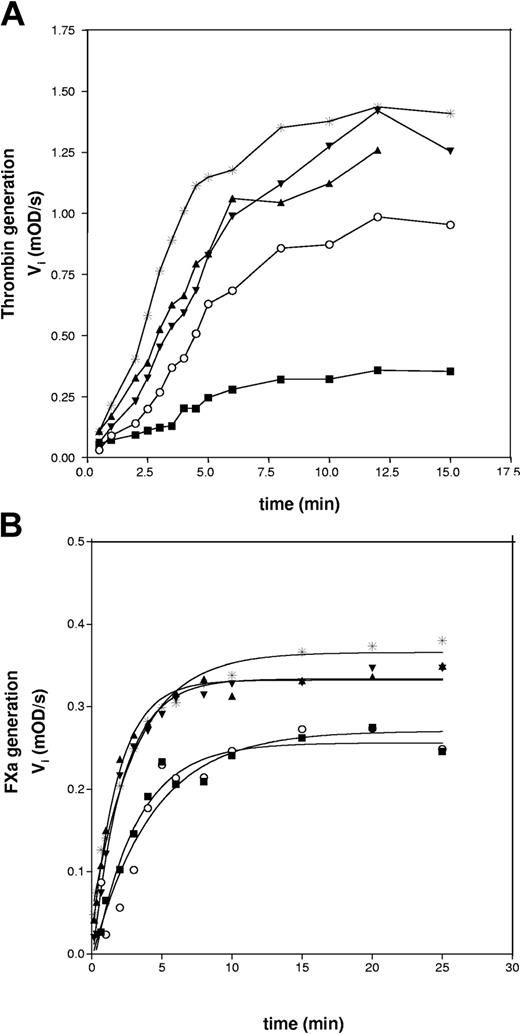
In a solid-phase assay, wild-type rPS, GlaFII-ΔTSR-PS, and GlaFII-PS bound to immobilized PC/PS/PE vesicles with respective Kd app values of 9.0 ± 0.5 nM, 17.0 ± 0.9 nM, and 21.1 ± 1.5 nM (Table 3). Because GlaFII-ΔTSR-PS and GlaFII-PS bound normally to phospholipid vesicles, we investigated the APC cofactor activity of the PS/FII chimeras in the FXa 1-stage plasma clotting assay, described by Smirnov et al.41 As shown in Figure 3A, wild-type rPS prolonged the clotting time in a concentration-dependent manner in the presence of 7.3 nM APC. In contrast, neither PS/FII chimera had APC cofactor activity (Figure 3A). Furthermore, GlaFII-ΔTSR-PS and GlaFII-PS were almost devoid of cofactor activity in an APTT assay (Figure 3B) sensitive to FVa and FVIIIa inactivation, pointing to defective interaction of the PS/FII chimeras with APC. This was further confirmed by assays using purified components in which the ability of PS to function as an APC cofactor in FVa and FVIIIa inactivation was examined (Figure 4). The FVa inactivation assay we used was highly sensitive to PS because APC alone (final concentration, 0.5 nM) had no discernible effect on thrombin generation, whereas the simultaneous addition of 100 nM wild-type rPS to 0.5 nM APC led to a marked decrease in thrombin generation (Figure 4A). In contrast, neither 100 nM GlaFII-ΔTSR-PS nor 100 nM GlaFII-PS affected thrombin generation (Figure 4A). In the FVIIIa inactivation assay, the amount of FXa generated by tenase activity was slightly reduced by the addition of APC alone (final concentration, 4 nM) (Figure 4B) and was further reduced by the simultaneous addition of 100 nM wild-type rPS to 4 nM APC in the reaction mixture (Figure 4B). As depicted in Figure 4B, the effect of 4 nM APC plus 100 nM GlaFII-ΔTSR-PS or 100 nM GlaFII-PS was nearly identical to that of APC alone. Under these conditions, wild-type rPS alone had no effect on thrombin and FXa generation in the FVa and FVIIIa inactivation assays, respectively (results not shown).
Comparison of the APC cofactor activity of wild-type rPS and PS/FII chimeras in plasma clotting assays. (A) The APC cofactor activity of each PS/FII chimera was investigated in an FXa 1-stage clotting assay. Increasing concentrations of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) were incubated with APC (7.3 nM) in the presence of PC/PS/PE phospholipid vesicles, RVV-X, and PS-deficient plasma. Clotting was triggered by the addition of Ca2+ ions. For all the PS mutants tested in the study, mean basal clotting time in the presence of 7.3 nM APC was 112 ± 12 seconds. Data represent mean values (±SD) of 2 separate experiments. (B) APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) was assessed in an APTT assay, as described in “Materials and methods.” For all PS mutants used in the study, mean basal clotting time in the presence of APC alone was 77 ± 2 seconds. In both plasma clotting assays, the APC cofactor activity of the different PS species was evaluated by determining the following ratio: clotting time with APC + PS/clotting time with APC only.
Comparison of the APC cofactor activity of wild-type rPS and PS/FII chimeras in plasma clotting assays. (A) The APC cofactor activity of each PS/FII chimera was investigated in an FXa 1-stage clotting assay. Increasing concentrations of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) were incubated with APC (7.3 nM) in the presence of PC/PS/PE phospholipid vesicles, RVV-X, and PS-deficient plasma. Clotting was triggered by the addition of Ca2+ ions. For all the PS mutants tested in the study, mean basal clotting time in the presence of 7.3 nM APC was 112 ± 12 seconds. Data represent mean values (±SD) of 2 separate experiments. (B) APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) was assessed in an APTT assay, as described in “Materials and methods.” For all PS mutants used in the study, mean basal clotting time in the presence of APC alone was 77 ± 2 seconds. In both plasma clotting assays, the APC cofactor activity of the different PS species was evaluated by determining the following ratio: clotting time with APC + PS/clotting time with APC only.
Comparison of the APC cofactor activity of wild-type rPS and PS/FII chimeras in purified systems. (A) The capacity of each PS species to act as an APC cofactor in FVa inactivation was quantified by determining the rate of FXa-catalyzed prothrombin activation in a reaction mixture containing 2 nM FV/FVa, 0.1 nM FXa, 100 nM prothrombin, and 10 μM PC/PS vesicles, as described in “Materials and methods.” In the absence of wild-type rPS, thrombin generation was measured in the presence (*) or absence (×) of 0.5 nM APC. APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) in the FVa inactivation assay was determined by measuring thrombin generation after the simultaneous addition of 100 nM PS to 0.5 nM APC. Experimental conditions were designed to optimize detection of PS APC cofactor activity rather than prothrombinase activity; visible lag phase presumably originated from FV activation during kinetics. (B) FX activation by tenase complex (FIXa, FVIIIa, and phospholipids) was monitored in the absence of APC and PS (×) or in the presence of APC alone (final concentration, 4 nM) (*), as described in “Materials and methods.” APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) in the FVIIIa inactivation assay was determined by measuring FXa generation after the simultaneous addition of 100 nM PS to 4 nM APC.
Comparison of the APC cofactor activity of wild-type rPS and PS/FII chimeras in purified systems. (A) The capacity of each PS species to act as an APC cofactor in FVa inactivation was quantified by determining the rate of FXa-catalyzed prothrombin activation in a reaction mixture containing 2 nM FV/FVa, 0.1 nM FXa, 100 nM prothrombin, and 10 μM PC/PS vesicles, as described in “Materials and methods.” In the absence of wild-type rPS, thrombin generation was measured in the presence (*) or absence (×) of 0.5 nM APC. APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) in the FVa inactivation assay was determined by measuring thrombin generation after the simultaneous addition of 100 nM PS to 0.5 nM APC. Experimental conditions were designed to optimize detection of PS APC cofactor activity rather than prothrombinase activity; visible lag phase presumably originated from FV activation during kinetics. (B) FX activation by tenase complex (FIXa, FVIIIa, and phospholipids) was monitored in the absence of APC and PS (×) or in the presence of APC alone (final concentration, 4 nM) (*), as described in “Materials and methods.” APC cofactor activity of wild-type rPS (▪), GlaFII-ΔTSR-PS (□), and GlaFII-PS (♦) in the FVIIIa inactivation assay was determined by measuring FXa generation after the simultaneous addition of 100 nM PS to 4 nM APC.
Thus, GlaFII-PS was unable to function as an APC cofactor despite normal phospholipid binding and the presence of TSR and EGF1 domains structurally comparable to the wild-type rPS counterparts. This implied that the Gla domain was directly involved in molecular interactions underlying the APC cofactor activity of PS.
Structural analysis
Superimposition of the PS and prothrombin Gla models revealed several striking features that might account for the APC cofactor activity specific to the PS Gla domain (Figure 5). Assuming that PS and prothrombin insert into the membrane up to the Ca2+ ions (insertion of the N-terminal ω-loop into the membrane), 42 we focused on differences in the solvent-exposed side chains of the portion encompassing residues 10 to 45. The main differences between the PS and prothrombin Gla domains involved 2 distinct clusters of solvent-exposed amino acid residues located on 2 opposite faces. The first cluster comprised residues 10, 33, 34, 35, 36, and 39 and is referred to here as face 1. The second cluster involved residues 21, 23, 24, 28, 33, 34, 35, 41, and 45 and is referred to as face 2. When replaced by the corresponding prothrombin amino acids, the substitutions occurring on face 1 and face 2 of PS would be of sufficient nature to disrupt putative interactions of the PS Gla domain with APC. Thus, these structural findings suggested that, individually or together, these clusters might mediate macromolecular interactions with APC.
Putative APC-binding sites at the surface of the PS Gla domain. The PS Gla domain is shown in red, the TSR in purple, and the EGF1 domain in white. Ca2+ ions are depicted as yellow spheres. The PS Gla domain is thought to insert into the membrane surface through its N-terminal ω-loop (approximately the first 10 residues). Solvent-exposed amino acid residues that differ significantly from the corresponding residues in prothrombin are shown in blue, and the nature of the substitution is indicated at each position (eg, human PS has a Gln at position 10 whereas human prothrombin has a Lys). Face 1 comprises amino acids Gln10, Asn33, Asp34, Pro35, Gla36, and Tyr39. Face 2 comprises amino acids Leu21, Asn23, Lys24, Arg28, Asn33, Asp34, Pro35, Tyr41, and Leu45. Residues at positions 33, 34, and 35 are at the interface and are, therefore, present on both faces. In the present study, Asn33 and Pro35 was considered part of face 1, whereas Asp34 was considered part of face 2.
Putative APC-binding sites at the surface of the PS Gla domain. The PS Gla domain is shown in red, the TSR in purple, and the EGF1 domain in white. Ca2+ ions are depicted as yellow spheres. The PS Gla domain is thought to insert into the membrane surface through its N-terminal ω-loop (approximately the first 10 residues). Solvent-exposed amino acid residues that differ significantly from the corresponding residues in prothrombin are shown in blue, and the nature of the substitution is indicated at each position (eg, human PS has a Gln at position 10 whereas human prothrombin has a Lys). Face 1 comprises amino acids Gln10, Asn33, Asp34, Pro35, Gla36, and Tyr39. Face 2 comprises amino acids Leu21, Asn23, Lys24, Arg28, Asn33, Asp34, Pro35, Tyr41, and Leu45. Residues at positions 33, 34, and 35 are at the interface and are, therefore, present on both faces. In the present study, Asn33 and Pro35 was considered part of face 1, whereas Asp34 was considered part of face 2.
Probing the involvement of faces 1 and 2 of the PS Gla domain in APC cofactor activity
To verify our hypothesis, we constructed 2 additional PS mutants in which faces 1 and 2 of the PS Gla domain were separately replaced by the corresponding faces of the prothrombin Gla domain (Figure 1). In F1FII-GlaPS-PS, Asn33, Pro35, Gla36, and Tyr39 were replaced by Ser33, Thr35, Ala36, and Val39. In F2FII-GlaPS-PS, Leu21, Asn23, Lys24, Arg28, Asp34, Tyr41, and Leu45 were replaced by Thr21, Ser23, Tyr24, Phe28, Ser34, Trp41, and Thr45. Although Gln10 was part of face 1 of the PS Gla domain, it belonged to the PS ω loop, thought to be a strong determinant of phospholipid binding in vitamin K–dependent proteins.42 We did not, therefore, mutate this residue in F1FII-GlaPS-PS because this might have impaired phospholipid binding. PS has a potentially structurally important Pro residue at position 35, and substitution at this position might have a destabilizing effect on backbone structure. However, the Pro35Leu substitution is recognized as a neutral polymorphism, 43 suggesting that the Pro-to-Leu substitution, and thereby the Pro-to-Thr substitution in F1FII-GlaPS-PS, would be tolerated and would not induce any structural changes.
F1FII-GlaPS-PS and F2FII-GlaPS-PS bound to immobilized C4BP with respective Kd app values of 2.4 ± 0.3 nM and 5.9 ± 0.4 nM (Table 3), supporting proper folding of the SHBG-like domain in these PS molecules. The ability of the F1FII-GlaPS-PS and F2FII-GlaPS-PS mutants to bind to phospholipid vesicles was then evaluated in a solid-phase assay. Surprisingly, F1FII-GlaPS-PS bound to PC/PS/PE vesicles with 9- to 10-fold lower affinity than wild-type rPS (ie, 84.8 ± 3.4 nM for F1FII-GlaPS-PS and 9.0 ± 0.5 nM for wild-type rPS) (Table 3), possibly reflecting a strong requirement of PS face 1 amino acid residues for high phospholipid affinity. In contrast, F2FII-GlaPS-PS bound efficiently to PC/PS/PE vesicles with a Kd app value of 9.5 ± 1.1 nM (Table 3), consistent with the hypothesis that the PS Gla domain face 2 residues are not implicated in membrane binding.
F2FII-GlaPS-PS did not express APC cofactor activity in the FXa 1-stage plasma clotting assay (Figure 6A). Again, the loss of APC cofactor activity in an APTT assay (Figure 6B) and in purified systems (Figure 7) pointed to defective interaction of the F2FII-GlaPS-PS mutant with APC. Correct overall folding of the F2FII-GlaPS-PS EGF1 domain and TSR, respectively, was supported by an enzyme-linked immunosorbent assay (ELISA) using mAb HPS54 (Table 3) and by analyzing FXa cleavage at Arg60 (results not shown), as described in “Results.” Taken together, these results suggest that PS loses its ability to function as an APC cofactor when the solvent-exposed face 2 residues of the PS Gla domain are mutated and that amino acid residues within face 2 may provide an interaction surface with APC.
Involvement of face 1 and face 2 PS residues for APC cofactor activity in plasma clotting assays. The ability of wild-type rPS (▪), F1FII-GlaPS-PS (▴), F2FII-GlaPS-PS (▾), and F12PS-GlaFII-PS (○) to function as APC cofactors was investigated in an FXa 1-stage plasma clotting assay (A) and in an APTT assay (B), as described in Figure 3. F1FII-GlaPS-PS and F2FII-GlaPS-PS were essentially devoid of cofactor activity, whereas F12PS-GlaFII-PS recovered significant APC cofactor activity.
Involvement of face 1 and face 2 PS residues for APC cofactor activity in plasma clotting assays. The ability of wild-type rPS (▪), F1FII-GlaPS-PS (▴), F2FII-GlaPS-PS (▾), and F12PS-GlaFII-PS (○) to function as APC cofactors was investigated in an FXa 1-stage plasma clotting assay (A) and in an APTT assay (B), as described in Figure 3. F1FII-GlaPS-PS and F2FII-GlaPS-PS were essentially devoid of cofactor activity, whereas F12PS-GlaFII-PS recovered significant APC cofactor activity.
Involvement of face 1 and face 2 PS residues for APC cofactor activity in purified systems. Wild-type rPS (▪), F1FII-GlaPS-PS (▴), F2FII-GlaPS-PS (▾), and F12PS-GlaFII-PS (○) were assessed in the FVa (A) and FVIIIa (B) inactivation assays, as described in Figure 4. In both inactivation assays, the effect of APC alone (*) on thrombin (A) and FXa (B) generation was also measured. As in the plasma clotting assays, APC cofactor activity was lost in F1FII-GlaPS-PS and F2FII-GlaPS-PS and was restored in the PS mutant F12PS-GlaFII-PS.
Involvement of face 1 and face 2 PS residues for APC cofactor activity in purified systems. Wild-type rPS (▪), F1FII-GlaPS-PS (▴), F2FII-GlaPS-PS (▾), and F12PS-GlaFII-PS (○) were assessed in the FVa (A) and FVIIIa (B) inactivation assays, as described in Figure 4. In both inactivation assays, the effect of APC alone (*) on thrombin (A) and FXa (B) generation was also measured. As in the plasma clotting assays, APC cofactor activity was lost in F1FII-GlaPS-PS and F2FII-GlaPS-PS and was restored in the PS mutant F12PS-GlaFII-PS.
F1FII-GlaPS-PS had virtually no APC cofactor activity (Figures 6, 7), possibly reflecting either the inability of F1FII-GlaPS-PS to bind to phospholipid vesicles with high affinity or the participation of face 1 residues in APC interaction. Contrary to the face 1 residues, whose replacement leads to impaired phospholipid binding, the face 2 residues do not appear to be required for high-affinity interaction with phospholipids.
Recovery of APC cofactor activity of GlaFII-PS by mutation of faces 1 and 2
Assuming that the solvent-exposed amino acid residues of face 2 of the PS Gla domain were involved in the APC cofactor activity of PS, we sought to restore the ability of GlaFII-PS to function as a cofactor for APC by replacing the face 2 residues of GlaFII-PS with those of the PS Gla domain. Although at least face 2 residues were thought to be required to restore the APC cofactor activity of GlaFII-PS, the PS face 1 residues appeared crucial for PS phospholipid binding and could not be specifically investigated for their role in PS APC cofactor activity. Thus, we decided to replace the face 1 and face 2 residues of GlaFII-PS. In this mutant, designated F12PS-GlaFII-PS, residues 21, 23, 24, 28, 33, 34, 35, 36, 39, 41, and 45 were replaced by the corresponding residues of PS (Figure 1). This mutant comprised an Ala residue of prothrombin origin at position 42, disrupting the integrity of the aromatic amino acid stack Pro35-Pro42 loop that is potentially important for PS functions.44 To restore the Pro35-Pro42 loop present in native PS, Ala42 was also replaced by a Pro residue in F12PS-GlaFII-PS.
Functional characterization of the PS mutant F12PS-GlaFII-PS showed that the SHBG-like domain and the EGF1 domain were correctly folded (Table 3). The structural integrity of the TSR was again supported by efficient FXa cleavage at Arg60 (results not shown). Moreover, F12PS-GlaFII-PS bound to PC/PS/PE vesicles with an affinity similar to that of wild-type rPS (8.6 ± 0.8 nM and 9.0 ± 0.5 nM, respectively (Table 3). In an FXa 1-stage plasma clotting assay, F12PS-GlaFII-PS had significant, though partial, APC cofactor activity (Figure 6A). At near-physiologic PS concentrations (230 nM), F12PS-GlaFII-PS expressed approximately 60% of wild-type rPS APC cofactor activity. The gain in APC cofactor activity was also observed in an APTT assay (Figure 6B) and in purified systems (Figure 7), suggesting that the interaction with APC was restored in F12PS-GlaFII-PS.
Overall, these findings showed that GlaFII-PS could gain APC cofactor activity by selective mutation of solvent-exposed amino acids in its Gla domain, probably through recovery of the interaction with APC.
Discussion
GlaFII-ΔTSR-PS and GlaFII-PS were initially constructed to assess the importance of the TSR for the APC cofactor activity of PS, but these chimeras unexpectedly proved helpful for demonstrating the direct involvement of the PS Gla domain in APC cofactor activity. Given that the PS/FII chimera GlaFII-PS was devoid of APC cofactor activity yet bound normally to phospholipids, we deduced that the important role of the Gla domain in the APC cofactor activity of PS was not restricted to mediating the interaction with phospholipid membranes but that the Gla domain was also directly involved in the molecular mechanisms underlying APC cofactor activity. GlaFII-ΔTSR-PS did not gain APC cofactor activity when the TSR was added, given that the 2 PS/FII chimeras exhibited similar loss of activity. Thus, the PS Gla domain appears to play as important a role as the TSR in APC cofactor activity.
The global effect of PS Gla domain replacement, both in plasma clotting assays and purified systems, indicated that the complete loss of APC cofactor activity of both PS/FII chimeras was caused by defective interaction with APC rather than with another component. The PS Gla domain might exert its effects on PS interaction with APC through various molecular mechanisms. In particular, the PS Gla domain might mediate the correct reorientation and positioning of adjacent PS domains involved in contacts with APC (TSR and EGF1 domains) above the membrane surface. Alternatively, the PS Gla domain might be directly involved in molecular interactions with APC.
It has been shown that replacing the protein C Gla domain with that of prothrombin in a protein C/prothrombin chimera45 causes a translocation of the APC active site toward the phospholipid surface from 9.4 to 8.9 nm compared with wild-type APC.46 As demonstrated for this chimera, it is possible that the prothrombin Gla domain of GlaFII-PS may similarly translocate PS toward the phospholipid surface, resulting in impaired alignment of the TSR and EGF1 domains with APC. However, this hypothetical effect did not seem of particular importance in our experiments. Indeed, possible translocation toward the membrane is insufficient to explain the complete loss of cofactor activity of the F2FII-GlaPS-PS mutant, whose Gla domain was of PS origin; translocation toward the membrane would not, therefore, have occurred in the latter mutant. The translocation that would have occurred in the F12PS-GlaFII-PS mutant, whose Gla domain was of prothrombin origin, would not fully have precluded correct positioning of the TSR and EGF1 domains for interaction with APC because the latter mutant expressed significant APC cofactor activity. Consequently, our results indicate that the prime molecular mechanism underlying the specific role of the PS Gla domain in APC cofactor activity is direct interaction with APC rather than simple reorientation of the PS domains involved in contacts with APC (TSR and EGF1 domains).
Our study further demonstrates the importance of solvent-exposed residues within the PS Gla domain. Although GlaFII-PS and F12PS-GlaFII-PS share a high degree of homology (differing in only 12 amino acid positions), the former PS mutant is devoid of APC cofactor activity whereas the latter expresses significant APC cofactor activity. Therefore, the replacement of specific amino acids in GlaFII-PS by their wild-type PS counterparts is sufficient to restore APC cofactor activity, making these residues candidates for mediating the molecular interaction with APC. Interestingly, these solvent-exposed residues are clustered on 2 opposite faces of the PS Gla domain (faces 1 and 2), suggesting that only 1 face is required for APC cofactor activity.
The study of F1FII-GlaPS-PS and F2FII-GlaPS-PS offered insights into the respective involvement of each face of the Gla domain in PS interaction with APC. Because F1FII-GlaPS-PS failed to bind efficiently to phospholipids, we could not specifically investigate the role of face 1 residues in APC cofactor activity. However, any engagement in membrane interaction would make them less available to interact with APC. In contrast, face 2 residues appeared to be crucial for APC cofactor activity but not for phospholipid binding. The participation of face 2 residues in PS interaction with APC is supported by the observation that some face 2 residues are in the vicinity of the solvent-exposed Arg49, a TSR residue previously suggested to participate in direct interaction with APC.16
As demonstrated here for PS, the Gla domains of other vitamin K–dependent proteins have been implicated in protein-protein and protein-membrane interactions.30,47-51 Interestingly, a chimeric protein C species in which the Gla domain was replaced by the prothrombin Gla domain has been found to be insensitive to PS in a plasma clotting assay45 ; in addition, PS dependence is associated with the C-terminal part of the protein C Gla domain.45 Thus, it was suggested that PS functions in plasma are mainly dependent on interaction with APC and that this interaction is disrupted by replacing the Gla domain of protein C with that of prothrombin.45 Our findings suggest that replacing the PS Gla domain with the prothrombin Gla domain results in similar disruption of the PS-APC interaction. Taken together, these results may indicate that the Gla domains of PS and APC interact at the membrane surface.
In conclusion, the present study highlights the importance of solvent-exposed amino acid residues of the PS Gla domain in the expression of APC cofactor activity, likely through direct interaction with APC. For the first time, we here extend the model assuming multiple interaction sites between PS and APC to another functionally important PS region, the Gla domain, in addition to the TSR and the EGF1 domains.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-06-2176.
Supported in part by INSERM, the Groupe d'Etude sur l'Hemostase et la Thrombose (GEHT) (F.S.), and the Fondation de France.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr B. Dahlbäck (Lund University, Malmö, Sweden) for kindly providing the monoclonal antibody HPS54 against human protein S and the polyclonal antibody against C4BP. We also thank bioMérieux (Marcy l'Etoile, France) for providing monoclonal antibody against human protein S 20C11. We thank Samira Chaib (Laboratoire de Biologie de la Nutrition, UFR des Sciences Pharmaceutiques et Biologiques, Université Paris V, France) for her technical assistance in the preparation of extruded phospholipid vesicles.