Abstract
Interleukin-6 (IL-6) triggers multiple myeloma (MM) cell proliferation and protects against apoptosis by up-regulating myeloid cell leukemia 1 (Mcl-1). Vascular endothelial growth factor (VEGF) induces modest proliferation of MM cells and induces IL-6 secretion in a paracrine loop involving MM cells and bone marrow stromal cells. Using murine embryonic fibroblast cell lines as a model (Mcl-1wt/wt and Mcl-1Δ/null MEFs), we here demonstrate that deletion of Mcl-1 reduces fetal bovine serum (FBS)-, VEGF-, and IL-6-induced proliferation. We also show that VEGF up-regulates Mcl-1 expression in a time- and dose-dependent manner in 3 human MM cell lines and MM patient cells. Importantly, we demonstrate that the pan-VEGF inhibitor, GW654652, inhibits VEGF-induced up-regulation of Mcl-1 and, as with Mcl-1 siRNA, is associated with decreased proliferation and induction of apoptosis. Finally, we show that VEGF protects MM patient cells against FBS starvation-induced apoptosis. Our studies therefore demonstrate that VEGF-induced MM cell proliferation and survival are mediated via Mcl-1, providing the preclinical framework for novel therapeutics targeting Mcl-1 and/or VEGF to improve patient outcome in MM.
Introduction
Multiple myeloma (MM) is a clonal B-cell malignancy characterized by the accumulation of malignant plasma cells within the bone marrow (BM). Binding of MM cells to bone marrow stromal cells (BMSCs) promotes tumor cell growth, survival, and drug resistance by both MM cell-BMSC contact and triggering of cytokine secretion.1 Among these cytokines, interleukin-6 (IL-6) produced by BMSCs plays a major role on both proliferation and survival of tumor cells.2-4 In turn, MM cells secrete vascular endothelial growth factor (VEGF), which further promotes production of IL-6 in BMSCs, as well as migration and proliferation of the tumor cells. Thus VEGF is both an autocrine growth factor and trigger of IL-6-mediated paracrine MM cell growth. Recent reports have highlighted the major role of VEGF in MM pathogenesis, demonstrating that VEGF also increases microvessel density in the BM.5-7 Moreover, VEGF increases bone resorbtion by osteoclasts and inhibits maturation of dendritic cells.8,9 Taken together, these reports have promoted preclinical MM studies that confirm the promise of VEGF targeting therapies.10-12 Although the impact of VEGF on MM cell proliferation and migration is well documented, its role in conferring protection against apoptosis remains unknown. As reported in other hematologic malignancies such as leukemia, VEGF up-regulates Bcl-2 and thus protects leukemia cells against chemotherapy.13,14 Furthermore, among the Bcl-2 family members induced by VEGF, Katoh et al have demonstrated that Mcl-1 was critical to protect leukemia cell lines against etoposide-induced apoptosis.15
Myeloid cell leukemia 1 (Mcl-1) is an antiapoptotic member of the Bcl-2 family, which is distinguished from other Bcl-2 family members, such as Bcl-2 or Bcl-xL, by its short half-life and ability to protect cells against a large variety of cytotoxic stimuli. Moreover, Mcl-1 down-regulation is a critical and pivotal check-point controlling mitochondrial apoptotic events, such as cytochrome c release and caspase activation.16,17 Recently, Opferman et al demonstrated that Mcl-1 is also required for development and maintenance of B and T lymphocytes.18 In MM, IL-6 activates the Janus kinase (JAK)/signal transducer and activator of transcription 3 (Stat-3) pathway leading to the up-regulation of Mcl-1 expression.19,20 Using oligonucleotide antisense technique (ASO), Zhang et al21 and Derenne et al22 demonstrated that specific inhibition of Mcl-1, but not of Bcl-2 or Bcl-xL, induces apoptosis of MM cells. Conversely, the antiapoptotic effect of IL-6 is mediated through Mcl-1 up-regulation.23 However, Zhang et al demonstrated that IL-6 failed to up-regulate Mcl-1 expression in almost two thirds of MM cell lines and primary MM cells, despite triggering phosphorylation of Stat-3.24 Thus, the mechanisms whereby cytokines regulate Mcl-1 may involve distinct signaling pathways. Mcl-1, besides triggering antiapoptotic effects, is also involved in cell cycle progression and pivotal in regulating cell homeostasis.25
In the present report, we investigated whether VEGF, as IL-6, can regulate Mcl-1 expression and thereby influence survival and proliferation of MM cells. Using Mcl-1wt/wt and Mcl-1Δ/null murine embryonic fibroblast cells lines (MEFs) as a model to investigate the role of Mcl-1, we first demonstrate that Mcl-1 is involved in both IL-6- and VEGF-induced cell proliferation. Second, we show that VEGF up-regulates Mcl-1 expression in human MM cell lines (HMCLs) and MM patient cells; and conversely, we confirm that specific down-regulation of Mcl-1 expression by siRNA inhibits proliferation and induces apoptosis. Furthermore, we demonstrate that VEGF protects MM patient cells against fetal bovine serum (FBS) starvation-induced apoptosis. Taken together, these data confirm the pivotal role of VEGF in survival of MM cells, thereby providing the preclinical rationale for targeting Mcl-1 and VEGF in novel therapeutics to improve patient outcome in MM.
Materials and methods
Cells and cell culture
The human MM cell lines (HMCLs) MM1s, MM1r, and U266, as well as patient MM cells were maintained in RPMI 1640 medium with 2 mM l-glutamine (Mediatech, Cellgro, AK) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 10 μg streptomycin (Mediatech). Murine embryonic fibroblast cell line Mcl-1wt/wt and Mcl-1Δ/null MEFs cells were kindly provided by J. Opferman (Howard Hughes Institute, Dana-Farber Cancer Institute, Boston, MA).18 MEFs were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 10 μg streptomycin, 2 mM l-glutamine, 2 mercapto-ethanol (Sigma, St Louis, MO) and modified essential medium nonessential amino acid (Gibco, Grand Island, NY). Approval for these studies was obtained from the Dana-Farber Cancer Institute institutional review board, and informed consent was provided according to the Declaration of Helsinki.
Isolation of patients' tumor cells
Patients' BM samples were harvested after informed consent. Mononuclear cells were obtained after Ficoll-Paque centrifugation (Pharmacia Biotech, Uppsala, Sweden), and MM patient cells (96% CD38+ CD45RA-) were separated by antibody-mediated selection using RossetteSep (StemCell Technologies, Vancouver, BC, Canada) as previously described.26
Reagent
Indazolylpyrimidine GW654652 (GlaxoSmithKline, Research Triangle Park, NC) is a tyrosine kinase inhibitor that inhibits all 3 VEGF receptors.12
Stimulation of cells
Cell lines were starved overnight in their respective culture medium supplemented with 0.25% or 0.5% FBS for MEFs and HMCLs, respectively. Cells were stimulated with FBS and mouse recombinant VEGF (m-rVEGF) or mouse recombinant IL-6 (m-rIL-6) for MEFs and human recombinant VEGF or IL-6 for HMCLs. All cytokines were obtained from R&D Systems (Minneapolis, MN). Duration of stimulation and doses of cytokines are indicated for each experiment.
DNA synthesis and cell proliferation assay
Cell growth was assessed by addition of 0.5 μCi (0.0185 MBq) 3H-thymidine per well during the last 8 hours of each experiment. Cells were harvested onto glass-fiber filtermates using an automatic cell harvester (Tomec Harvester 96 Mach III; Hamden, CT), and radioactivity was counted using the Wallac Trilux Betaplate scintillation counter (Turku, Finland). Each condition was performed in quadruplicate.
Cell viability assays
Cell viability was assessed by 3-(4,5 dimethylthiozol-2-y)-2,5 diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) assay, according to manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN). Cells were seeded in 96-well plates. Cell viability was evaluated as previously described.27,28 Cell survival was estimated as a percentage of the value of untreated control.
Flow cytometry and cell cycle analysis
For cell cycle analysis, DNA was stained with propidium iodide. Briefly, 1 × 106 cells were washed with 1 × PBS, resuspended in 70% ethanol, and then incubated for 30 minutes on ice. After incubation, cells were washed twice with 1 × PBS and resuspended in the presence of RNase for 20 minutes at 37°C. After 2 washes in 1 × PBS, cells were resuspended in propidium iodide on ice for 20 minutes for cytometric analysis. Apoptotic cells were detected as a subdiploid peak, as described by Zamai et al.29 Flow cytometry was analyzed using Cytomics RXP program (Beckman Coulter, Hialeah, FL).
Apoptotic MM patient cells were assayed with double staining using CD38 and Apo 2.7 monoclonal antibodies (mAbs) coupled to fluorescein isothiocyanate (FITC) and phycoerythrin (PE), respectively (Immunotech, Marseille, France). After staining, MM and non-MM patient cells were gated according to their CD38 expression (CD38++ and CD38+/- for MM cells and non-MM cells, respectively), and apoptotic cells were assessed by Apo 2.7 expression, as previously described.30 Thus, the percentage of apoptotic cells in each cell subset was separately measured.
Cell lysis and Western blot
Cells were washed 2 times with 1 × PBS and suspended in lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane, pH 7.6], 150 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid], 1% Triton X-100, 1 mM sodium vanadate, 1 mM N-phenylmethyl sulfonyl fluoride, and 2 mg/mL aprotinin). After 40 minutes on ice, lysates were cleared by centrifugation at 13 000g/min for 30 minutes at 4°C and were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, prior to electrophoretic transfer onto Hybond C super membrane (Amersham, Arlington Heights, IL). The blots were probed overnight with either Mcl-1, Bcl-2, Actin, extracellular signal-related kinase 1/2 (Erk-1/2) (Santa Cruz Biotechnology, Santa Cruz, Ca), Bax, Bad, Bcl-Xl, X-linked inhibitor of apoptosis protein (XIAP), survivin, or cellular inhibitor of apoptosis protein (cIAP) antisera (Cell Signaling Technology, Beverly, MA) prior to incubation with secondary antibodies and exposure to enhanced chemoluminescence substrate.
Transfection of Mcl-1 siRNA
MM1s cells were transiently transfected with indicated amounts of Mcl-1 duplex (5′-UAA CAC CAG TAC GGA CGG G dTdT; dTdT AUU GUG GUC AUG CCU GCC C-5′ targeting 5′-TAA CAC CAG TAC GGA CGG C-3′)31 or nonspecific control duplexes (pool of 4) using the Cell line Nucleofector Kit V Solution (Amaxa Biosystems, Cologne, Germany), as previously described.32 Following transfection, MM1s cells were subjected to Western blot analysis, MTT assays, and 3H[dT] uptake assays.
Results
Mcl-1 contributes to FBS-induced proliferation and mediates both IL-6- and VEGF-induced proliferation in MEFs
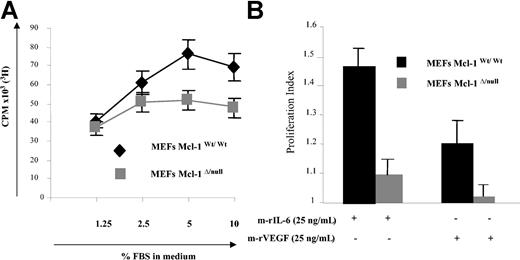
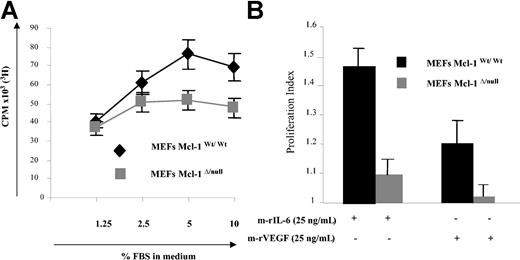
Recently, Opferman et al generated a Mcl-1null allele and Mcl-1Δ/null MEFs that failed to express Mcl-1 protein; using this model system, they demonstrated that Mcl-1 is required for the development of B and T lymphocytes, since deletion of Mcl-1 led to a profound reduction of these hematopoietic cells.18 In the present study, Mcl-1wt/wt and Mcl-1Δ/null MEF cell lines were used as a model to study Mcl-1 involvement in proliferation. To study the consequences of Mcl-1 deletion on growth factor- and cytokine-mediated proliferation, Mcl-1wt/wt and Mcl-1Δ/null MEFs were starved overnight in DMEM with 0.25% FBS, followed by culture in the absence or presence of various doses of FBS. As in Figure 1A, low-dose FBS (1.25%) triggered MEF proliferation similarly in these 2 cell lines. However, at higher concentration of FBS the number of proliferating cells was significantly lower in Mcl-1Δ/null compared with Mcl-1wt/wt MEFs. In addition, we compared the cell cycle distribution of Mcl-1Δ/null and Mcl-1wt/wt MEFs (Table 1). Interestingly, the percentage of cells in S phase was significantly lower in Mcl-1Δ/null MEFs versus Mcl-1wt/wt MEFs (21% and 30%, respectively), demonstrating that lack of Mcl-1 reduces DNA synthesis in MEFs. Next, we compared the response of these cell lines with IL-6 and VEGF. Mcl-1wt/wt and Mcl-1Δ/null MEFs were starved in DMEM with 0.25% FBS overnight, followed by culture for 2 days in the absence or presence of 25 ng/mL m-rIL-6 or m-rVEGF for 2 days (Figure 1C). Both m-rIL-6 and m-rVEGF significantly increased proliferation of Mcl-1wt/wt MEF cells (increase of 45% and 20%, respectively, compared with unstimulated cells). In contrast, Mcl-1Δ/null MEFs did not respond to m-rVEGF, and proliferation induced by m-rIL-6 was only modest (10% increase).
Deletion of Mcl-1 in MEFs modifies FBS-induced proliferation. (A) Deletion of Mcl-1 in MEFs reduces FBS-induced proliferation. Mcl-1wt/wt and Mcl-1Δ/null MEFs were cultured in 96-well plates overnight in DMEM with 0.25% FBS. They were then cultured without or with the indicated percentages of FBS for 48 hours; proliferation was determined by 3H-thymidine uptake during the last 8 hours. Data represent mean ± SD for quadruplicate samples. Shown is 1 representative experiment of 3. CPM indicates counts per minute. (B) Mcl-1 is required for m-rIL-6- or m-rVEGF-induced proliferation. Mcl-1wt/wt and Mcl-1Δ/null MEFs were starved overnight in DMEM with 0.25% FBS followed by culture with or without m-rIL-6 or m-rVEGF (25 ng/mL) for 48 hours; cell growth was assessed as described in “Materials and methods.” Proliferation index is defined as the number of CPM in the stimulated cell divided by the CPM in the control unstimulated cells. Data represent mean ± SD of 3 different experiments.
Deletion of Mcl-1 in MEFs modifies FBS-induced proliferation. (A) Deletion of Mcl-1 in MEFs reduces FBS-induced proliferation. Mcl-1wt/wt and Mcl-1Δ/null MEFs were cultured in 96-well plates overnight in DMEM with 0.25% FBS. They were then cultured without or with the indicated percentages of FBS for 48 hours; proliferation was determined by 3H-thymidine uptake during the last 8 hours. Data represent mean ± SD for quadruplicate samples. Shown is 1 representative experiment of 3. CPM indicates counts per minute. (B) Mcl-1 is required for m-rIL-6- or m-rVEGF-induced proliferation. Mcl-1wt/wt and Mcl-1Δ/null MEFs were starved overnight in DMEM with 0.25% FBS followed by culture with or without m-rIL-6 or m-rVEGF (25 ng/mL) for 48 hours; cell growth was assessed as described in “Materials and methods.” Proliferation index is defined as the number of CPM in the stimulated cell divided by the CPM in the control unstimulated cells. Data represent mean ± SD of 3 different experiments.
Taken together, these experiments demonstrate that deletion of Mcl-1 reduces FBS- and inhibits VEGF-induced proliferation.
VEGF up-regulates Mcl-1 expression in HMCLs
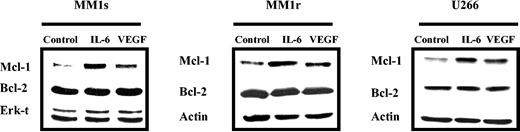
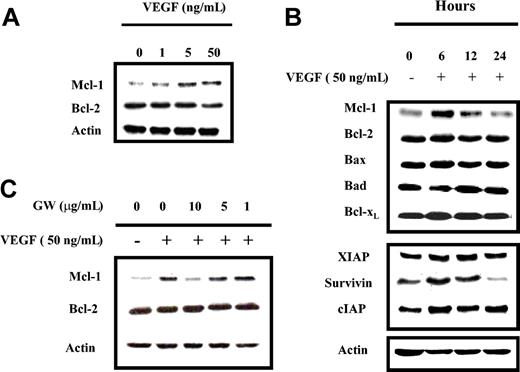
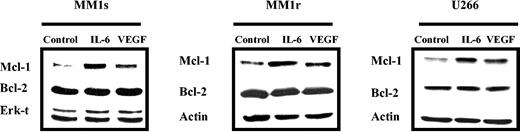
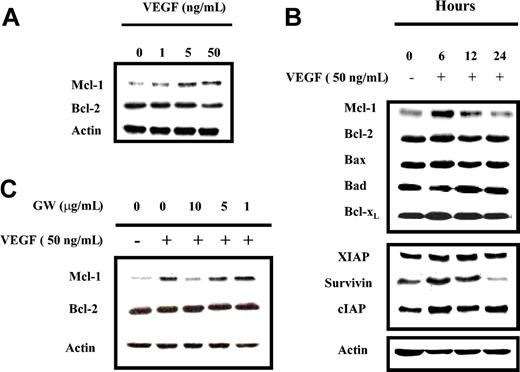
Since both VEGF and IL-6 promote MM cell proliferation, and IL-6 up-regulates Mcl-1, we next investigated whether VEGF could also up-regulate Mcl-1 expression. MM1s, MM1r, and U266 cells were starved overnight in RPMI 0.5% FBS, followed by culture in the absence or presence of either 50 ng/mL IL-6 or VEGF. After 6-hour stimulation, cells were lysed and Mcl-1 expression was determined by Western blot analysis (Figure 2). IL-6 and VEGF, to a lesser extent, up-regulated Mcl-1, but not Bcl-2, expression in these 3 HMCLs. MM1s cells were the most sensitive, and MM1r cells were the least sensitive. Thus, we conducted further experiments on HMCLs in MM1s cells. Time- and dose-dependent Mcl-1 up-regulation by VEGF was observed in MM1s cells (Figure 3A-B): Mcl-1 expression was slightly up-regulated at 1 ng/mL and peaked at 5 ng/mL VEGF. Moreover, time course experiments show that VEGF-triggered up-regulation of Mcl-1 is transient, peaking at 6 hours and returning to baseline after 24 hours (Figure 3B).
VEGF triggers up-regulation of Mcl-1 in HMCLs. HMCLs (MM1s, MM1r, and U266) were starved overnight in RPMI 1640 with 0.5% FBS and then cultured in the absence or presence of VEGF (50 ng/mL) or IL-6 (50 ng/mL) for 6 hours. Cell lysates (30 μg in each lane) were analyzed by Western blot analysis with Mcl-1, Bcl-2, and Actin or Erk-1/2 antisera. Actin and Erk-1/2 were used as loading controls. Shown is 1 representative experiment of 3.
VEGF triggers up-regulation of Mcl-1 in HMCLs. HMCLs (MM1s, MM1r, and U266) were starved overnight in RPMI 1640 with 0.5% FBS and then cultured in the absence or presence of VEGF (50 ng/mL) or IL-6 (50 ng/mL) for 6 hours. Cell lysates (30 μg in each lane) were analyzed by Western blot analysis with Mcl-1, Bcl-2, and Actin or Erk-1/2 antisera. Actin and Erk-1/2 were used as loading controls. Shown is 1 representative experiment of 3.
VEGF triggers time- and dose-dependent protein expression in MM1s cells, which is specifically inhibited by GW654652. (A) VEGF triggers dose-dependent up-regulation of Mcl-1 expression in MM1s cells. MM1s cells were starved overnight in RPMI 1640 supplemented with 0.5% FBS, followed by culture in the presence or absence of the indicated doses of VEGF for 6 hours. Cell lysates (30 μg) were investigated by Western blot analysis with indicated antisera. Actin was used as a loading control. (B) VEGF triggers time-dependent modulation of protein expression in MM1s cells. MM1s cells were cultured overnight in RPMI 1640 supplemented with 0.5% FBS, followed by culture with VEGF (50 ng/mL) for 6 hours. Cell lysates (30 μg) were studied by Western blot analysis with indicated antisera. Actin served as a loading control. (C) VEGF-triggered Mcl-1 up-regulation is inhibited by GW654652. After overnight starvation followed by addition of the indicated doses of GW654652 (1 hour), cells were cultured in presence or absence of 50 ng/mL VEGF (6 hours). Cells lysates (30 μg) were analyzed by Western blot analysis. Shown is 1 representative experiment of 3.
VEGF triggers time- and dose-dependent protein expression in MM1s cells, which is specifically inhibited by GW654652. (A) VEGF triggers dose-dependent up-regulation of Mcl-1 expression in MM1s cells. MM1s cells were starved overnight in RPMI 1640 supplemented with 0.5% FBS, followed by culture in the presence or absence of the indicated doses of VEGF for 6 hours. Cell lysates (30 μg) were investigated by Western blot analysis with indicated antisera. Actin was used as a loading control. (B) VEGF triggers time-dependent modulation of protein expression in MM1s cells. MM1s cells were cultured overnight in RPMI 1640 supplemented with 0.5% FBS, followed by culture with VEGF (50 ng/mL) for 6 hours. Cell lysates (30 μg) were studied by Western blot analysis with indicated antisera. Actin served as a loading control. (C) VEGF-triggered Mcl-1 up-regulation is inhibited by GW654652. After overnight starvation followed by addition of the indicated doses of GW654652 (1 hour), cells were cultured in presence or absence of 50 ng/mL VEGF (6 hours). Cells lysates (30 μg) were analyzed by Western blot analysis. Shown is 1 representative experiment of 3.
The effects of VEGF on other Bcl-2 family members were similarly investigated. No modulation of Bcl-2, Bax, Bad, or Bcl-xL protein expression was observed, suggesting that Mcl-1, among Bcl-2 family members, is specifically targeted by VEGF. Other antiapoptotic proteins, such as survivin and cIAP, were also up-regulated, whereas XIAP expression was unchanged. Interestingly, both Mcl-1 and survivin protein expression were significantly down-regulated after overnight starvation, while expression of the other antiapoptotic proteins remained stable. This suggests that starvation, as a proapoptotic stimulus, acts mainly through down-regulation of Mcl-1 and/or survivin (Figure 3B).
To confirm the specific link between VEGF stimulation and Mcl-1 up-regulation, VEGF receptor (VEGF-R) was inhibited using GW654652, a pan-VEGF-R inhibitor.12 After overnight starvation, MM1s cells were cultured for one hour in the absence or presence of various doses of GW654652, followed by culture without or with 50 ng/mL VEGF for 6 hours. As shown in Figure 3C, up-regulation of Mcl-1 triggered by VEGF was blocked by GW654652 in a dose-dependent manner, thereby confirming the link between VEGF stimulation and Mcl-1 up-regulation.
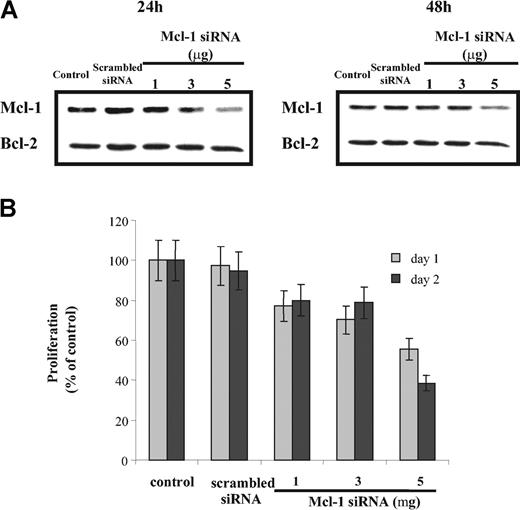
Inhibition of Mcl-1 by Mcl-1 siRNA induces apoptosis and inhibits proliferation of MM1s cells
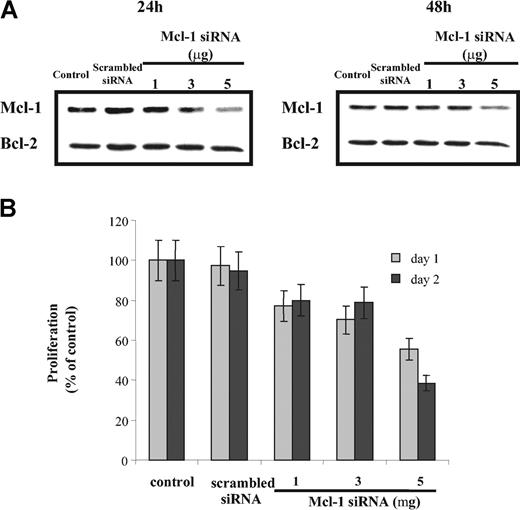
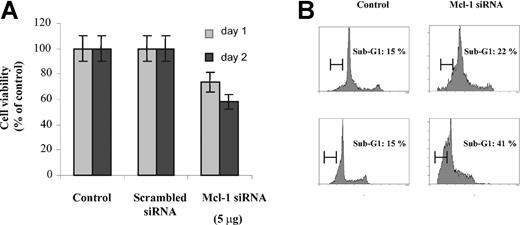
To investigate the consequences of specific inhibition of Mcl-1 on both proliferation and apoptosis, we performed Mcl-1 siRNA transfection in MM1s cells. Mcl-1 protein expression (using Western blot analysis), proliferation (using 3H-thymidine incorporation), and cell viability (using MTT assay) were determined 24 and 48 hours after transfection. Mcl-1 siRNA transfection down-regulated Mcl-1 expression in a dose-dependent manner (Figure 4A), without any modification of Bcl-2 or Bcl-2 family member expression (data not shown). Mcl-1 down-regulation also inhibited 3H-thymidine incorporation (42% and 61% decrease at 24 and 48 hours, respectively; Figure 4B). Similarly, cell viability after Mcl-1 down-regulation was 75% and 60% relative to control cultures at 24 and 48 hours, respectively (Figure 5A).
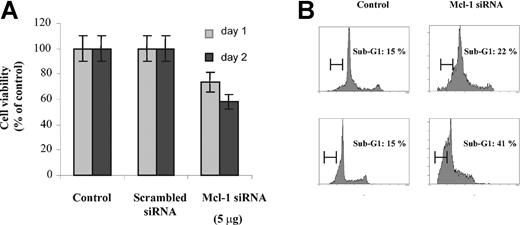
Treatment with Mcl-1 siRNA decreases MM cell proliferation. (A) Down-regulation of Mcl-1 expression in a dose-dependent manner by Mcl-1 siRNA. MM1s cells were transfected with indicated doses of Mcl-1 siRNA (and scrambled siRNA, 5 μg). Mcl-1 expression was determined by Western blot analysis at 24 hours (left) and 48 hours (right). Whole-cell lysates of MM1s cells served as an additional control. Because no change in Bcl-2 expression was observed, Bcl-2 is used as a loading control. (B) Transfection of Mcl-1 siRNA inhibits MM1s proliferation. After transfection with Mcl-1 siRNA or scrambled siRNA (5 μg), 2 × 104 MM1s cells per well were cultured for 24 and 48 hours. Proliferation assays were performed as described in “Materials and methods.” Nontransfected cells served as an additional control. Results shown are the percentage of 3H-thymidine incorporation compared with control. Shown is 1 representative experiment of 3; error bars indicate standard deviation.
Treatment with Mcl-1 siRNA decreases MM cell proliferation. (A) Down-regulation of Mcl-1 expression in a dose-dependent manner by Mcl-1 siRNA. MM1s cells were transfected with indicated doses of Mcl-1 siRNA (and scrambled siRNA, 5 μg). Mcl-1 expression was determined by Western blot analysis at 24 hours (left) and 48 hours (right). Whole-cell lysates of MM1s cells served as an additional control. Because no change in Bcl-2 expression was observed, Bcl-2 is used as a loading control. (B) Transfection of Mcl-1 siRNA inhibits MM1s proliferation. After transfection with Mcl-1 siRNA or scrambled siRNA (5 μg), 2 × 104 MM1s cells per well were cultured for 24 and 48 hours. Proliferation assays were performed as described in “Materials and methods.” Nontransfected cells served as an additional control. Results shown are the percentage of 3H-thymidine incorporation compared with control. Shown is 1 representative experiment of 3; error bars indicate standard deviation.
Down-regulation of Mcl-1 by Mcl-1 siRNA induces apoptosis in MM cells. (A) Mcl-1 down-regulation by Mcl-1 siRNA induces cytotoxicity. MM1s cells (2 × 104 cells per well) were transfected with Mcl-1 siRNA or scrambled siRNA (5 μg), followed by culture for 24 and 48 hours in 96-well plates. MM1s cell survival was determined at 24 and 48 hours by MTT assay. Results shown are percentage of viability compared with control. Error bars indicate standard deviation. (B) Mcl-1 down-regulation by Mcl-1 siRNA induces apoptosis. Cell cycle analysis was performed at 24 and 48 hours (top row and bottom row, respectively) following transfection with Mcl-1 siRNA or scrambled siRNA (5 μg) (right column and left column, respectively). A sub-G1 peak represents apoptotic cells. Shown is 1 representative experiment of 3.
Down-regulation of Mcl-1 by Mcl-1 siRNA induces apoptosis in MM cells. (A) Mcl-1 down-regulation by Mcl-1 siRNA induces cytotoxicity. MM1s cells (2 × 104 cells per well) were transfected with Mcl-1 siRNA or scrambled siRNA (5 μg), followed by culture for 24 and 48 hours in 96-well plates. MM1s cell survival was determined at 24 and 48 hours by MTT assay. Results shown are percentage of viability compared with control. Error bars indicate standard deviation. (B) Mcl-1 down-regulation by Mcl-1 siRNA induces apoptosis. Cell cycle analysis was performed at 24 and 48 hours (top row and bottom row, respectively) following transfection with Mcl-1 siRNA or scrambled siRNA (5 μg) (right column and left column, respectively). A sub-G1 peak represents apoptotic cells. Shown is 1 representative experiment of 3.
To confirm that cytotoxicity was due to apoptosis, cell cycle analysis was performed. After 24 and 48 hours, the percentage of apoptotic cells (sub-G1 peak) was 22% and 41% in Mcl-1 siRNA-treated cells, versus 15% and 15%, respectively, in control cultures.
Taken together, these experiments demonstrate that specifically targeting Mcl-1 inhibits proliferation and induces apoptosis in MM1s HMCLs. Thus, down-regulation of Mcl-1 is sufficient by itself to inhibit proliferation and induce apoptosis in MM cells.
VEGF overcomes inhibition of DNA synthesis induced by FBS starvation in MM1s cells
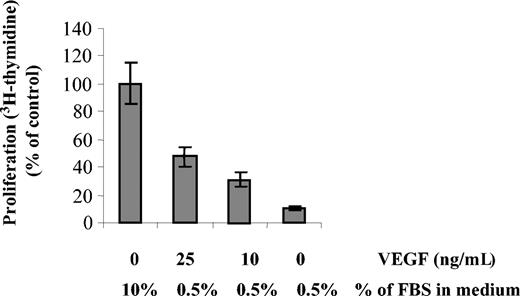
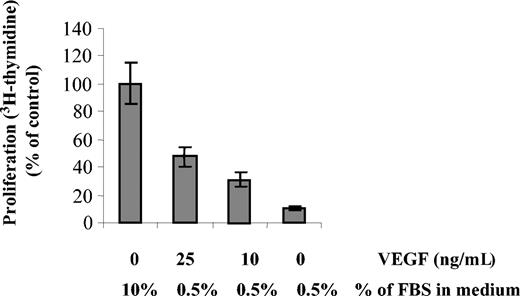
We next investigated whether VEGF could overcome FBS starvation in MM1s cells. MM1s cells were starved in RPMI 0.5% FBS overnight, followed by culture with or without various doses of VEGF. DNA synthesis was evaluated by 3H-thymidine incorporation (Figure 6). Only 12% of starved cells incorporated 3H-thymidine in the absence of VEGF. In contrast, 33% and 49% of cells still incorporated 3H-thymidine in presence of 10 and 25 ng/mL VEGF, respectively. Higher concentrations of VEGF did not increase the percentage of proliferating cells (data not shown). These results demonstrate that VEGF overcomes, at least in part, FBS starvation, thereby maintaining MM cell viability and proliferation.
FBS starvation-induced cell death is partially rescued by VEGF. MM1s cells were starved overnight in RPMI 1640 supplemented with 0.5% FBS in 96-well plates, followed by culture for 48 hours with the indicated doses of VEGF and FBS. Proliferation was measured by 3H-thymidine incorporation. Results shown are compared with control cells cultured in RPMI 1640 supplemented with 10% FBS. Error bars indicate standard deviation.
FBS starvation-induced cell death is partially rescued by VEGF. MM1s cells were starved overnight in RPMI 1640 supplemented with 0.5% FBS in 96-well plates, followed by culture for 48 hours with the indicated doses of VEGF and FBS. Proliferation was measured by 3H-thymidine incorporation. Results shown are compared with control cells cultured in RPMI 1640 supplemented with 10% FBS. Error bars indicate standard deviation.
VEGF up-regulates Mcl-1 expression and protects patient MM cells against FBS starvation-induced apoptosis
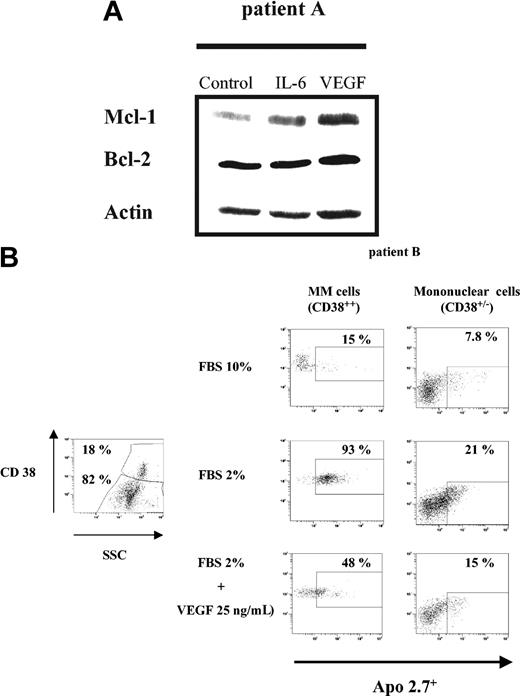
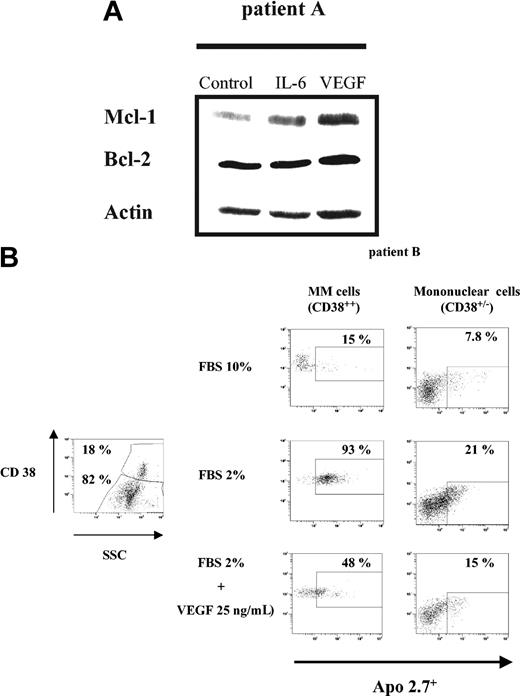
We then investigated the effects of VEGF in 2 patient MM cells. After selection, MM cells (patient A) were starved overnight in RPMI 0.5% FBS, followed by culture in the absence or presence of 50 ng/mL IL-6 or VEGF for 6 hours. Mcl-1 expression was determined by Western blot analysis (Figure 7A). As in HMCLs, both IL-6 and VEGF induced Mcl-1 up-regulation. In contrast to HMCLs, Mcl-1 up-regulation triggered by VEGF was stronger than by IL-6; like HMCLs, Bcl-2 expression remained unchanged.
VEGF up-regulates Mcl-1 and protects patient MM cells from FBS starvation-induced apoptosis. (A) VEGF up-regulates Mcl-1 expression in patient MM cells. Patient MM cells were starved overnight in RPMI 1640 with 0.5% FBS, followed by culture for 6 hours in the absence or presence of either IL-6 (50 ng/mL) or VEGF (50 ng/mL). Cells lysates (30 μg per lane) were analyzed by Western blot analysis using indicated antisera. Actin was used as a loading control. (B) FBS starvation-induced apoptosis is inhibited by VEGF. Patient BM mononuclear cells were cultured for 48 hours in RPMI 1640 with 10% or 2% FBS, in the presence or absence of VEGF (25 ng/mL). The subset of MM cells (CD38++ cells; left panel) and non-MM cells (CD38+/- cells; right panel) was evaluated for apoptosis using Apo 2.7 staining. The percentage of apoptotic MM cells or non-MM cells cultured in control FBS 10% (upper lane), in FBS 2% (middle lane), and in FBS 2% supplemented with VEGF 25 ng/mL (bottom lane) is indicated.
VEGF up-regulates Mcl-1 and protects patient MM cells from FBS starvation-induced apoptosis. (A) VEGF up-regulates Mcl-1 expression in patient MM cells. Patient MM cells were starved overnight in RPMI 1640 with 0.5% FBS, followed by culture for 6 hours in the absence or presence of either IL-6 (50 ng/mL) or VEGF (50 ng/mL). Cells lysates (30 μg per lane) were analyzed by Western blot analysis using indicated antisera. Actin was used as a loading control. (B) FBS starvation-induced apoptosis is inhibited by VEGF. Patient BM mononuclear cells were cultured for 48 hours in RPMI 1640 with 10% or 2% FBS, in the presence or absence of VEGF (25 ng/mL). The subset of MM cells (CD38++ cells; left panel) and non-MM cells (CD38+/- cells; right panel) was evaluated for apoptosis using Apo 2.7 staining. The percentage of apoptotic MM cells or non-MM cells cultured in control FBS 10% (upper lane), in FBS 2% (middle lane), and in FBS 2% supplemented with VEGF 25 ng/mL (bottom lane) is indicated.
BM mononuclear cells from another patient (patient B), were cultured in RPMI 10% FBS or starved with RPMI 2% FBS, with or without 25 ng/mL VEGF for 2 days. Cells were then stained with both CD38-FITC and Apo 2.7-PE. Apoptosis was assessed by flow cytometry, in MM cells as CD38 bright cells (CD38++) and in non-MM cells as CD38 low/negative cells (CD38+/-) (Figure 7B). In the BM sample, 18% CD38++ MM cells were present. Gated analysis showed that a total of 15% of MM cells in the control group (cultured in RPMI supplemented with 10% FBS) versus 93% in the FBS-starved group without VEGF and 48% in the FBS-starved group with VEGF were apoptotic (Apo 2.7+). Gated analysis on non-MM mononuclear cells showed that VEGF also reduced FBS starvation-induced apoptosis from 21% to 15%. Thus, these results demonstrate that VEGF protects MM patient cells against FBS starvation-induced apoptosis.
Discussion
VEGF induces angiogenesis, vasculogenesis, and vasodilatation, and increases vascular permeability.33 Because of its ability to promote the growth of tumor vascular environment, VEGF is a major growth factor mediating tumor progression.34 In MM, VEGF increases microvessel density (MVD), reflecting bone marrow angiogenesis. Thus, MVD has been reported to increase during disease progression from monoclonal gammopathy of undetermined significance (MGUS) to active MM.5,35,36 In addition, a high BM MVD is an adverse prognosis factor.37 Taken together, these reports demonstrate that VEGF, by its effects on the BM microenvironment of MM cells, is a major factor in MM progression. Furthermore, by inducing IL-6 in the BM milieu, VEGF is involved in both autocrine or paracrine MM cell growth. In addition, VEGF also directly targets MM cells. Indeed, Bellamy et al38 and Podar et al6 reported expression of VEGF-R1 on MM cells and primary patient MM cells; Kumar et al39 demonstrated that VEGF-R2 was also expressed on these cells. Importantly, VEGF triggers signaling cascades in MM cells including ERK pathway mediating cell growth and phosphatidylinositol-3 kinase/protein kinase C-dependent cascade mediating migration.6 To date, however, the impact of VEGF protecting against MM cell apoptosis remains unclear. In the present study, we demonstrate that VEGF up-regulates expression of antiapoptotic proteins, including Mcl-1, survivin, and cIAP. Furthermore, we demonstrate that FBS starvation-induced apoptosis in MM cells is partially blocked by VEGF, confirming that VEGF is a potent antiapoptotic cytokine in MM.
Up-regulation of Mcl-1 triggered by VEGF, also reported in chronic lymphocytic leukemia cells, may account, at least in part, for the VEGF antiapoptotic effect.40 Among the Bcl-2 family members, Mcl-1 is a key antiapoptotic protein in the intrinsic pathway of apoptosis. Indeed, Mcl-1 interacts with Bim, a proapoptotic BH3-only protein of the Bcl-2 family, thereby protecting against its proapoptotic effect.18 Furthermore, Nijhawan et al demonstrated that down-regulation of Mcl-1 is a required and early event in ultraviolet (UV) radiation-induced apoptosis.17 Thus, Mcl-1 down-regulation is a pivotal and early checkpoint for some proapoptotic stimuli.41 As reported by Derenne et al, we confirm in the present study that Mcl-1 down-regulation by Mcl-1 siRNA induces apoptosis of MM cells, confirming that Mcl-1 is required for MM cell survival.22
In MM, the main cytokine reported to up-regulate Mcl-1 is IL-6. Relative to IL-6, Mcl-1-induced up-regulation triggered by VEGF was weaker in HMCLs, but stronger in patient MM cells, suggesting that signaling pathway(s) triggered by VEGF mediating up-regulation of Mcl-1 may differ from those induced by IL-6. Similarly to Podar et al,6 we found that VEGF did not activate the JAK/Stat-3 pathway, the main signaling pathway whereby IL-6 up-regulates Mcl-1.19 Furthermore, because Mcl-1-induced up-regulation was stronger by VEGF than by IL-6 in patient MM cells, VEGF may be a more potent in vivo stimulus to up-regulate Mcl-1 than IL-6. Ongoing studies of additional patient MM cell samples are delineating the respective roles of VEGF versus IL-6 in Mcl-1 regulation in vivo.
Like other proteins, including p53, the E2F family proteins, and survivin, Mcl-1 is involved not only in apoptosis but also in cell cycle regulation.42-45 Indeed, Mcl-1 is a cell cycle regulator through its interaction with proliferating cell nuclear antigen (PCNA), a cell cycle regulatory protein essential for G1 to S phase transition.45 Fujise et al45 demonstrated that Mcl-1 interacts functionally and physically with PCNA through a specific amino acid motif. Interestingly, among the Bcl-2 family proteins only Mcl-1 has this motif and interacts with PCNA. In this report, the authors suggested that Mcl-1 is up-regulated by DNA damage, thereby slowing cell cycle progression by binding PCNA. Thus Mcl-1 would slow cell cycle and act as an antiapoptotic protein, ensuring cell survival until DNA repair is completed. By its dual function on apoptosis and cell cycle regulation, Mcl-1 would prevent replication of altered DNAs.45 In contrast, our study demonstrates that FBS-triggered proliferation is significantly reduced in Mcl-1Δ/null MEFs compared with Mcl-1wt/wt MEFs. Importantly, we also demonstrate a decreased percentage of S phase cells in Mcl-1Δ/null MEFs, suggesting that Mcl-1 is required for cell cycle progression. Furthermore, we demonstrate that VEGF fails to promote cell growth and that IL-6-induced proliferation is significantly reduced in deleted MEFs. Moreover, Mcl-1 siRNA inhibits HMCL proliferation. Taken together, our results demonstrate that Mcl-1 promotes IL-6- and VEGF-triggered cell growth and cell cycle progression.
We believe that the report of Fujise et al45 and our present study highlight the complexity of the role of Mcl-1 in cell cycle regulation. As reported by Craig et al, Mcl-1 interacts with a large panel of proteins involved in cell cycle and/or apoptosis, including other Bcl-2 family members, and thus creates a “coordinated network” influencing viability, proliferation, and differentiation.25 Because of its rapid and inducible expression, short-term effects, ability to interact with others proteins, and rapid turnover, Mcl-1 has the “parfait profil” of a protein responsive to a large spectrum of stimuli influencing differentiation, proliferation, and survival.
In conclusion, we show in the present report that VEGF protects patient MM cells against FBS starvation-induced apoptosis and that VEGF, like IL-6, is not only a growth, but also an antiapoptotic, factor. Indeed, VEGF up-regulates Mcl-1 expression in MM cells, thereby mediating, at least in part, its antiapoptotic capacity. As further confirmation of its potent antiapoptotic role, we also demonstrate that VEGF up-regulates survivin and cIAP antiapoptotic proteins. Moreover, we show that Mcl-1 mediates proliferation and cell cycle progression, and is required for IL-6- or VEGF-induced MM cell proliferation. Taken together, these results provide the preclinical framework for targeting VEGF and Mcl-1 in novel MM therapeutics.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-05-1760.
Supported by National Institutes of Health grants PO-1 78378 and PO-1 50947, and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.). S. Le G. is supported by the “Lavoisier” grant from the Ministère des Affaires Etrangères, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are thankful to L. Popitz, M. Simoncini, and G. Li for support.