Abstract
Pediatric acute myelogenous leukemia (AML) has a poor prognosis, and novel therapies are needed. The FLT3 tyrosine kinase represents a promising target in pediatric AML. FLT3 is constitutively activated either by an internal tandem duplication (ITD) or by a point mutation (PM) in 17% to 24% of pediatric AML cases. Autocrine stimulation of wild-type (WT) FLT3 by coexpressed FLT3 ligand (FL) occurs in many other cases. FLT3/ITD mutations confer a particularly poor prognosis in pediatric AML patients. Inhibitors of FLT3 are being tested in adult AML patients, with promising preliminary results. In this study, cytotoxicity and apoptosis assays were performed on 44 diagnostic pediatric AML blast samples (14 FLT3/WT, 15 FLT3/ITD, 15 FLT3/PM) using CEP-701, a potent and selective FLT3 inhibitor. Pronounced cytotoxicity and induction of apoptosis were observed in a higher percentage of FLT3/ITD samples (93%) than FLT3/PM (27%) or FLT3/WT (29%). The cytotoxicity was greatest in samples with a high FLT3/ITD mutant-to-wild-type allelic ratio. The addition of FL enhanced the survival and augmented the sensitivity to FLT3 inhibition for the CEP-701–responsive subset of FLT3/WT and FLT3/PM samples. Clinical testing of FLT3 inhibitors as molecularly targeted agents for the improvement of outcome of pediatric AML patients is warranted.
Introduction
The marked improvement in the prognosis of childhood leukemia over the past few decades has been limited primarily to children with acute lymphoblastic leukemia (ALL).1 On the other hand, in pediatric acute myelogenous leukemia (AML), overall survival is approximately 30% to 40%, despite intensification of standard chemotherapy regimens.2,3 Clearly, novel therapies are needed, and molecularly targeted agents represent a promising new therapeutic strategy.
One molecular target showing great promise for the treatment of leukemia is the receptor tyrosine kinase FLT3 (FMS-like tyrosine kinase 3).4-8 FLT3 is aberrantly expressed in most human leukemias, including more than 90% of cases of AML.4,9-11 Nearly all primary leukemic cells and cell lines examined also express FLT3 ligand (FL), and in a number of cases this leads to constitutive FLT3 phosphorylation and activation through autocrine, paracrine, or intracrine mechanisms.12-15 Exogenous FL also has been shown to enhance proliferation of primary leukemic blasts.16
In addition to ligand stimulation, FLT3 also can be activated through 2 types of activating mutations. Internal tandem duplication mutations (FLT3/ITD) are in-frame duplications of variable numbers of base pairs in the juxtamembrane region. Point mutations (FLT3/PM) occur within the “activation loop” of the kinase domain and most commonly consist of single amino acid substitutions at positions D835 and I836.17-19 Both types of mutations result in ligand-independent autophosphorylation and activation of the receptor with subsequent activation of downstream targets, including proteins in the STAT, MAP kinase (MAPK), and AKT pathways, which are involved in proliferation, differentiation, and survival.20-27 The mutations have been found in many adult and pediatric leukemias, most notably in AML.4,6,7 FLT3/ITD is the most common type of mutation, with a prevalence of 17% to 34% in adult AML cases and 10%-17% in pediatric AML cases. FLT3/PM occurs in 7%-9% of both adult and pediatric AML cases. Thus, a significant proportion (17%-26%) of children with AML has activating mutations of the FLT3 tyrosine kinase, making it the most common somatic genetic alteration in this disease.
Constitutive activation of FLT3 has been shown in many model systems (transfected cell lines and primary murine cells) to contribute to the leukemic phenotype.4,6,7 Further, a number of retrospective studies have demonstrated that adult and pediatric AML patients with FLT3/ITD have a worse prognosis. For example, in a United States pediatric AML cooperative group trial, the overall survival rate was 13% for FLT3/ITD+ patients (n = 15) and 50% for FLT3/ITD– patients (n = 76).28 Other studies of pediatric AML patients also have shown poor prognosis associated with FLT3/ITD expression.29-34 The same prognostic impact has not been demonstrated for FLT3/PM. For example, of the same 91 patients discussed above, 7 were FLT3/PM+, and these patients had an excellent outcome (overall survival rate, 86%).35
The demonstrated importance of FLT3 signaling in the pathogenesis of human leukemia has led to the development of several small-molecule tyrosine kinase inhibitors with activity against FLT3.4,5,36 CEP-701 is an indolocarbazole derivative with an IC50 of 3 nM for inhibition of phosphorylation of ITD, pm, and wild-type (WT) FLT3.37 It is orally bioavailable and selective for FLT3, inhibiting the most closely related kinases (PDGFR-β, FMS, and KIT) only at concentrations of 500 nM to 1000 nM or greater.37 We previously have shown that CEP-701 is selectively cytotoxic to primary adult AML samples with FLT3/ITD.37 A number of other groups now have identified and validated small molecule inhibitors of FLT3 with similar preclinical data using primary adult AML samples.38-42 Based on these data, several FLT3 inhibitors are now in various stages of clinical development for adult AML. CEP-701 and PKC-412, for example, have undergone phase 2 trials as monotherapy in adults with relapsed/refractory FLT3 mutant AML.43,44 Both have shown clinical activity with clearance of peripheral blasts and, less frequently, major bone marrow responses.
Thus, convincing data now have been generated in the laboratory and the clinic that support FLT3 as a validated target against which inhibitors should be developed clinically for adult AML.5-7 As discussed earlier in this section, FLT3 activating mutations occur in approximately 17% to 26% of pediatric AML cases.4,6 Most of these are FLT3/ITD, signifying a poor prognosis. In other cases, coexpression of FL results in autocrine activation of FLT3.12 Thus, a significant percentage of pediatric AML blasts express activated FLT3, perhaps reflecting a dependence on FLT3 signaling for the survival of the leukemic blasts. There have been no studies to date examining the in vitro effects of FLT3 inhibition on primary pediatric AML blasts and on the comparative cytotoxic effects of FLT3 inhibition on primary FLT3/ITD versus FLT3/PM versus FLT3/WT AML samples. Thus, we investigated the potential for FLT3 inhibitors to induce cytotoxicity in primary pediatric AML blasts. We hypothesize that FLT3 inhibitors will be selectively cytotoxic to that subset of pediatric AML blasts that are most dependent on FLT3 signaling for survival and that this subset defines the patients most likely to benefit from the incorporation of FLT3 inhibitors into their therapy.
Patients, materials, and methods
Reagents and cell culture
CEP-701 was stored as a 4-mM stock solution in dimethyl sulfoxide (DMSO) at –20° C and was diluted into medium just before use. For every experiment described in this report, each sample, including the controls, contained an identical amount of DMSO. Cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine, and penicillin/streptomycin. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA) except for FBS, which was from Gemini (Woodland, CA). Phycoerythrin (PE)–conjugated annexin V, 7-amino actinomycin D (7-AAD), and PE-conjugated CD135 were obtained from BD Biosciences (San Diego, CA); anti-FLT3 S-18 and anti-STAT5 C-17 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); 4G10 anti–phospho-tyrosine antibody and protein A agarose were from Upstate Biotechnology (Lake Placid, NY); and anti–phospho-STAT5, anti–phospho-MAPK, and anti-MAPK antibody were from Cell Signaling Technologies (Beverly, MA). Recombinant human FL was obtained from Peprotech (Rocky Hill, NJ).
Cytotoxicity and apoptosis assays
Cytotoxicity assays were performed using 3-4,5-dimethylthiazol-2,5-diphenyltetrazolium (MTT). Fifty microliters of culture medium with 2 × concentration of CEP-701 was aliquoted into triplicate wells of 96-well plates for each CEP-701 concentration point. Fifty microliters of cells suspended in culture medium was then added to each well (200 000 viable cells/well). Plates were incubated for 48 hours at 37° C, 5% CO2, then the MTT assay was performed according to the manufacturer's instructions (Roche, Indianapolis, IN). For the annexin V binding apoptosis assay, cells were incubated for 48 hours in medium with increasing CEP-701 concentrations, then washed in phosphate-buffered saline and resuspended in annexin V binding buffer. Annexin V–PE and 7-AAD were added, and after incubation for 15 minutes, cells were then sorted by flow cytometry (FACSCalibur, BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
Immunoprecipitation, immunoblotting, and CD135 FACS analysis
For Western blots, cells were incubated for one hour with 50 nM of CEP-701 or DMSO control (untreated). The cells were then lysed with lysis buffer (1% Nonidet P-40 [NP-40], 0.2 M Tris [tris(hydroxymethyl)aminomethane], 0.15 M NaCl, 0.1 M NaF, 0.01 M EDTA (ethylenediaminetetraacetic acid) with mini-Complete protease inhibitor cocktail tablet (Roche, Mannheim, Germany) and 5 mM sodium orthovanadate, and lysate was clarified by centrifugation. For immunoprecipitations, 500 μg of protein was immunoprecipitated with anti-FLT3 antibody overnight at 4° C, then protein A agarose was added for 2 hours. For analysis of downstream signaling proteins, 50 μg of whole cell lysate was used. After electrophoresis through 8% polyacrylamide gels and transfer to nitrocellulose, membranes were blotted sequentially with anti–phospho-tyrosine (4G10) anti–phospho-STAT5, or anti–phospho-MAPK antibody, followed by stripping and reblotting with anti-FLT3, anti-STAT5, or anti-MAPK antibody. Protein bands were visualized using chemiluminescence (ECL; Amersham, Piscataway, NJ) and scanned with an AGFA Arcus 1200 laser scanner (Agfa, Ridgefield Park, NJ). For assessment of cell surface FLT3 expression, cells were stained with PE-conjugated CD135 and analyzed by flow cytometry.
Determination of FLT3 ITD/WT allelic ratio
Mutational analysis of the FLT3/ITD and FLT3/PM are detailed elsewhere.18,28 Patients who were FLT3/ITD positive were further examined using Genescan analysis (Applied Biosystems, Foster City, CA) to determine the ratio between mutant and wild-type FLT3 alleles. The 2 primers used were 5FLT3/ITD, 5′-6FAM-GCA ATT TAG GTA TGA AAG CCA GC-3′ and 3FLT3/ITD, 5′-CCT TCA GCA TTT TGA CGG CAA CC-3′ (Applied Biosystems, Foster City, CA). Polymerase chain reaction (PCR) was carried out in duplicate in a final volume of 50 μL containing 10 and 25 ng of genomic DNA, using previously described parameters.28 PCR products were diluted 1:100 with filtered Nanopure water. A 1-μL sample was then diluted in a 10-μL cocktail of formamide and size standard (0.05-μL size standard/sample) and analyzed on the ABI Prism 3100 Genetic Analyzer with the POP-4 Polymer using Genescan software (Applied Biosystems).
Statistical methods
Clinical data obtained in CCG-2941 through March 2002 and CCG-2961 through January 2004 were used in the analyses. Statistical significance of the difference in the means of age and white blood cell count (WBC) was determined by one-way analysis of variance (ANOVA). Statistical significance of the difference in cytogenetics, FAB subtypes, and FLT3 inhibitor response rates by group was determined by chi-square independence test. Statistical significance of the difference in induction remission rate was determined by Fisher exact test. Actuarial estimates of overall survival (OS) from diagnosis and relapse-free survival (RFS) from remission induction were calculated using the Kaplan-Meier method.45 RFS was defined as the time from the end of induction to relapse or death due to progressive disease (PD), censoring non-PD deaths. Patients lost to follow-up were censored at their last known point of study, with a cutoff of September 2001 for CCG-2941 and July 2003 for CCG-2961. Statistical significance of the difference in survival curves was determined by log-rank test.46 Statistical significance of the difference in mean MTT cytotoxicity responses by group was determined by Student t test. The degree of correlation of MTT cytotoxicity and annexin V binding (AVB) apoptosis assays was determined using Pearson correlation coefficient.
Patients and treatments
Samples were from patients registered on Children's Oncology Group (COG) pediatric AML protocols CCG-2941 and CCG-2961 and were collected under international review board–approved protocols from each patient's hospital. Informed consent was provided according to the Declaration of Helsinki. The median percentage of blasts in the fresh bone marrow samples was 88% (range, 52%-100%). Patients received 2 4-day cycles of intensively timed induction chemotherapy (idarubicin or daunorubicin, dexamethasone, Ara-C, 6-thioguanine, and etoposide [I/DCTER]). On CCG-2961, patients were then randomly assigned to receive 1 of 2 consolidation regimens, either an additional 2 cycles of I/DCTER or an 8-day cycle of fludarabine, Ara-C, and idarubicin. On CCG-2941, all patients were consolidated with an additional 2 cycles of I/DCTER. At the end of consolidation on both trials, patients who achieved remission and had a compatible matched related donor went on to receive an allogeneic bone marrow transplant (BMT). Patients without related donors received further chemotherapy followed by either interleukin-2 immune modulation therapy or standard follow-up care.47,48
Results
Patients with FLT3/ITD have a worse outcome and higher-risk clinical features compared to those with FLT3/PM and FLT3/WT
Sixty-three diagnostic bone marrow specimens from patients with de novo AML treated on CCG-2941 or CCG-2961 were selected from the COG myeloid reference laboratory leukemia bank based on FLT3 mutation status, which had been determined by PCR on genomic DNA isolated from the blasts at diagnosis.28,35 Twenty-one samples from each FLT3 genotype group (FLT3/ITD, FLT3/PM, and FLT3/WT) were selected randomly from all of the samples in each group that had adequate numbers of cells in the leukemia bank. Investigators were blinded to the FLT3 mutation status and clinical status of the study patients until after the cytotoxicity assays were performed. Cytotoxicity assays were successful for 44 of the 63 samples, as described in the next section. The study population therefore consisted of 15 patients with FLT3/ITD, 15 patients with FLT3/PM, and 14 patients with FLT3/WT (Tables 1, 2 and 3).
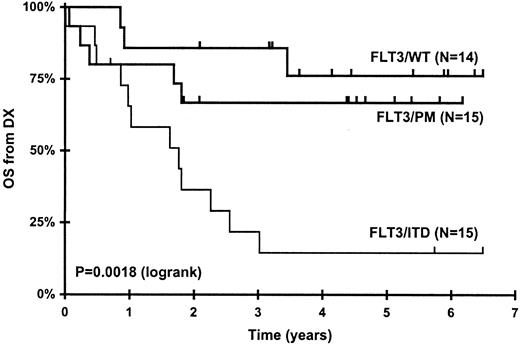
Patients with FLT3/ITD had a significantly higher median diagnostic WBC and significantly lower overall and relapse-free survival compared to those with FLT3/PM and FLT3/WT (Table 4; Figure 1). The differences in survival and diagnostic WBCs between FLT3/ITD+ and FLT3/ITD–patients are consistent with what has been reported for the overall CCG-2941/2961 study population and similar studies of pediatric AML patients reported by a number of groups.4,28,30-33,48,49
Patients with FLT3/ITD mutations have a worse outcome than patients with FLT3/PM (P = .019) and FLT3/WT (P = .0008). Kaplan-Meier curves for overall survival from diagnosis for all patients whose leukemia cells were studied in this report, stratified by FLT3 mutation status. P values were determined by log-rank analysis.
Patients with FLT3/ITD mutations have a worse outcome than patients with FLT3/PM (P = .019) and FLT3/WT (P = .0008). Kaplan-Meier curves for overall survival from diagnosis for all patients whose leukemia cells were studied in this report, stratified by FLT3 mutation status. P values were determined by log-rank analysis.
CEP-701 is differentially cytotoxic to primary pediatric AML blasts with FLT3/ITD, particularly those with high FLT3/ITD-to-wild-type ratios
Forty-eight–hour MTT cytotoxicity assays were performed in triplicate on all samples using CEP-701, a potent (IC50 = 3 nM) and selective inhibitor of mutant and wild-type FLT3. Samples were considered evaluable if there was adequate viability in the untreated control wells at the end of the 48-hour assay period (ie, a mean optical density of at least 0.100). By these criteria, 44 (70%) of 63 samples were evaluable. There were no differences in the fraction evaluable between the 3 groups.
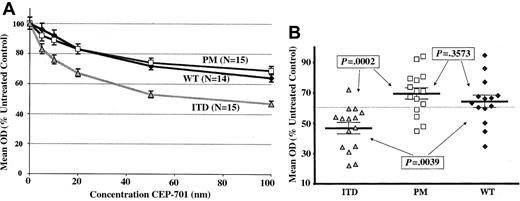
Figure 2A shows dose-response curves grouped by FLT3 mutation status of the mean MTT responses to CEP-701 treatment for all of the assayed samples normalized to untreated controls. The results clearly show that more cytotoxicity is induced for FLT3/ITD samples by CEP-701 compared to FLT3/PM or FLT3/WT samples. At every dose level of CEP-701, significantly higher levels of cytotoxicity are seen for FLT3/ITD samples compared to both FLT3/PM samples and FLT3/WT samples (P value range, .0002-.0222). By contrast, there is no significant difference between the cytotoxicity observed for FLT3/PM and FLT3/WT samples (P value range, .236-.9373). The mean cytotoxic response at 100 nM CEP-701 for the entire sample set was a mean optical density (OD) of 60% of the untreated control. A “responding” sample was therefore defined as one inhibited to less than 60% untreated control at 100 nM CEP-701. Using this definition, Table 5 shows the inhibitor response rate is 14 (93%) of 15 for FLT3/ITD, 4 (27%) of 15 for FLT3/PM, and 4 (29%) of 14 for FLT3/WT. Figure 2B is a dot plot demonstrating the individual samples' cytotoxic response (mean of triplicate wells) to CEP-701 at the 100-nM dose level. The dotted line represents the mean cytotoxic response of the entire sample set (60% of untreated control).
CEP-701 is more cytotoxic to primary pediatric AML blasts with FLT3/ITD mutations than those with FLT3/PM or FLT3/WT. (A) Dose-response curves showing mean cytotoxic response to CEP-701 for all assayed samples, grouped by FLT3 mutation status, normalized to untreated controls. Error bars represent standard error of the mean (SEM). (B) Dot plot of individual samples' cytotoxic response (mean of triplicate experiments) to CEP-701 at the 100-nM dose level, grouped by FLT3 mutation status. Error bars show the group mean ± SEM. P values are from Student t test. The dotted line represents the mean cytotoxic response of the entire sample set (60% of untreated control).
CEP-701 is more cytotoxic to primary pediatric AML blasts with FLT3/ITD mutations than those with FLT3/PM or FLT3/WT. (A) Dose-response curves showing mean cytotoxic response to CEP-701 for all assayed samples, grouped by FLT3 mutation status, normalized to untreated controls. Error bars represent standard error of the mean (SEM). (B) Dot plot of individual samples' cytotoxic response (mean of triplicate experiments) to CEP-701 at the 100-nM dose level, grouped by FLT3 mutation status. Error bars show the group mean ± SEM. P values are from Student t test. The dotted line represents the mean cytotoxic response of the entire sample set (60% of untreated control).
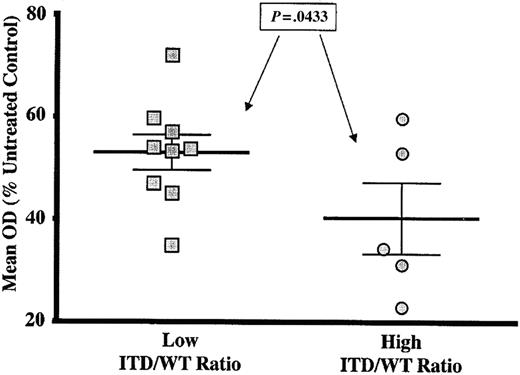
In studies of both adult and pediatric AML, high ITD/WT ratios have been associated with especially poor prognosis.50-52 Therefore, the relationship between the ITD/WT ratio and the cytotoxic response induced by 100 nM CEP-701 in the FLT3/ITD samples was examined. Table 1 shows the nucleotide range of the ITD sequence and the mutant-to-wild-type allelic ratio (ITD/WT ratio) for each FLT3/ITD sample. CEP-701 “responders” are indicated with asterisks. One sample (patient number I-4) had an extremely low ITD/WT ratio (0.01, 40-fold less than the next lowest ratio) and was excluded from the analysis because the FLT3/ITD mutation was present in such a minor subclone that cytotoxicity results would not be reflective of FLT3/ITD signaling. The mean ITD/WT ratio for the sample set was 0.721, and this value was used to separate the samples into 2 groups. As shown in Figure 3, samples with a “low” ITD/WT ratio (defined as less than the mean ITD/WT ratio of 0.721) demonstrated significantly less CEP-701–induced cytotoxicity compared to the samples with a ITD/WT ratio higher than the mean (P = .0433). We also analyzed these data by calculating a Pearson correlation coefficient and found that there was, in fact, a significant correlation between the ITD/WT ratio and the cytotoxic response (r2 = 0.3691, P = .0212).
High FLT3/ITD mutant-to-wild-type ratio correlates with increased sensitivity to FLT3 inhibition by CEP-701. Dot plot showing cytotoxic response of FLT3/ITD samples to 100-nM CEP-701 stratified by ITD/WT ratio. “Low” ratios were those less than the mean ITD/WT ratio for the sample set (0.721). “High” ratios were those greater than the mean. P value is from Student t test. Error bars represent standard error of the mean (SEM).
High FLT3/ITD mutant-to-wild-type ratio correlates with increased sensitivity to FLT3 inhibition by CEP-701. Dot plot showing cytotoxic response of FLT3/ITD samples to 100-nM CEP-701 stratified by ITD/WT ratio. “Low” ratios were those less than the mean ITD/WT ratio for the sample set (0.721). “High” ratios were those greater than the mean. P value is from Student t test. Error bars represent standard error of the mean (SEM).
We further investigated whether cytotoxic response to FLT3 inhibition, regardless of FLT3 mutation status, correlated significantly with outcome. We found a trend toward decreased survival in the CEP-701 responders (OS 41%, n = 22) versus the nonresponders (OS 63%, n = 22), but this difference was not statistically significant (P = .1687, log rank).
The mechanism of the cytotoxic effects of CEP-701 on pediatric AML blasts is the induction of apoptosis
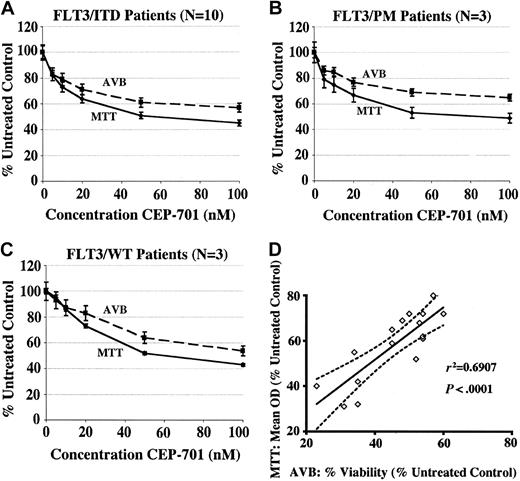
The MTT assay is a convenient and sensitive measure of cytotoxicity, but it does not directly measure cell death. A relative decrease in the mean optical density in a drug-treated well could be due to varying degrees of 3 different processes: inhibition of proliferation, reduction of metabolic activity, and induction of apoptosis.53 Primary myeloid leukemic cells tend not to proliferate significantly in culture medium, so it is likely that most of the cytotoxic effect seen in MTT assays on primary cells is due to either apoptosis or decreased metabolism. To determine the relative contribution of apoptosis, we performed 48-hour AVB flow cytometric assays in parallel with MTT assays on samples where sufficient cells were available. Cells that did not bind annexin or 7-AAD were considered viable. The percentage of viable cells was normalized to the untreated controls and plotted. Figures 4A-C show the aggregate results of the MTT and AVB assays for the CEP-701–responsive samples grouped by FLT3 mutation status, and Figure 4D shows the correlation of the MTT and AVB results at the 100-nM CEP-701 dose level for all samples represented in Figures 4A-C. There is a strong correlation between the MTT and AVB assays, confirming that the predominant mechanism of cytotoxicity of CEP-701 in these samples is the induction of apoptosis.
The mechanism of the cytotoxic effects of CEP-701 on pediatric AML blasts is the induction of apoptosis. Dose-response curves showing correlation between MTT and annexin V binding (AVB) assays for CEP-701–responsive samples. MTT curves show the average of the mean OD for each sample at each dose level, normalized to untreated controls. AVB curves show the mean percentage of viable (ie, annexin V and 7-AAD negative) cells for each sample at each dose level, normalized to untreated controls. Error bars represent SEM. (A) FLT3/ITD samples (N = 10), (B) FLT3/PM samples (N = 3), (C) FLT3/WT samples (N = 3). (D) Correlation of MTT and AVB results at the CEP-701 100-nM dose level. r2 = Pearson correlation coefficient. Solid line represents best-fit line by linear regression; dotted lines represent 95% confidence interval of best-fit line.
The mechanism of the cytotoxic effects of CEP-701 on pediatric AML blasts is the induction of apoptosis. Dose-response curves showing correlation between MTT and annexin V binding (AVB) assays for CEP-701–responsive samples. MTT curves show the average of the mean OD for each sample at each dose level, normalized to untreated controls. AVB curves show the mean percentage of viable (ie, annexin V and 7-AAD negative) cells for each sample at each dose level, normalized to untreated controls. Error bars represent SEM. (A) FLT3/ITD samples (N = 10), (B) FLT3/PM samples (N = 3), (C) FLT3/WT samples (N = 3). (D) Correlation of MTT and AVB results at the CEP-701 100-nM dose level. r2 = Pearson correlation coefficient. Solid line represents best-fit line by linear regression; dotted lines represent 95% confidence interval of best-fit line.
CEP-701 potently inhibits constitutively activated FLT3 but does not always induce a cytotoxic response
While the FLT3/ITD samples as a group demonstrated significantly greater CEP-701–induced cytotoxicity than the FLT3/PM or FLT3/WT groups, there was variability in cytotoxic responses between samples in each group, particularly in the FLT3/PM and FLT3/WT groups (Figure 2B). For CEP-701–responsive samples, the observed cytotoxicity may be the result of inhibition of activated FLT3 and the resultant changes in downstream survival signals. However, neither the MTT nor the apoptosis assays constitute direct biochemical evidence that CEP-701 is inhibiting FLT3 activity in these samples. For CEP-701–resistant samples, potential explanations for the lack of cytotoxic response include lack of FLT3 expression by the leukemic blasts, lack of FLT3 activation (due to lack of FLT3 mutation or lack of coexpression of FL), inability of CEP-701 to inhibit FLT3 activity in a given sample (ie, inherent FLT3 drug resistance), and relatively greater importance of FLT3-independent signaling for the survival of the blasts.
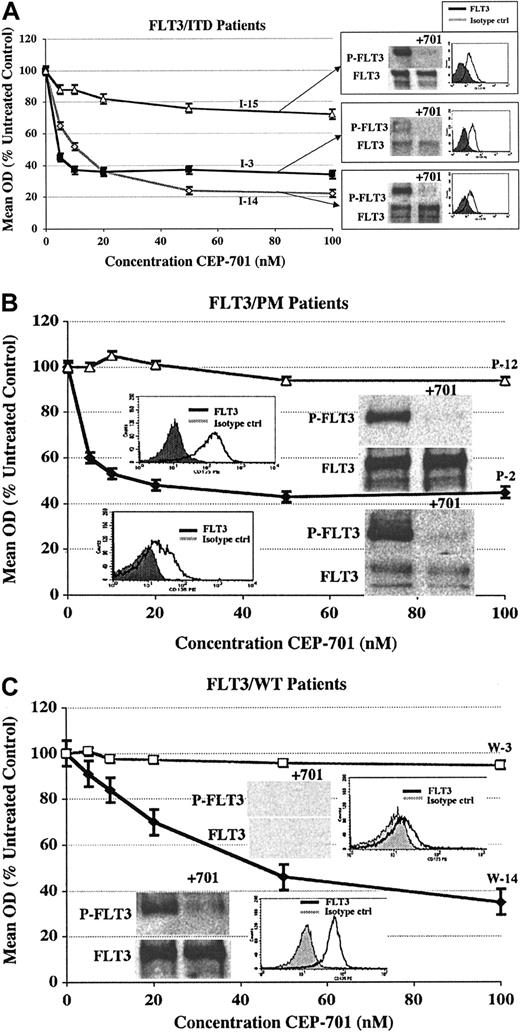
To further investigate the biologic basis for the variability in cytotoxic response to CEP-701, we performed FLT3 immunoprecipitation and Western blotting on at least one CEP-701 responder and nonresponder from each group. Surface FLT3 expression also was assessed by flow cytometry after staining with PE-conjugated CD135 (anti-FLT3). Figure 5 shows the results of these assays correlated with the dose-response cytotoxicity curve for each sample.
CEP-701 potently inhibits constitutively activated FLT3 but does not always induce a cytotoxic response. Immunoprecipitation/Western blotting and CD135-FACS analysis are shown with corresponding MTT cytotoxicity dose-response curves for selected CEP-701–responsive and nonresponsive samples from each group. (A) FLT3/ITD samples (2 responders and 1 nonresponder). (B) FLT3/PM samples (1 responder and 1 nonresponder). (C) FLT3/WT samples (1 responder and 1 nonresponder). Patient numbers (from Tables 1, 2 and 3) are noted next to each curve. Error bars represent SEM of triplicate wells.
CEP-701 potently inhibits constitutively activated FLT3 but does not always induce a cytotoxic response. Immunoprecipitation/Western blotting and CD135-FACS analysis are shown with corresponding MTT cytotoxicity dose-response curves for selected CEP-701–responsive and nonresponsive samples from each group. (A) FLT3/ITD samples (2 responders and 1 nonresponder). (B) FLT3/PM samples (1 responder and 1 nonresponder). (C) FLT3/WT samples (1 responder and 1 nonresponder). Patient numbers (from Tables 1, 2 and 3) are noted next to each curve. Error bars represent SEM of triplicate wells.
For the FLT3/ITD group (Figure 5A), all 3 samples demonstrate expression of constitutively activated FLT3 that is nearly completely inhibited by 1-hour exposure to 50-nM CEP-701. It is likely that in the one nonresponding sample (which was the only nonresponder of 15 FLT3/ITD samples), the survival of the blasts is not primarily dependent on FLT3 signaling but may be more dependent upon activation of one or more FLT3-independent prosurvival signaling pathways. This occurs in approximately 10% of FLT3/ITD samples we have examined (data not shown).
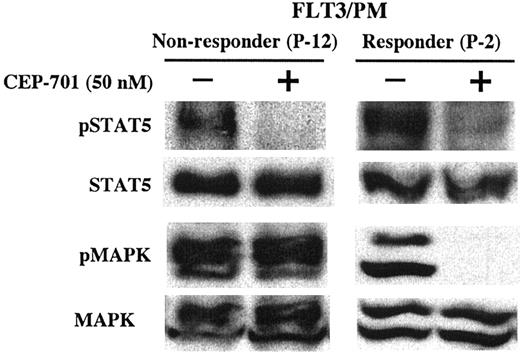
For the FLT3/PM group (Figure 5B), both samples express constitutively active FLT3 that is inhibited by CEP-701, again suggesting a lack of dependence on FLT3 signaling for survival in the unresponsive blast sample. For these 2 FLT3/PM samples, further analysis of downstream signaling proteins known to be activated by FLT3 was performed by Western blotting (Figure 6). Both samples express activated STAT5, which is potently inhibited by CEP-701. Both samples also express activated p44/42 MAPK (ERK1/2). However, only the responding sample demonstrates inhibition of activated MAPK by CEP-701, suggesting that in these blasts MAPK is activated by an FLT3-independent signaling cascade that may play a more important role than FLT3 in the survival of these blasts.
Lack of cytotoxic response to CEP-701 in a FLT3/PM sample is associated with lack of inhibition of activated p44/42 MAPK (ERK1/2). Whole-cell lysates from the 2 FLT3/PM samples shown in Figure 5B were examined by Western blotting for STAT5 and MAPK phosphorylation status. The blots were then stripped and reprobed to determine the STAT5 and MAPK protein expression. Patient numbers (from Tables 1, 2 and 3) are noted for each sample.
Lack of cytotoxic response to CEP-701 in a FLT3/PM sample is associated with lack of inhibition of activated p44/42 MAPK (ERK1/2). Whole-cell lysates from the 2 FLT3/PM samples shown in Figure 5B were examined by Western blotting for STAT5 and MAPK phosphorylation status. The blots were then stripped and reprobed to determine the STAT5 and MAPK protein expression. Patient numbers (from Tables 1, 2 and 3) are noted for each sample.
For the FLT3/WT group (Figure 5C), the responding sample demonstrates a high level of expression of activated FLT3 (possibly on the basis of FLT3-ligand coexpression) that is potently inhibited by CEP-701. The nonresponding sample, on the other hand, does not express an appreciable level of FLT3 protein.
Overall, 6 of 7 primary pediatric AML samples (3 of 3 FLT3/ITD, 2 of 2 FLT3/PM, and 1 of 2 FLT3/WT) express FLT3 protein, which in all 6 cases is constitutively activated. CEP-701 potently inhibits activated FLT3 in every case. Thus, it appears that lack of a cytotoxic response to CEP-701 in diagnostic primary pediatric AML blasts is not due to inability of CEP-701 to inhibit activated mutant or wild-type FLT3.
FLT3-ligand enhances survival and increases sensitivity to CEP-701 in CEP-701–responsive FLT3/PM and FLT3/WT samples
Since FLT3 ligand (FL) is widely expressed, particularly in the bone marrow stromal microenvironment, and the leukemia cells frequently coexpress FL, they are likely to be exposed to significant levels of FL in vivo.12,54 FL could activate ligand-responsive surface FLT3 on leukemic blasts and activate the FLT3 signaling cascade. This in turn could affect the proliferative rate and survival of the blasts, which have been demonstrated in vitro,16 as well as the response of the blasts to FLT3 inhibition. Since FLT3 mutations are in most cases heterozygous, FLT3 mutant blasts usually coexpress mutant and wild-type FLT3 receptors.17,18 Thus, even if mutant FLT3 receptors do not require FL for activation, FLT3 mutant samples may show some response to FL.
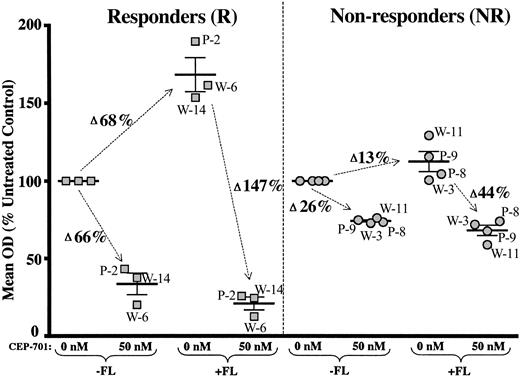
To test the potential effects of FL on pediatric AML blast survival and response to FLT3 inhibition, MTT and annexin V binding apoptosis assays were performed in culture medium with and without added FL (100 ng/mL) on some samples for which adequate cells were available. The only samples that responded significantly to FL were the FLT3/PM and FLT3/WT samples, which were CEP-701 responders in the absence of FL (Figure 7 and Table 6). For these samples, the presence of FL had 2 effects: (1) it increased the mean OD (MTT assay) and percent viability (annexin V binding assay) in the absence of CEP-701, reflecting enhanced survival of these blasts in the presence of FL alone, and (2) it increased the cytotoxicity observed with CEP-701 treatment.
FLT3-ligand enhances survival and increases sensitivity to CEP-701 in responsive FLT3/PM and FLT3/WT leukemic blasts. 48-hour MTT assays were performed with CEP-701 on selected samples both in standard culture medium (–FL) and in culture medium supplemented with 100 ng/mL of human FL (+FL). The mean OD (percent of untreated controls) is shown for each sample, grouped by CEP-701 responsiveness. Error bars represent the mean of the group ± SEM. The change in mean OD is noted next to the arrows. Patient numbers (from Tables 1, 2 and 3) are noted for each sample (P = FLT3/PM, W = FLT3/WT).
FLT3-ligand enhances survival and increases sensitivity to CEP-701 in responsive FLT3/PM and FLT3/WT leukemic blasts. 48-hour MTT assays were performed with CEP-701 on selected samples both in standard culture medium (–FL) and in culture medium supplemented with 100 ng/mL of human FL (+FL). The mean OD (percent of untreated controls) is shown for each sample, grouped by CEP-701 responsiveness. Error bars represent the mean of the group ± SEM. The change in mean OD is noted next to the arrows. Patient numbers (from Tables 1, 2 and 3) are noted for each sample (P = FLT3/PM, W = FLT3/WT).
The FLT3/ITD samples examined (2 responders and 1 nonresponder) showed no effects of FL on either survival or CEP-701 responsiveness (data not shown), and the nonresponder FLT3/PM and FLT3/WT samples likewise showed no significant effects of exogenous FL (Figure 7 and Table 6). The annexin V binding assays demonstrated the same patterns of enhanced survival and CEP-701 sensitivity from the responder FLT3/PM and FLT3/WT groups, but no effects for FLT3/ITD or nonresponder FLT3/PM or FLT3/WT samples (data not shown).
In summary, for the subset of FLT3/WT and FLT3/PM samples for which CEP-701 induced a cytotoxic response in the absence of FL, the presence of FL enhanced the survival of untreated blasts and augmented the cytotoxicity observed with CEP-701 treatment. These effects were not seen for FLT3/WT and FLT3/PM samples that were unresponsive to CEP-701 in the absence of FL. There was also no significant effect of FL on FLT3/ITD samples, regardless of responsiveness to CEP-701.
Discussion
Children with AML with FLT3/ITD mutations have an especially dismal prognosis, with survival rate in the 7% to 20% range.28-34 The results reported here show for the first time that the diagnostic blasts from the majority (93%) of these patients are highly dependent upon FLT3 signaling for their survival, as evidenced by the marked degree of cytotoxicity and apoptosis induced in these samples by a potent FLT3 inhibitor (Figures 2 and 4A). By contrast, blasts from most (70%-75%) patients with FLT3/PM mutations or wild-type FLT3 are significantly less dependent on FLT3 signaling, demonstrating only modest cytotoxicity with CEP-701. These data are similar to those reported for primary adult AML samples with FLT3/ITD,36,37 although this is the first study to systematically compare large numbers of FLT3/ITD, FLT3/pm, and FLT3/WT samples. Clinical trials of CEP-701, PKC-412, and other small molecule FLT3 inhibitors are showing clinical and biologic activity in adult AML patients with FLT3/ITD.43,44 Therefore, clinical trials of FLT3 inhibitors in pediatric AML patients with FLT3/ITD are clearly warranted.
As mentioned previously, high FLT3/ITD mutant-to-wild-type allelic (ITD/WT) ratios have been associated with even worse prognosis in both adult and pediatric AML cases.50-52 It is possible that the biologic basis for the worse prognosis in patients with a high ITD/WT ratio is increased dependence on activated FLT3 signaling. The ITD/WT allelic ratio provides a rough estimate of the percentage of leukemic blasts in a sample whose genomic DNA contains a mutant FLT3 allele. If the expression of these mutant alleles is relatively constant from sample to sample, then a high allelic ratio may imply quantitatively greater expression of constitutively active mutant FLT3 protein. This in turn may signify a greater dependence on FLT3 signaling for the survival of these samples compared to those with lower ITD/WT ratios. Our data provide evidence that this may be the case. Patients whose samples had ITD/WT ratios higher than the mean for the sample set demonstrated more pronounced cytotoxicity with CEP-701 than those with ITD/WT ratios lower than the mean (Figure 3). While the difference between the groups was statistically significant, it was relatively modest, and this finding will need to be confirmed in a larger sample set. It is possible that by using FLT3 inhibitors to eliminate the deleterious effects of FLT3/ITD signaling in a FLT3/ITD+ patient's leukemic blasts, particularly in those with high ITD/WT ratios, their disease might be rendered as curable by chemotherapy as those patients who lack FLT3/ITD mutations. Based on clinical data, this would markedly improve their prognosis.
FLT3/PM and FLT3/WT samples as a group showed significantly less dependence on FLT3 signaling for their survival, with CEP-701 response rates of 27% and 29%, respectively. It is interesting that there is no difference in the cytotoxic response to FLT3 inhibition between FLT3/PM and FLT3/WT sample groups. One might assume that the presence of an activating mutation in a putative oncogene in a leukemic sample would in general result in a dependence on the activity of that activated oncogenic protein for maintenance of the leukemic phenotype. These data suggest that in the case of point mutations in the kinase domain of FLT3, this is not necessarily the case. A possible clinical correlate is the fact that unlike FLT3/ITD, FLT3/PM does not confer a poor prognosis in pediatric AML patients. This suggests that the point mutations in the kinase domain of FLT3 and the resultant activation of the FLT3 signaling cascade may not be the predominant survival signal in most of these cases. An example of this is shown in Figure 6, where a nonresponding FLT3/PM sample is shown to maintain MAPK activation despite potent inhibition of activated FLT3 by CEP-701. In addition, we have previously observed a nonresponding FLT3/PM sample in which STAT5 activation was maintained by alternative pathways when activated FLT3 was inhibited with CEP-701.37 In contrast, the responding FLT3/PM sample in Figure 6 shows potent inhibition of both MAPK and STAT5. Furthermore, there may be biologically significant differences in the downstream signaling initiated by FLT3/ITD and FLT3/PM. A variety of published data suggests that ITD mutation-activated FLT3 may have different signaling properties compared with the wild-type receptor.25,55 It is certainly possible that there are similar signaling differences between FLT3/ITD and FLT3/PM mutations. These observations require further study.
While FLT3/PM and FLT3/WT samples as a group showed less dependence on FLT3 signaling for their survival, approximately 30% of the samples in each of these groups demonstrated a pronounced cytotoxic response to FLT3 inhibition. In the presence of exogenous FL, the viability and cytotoxic response to CEP-701 of the responding samples was enhanced. Since FL is produced in vivo in the bone marrow stromal microenvironment54 and in an autocrine fashion by the leukemic blasts,12 the addition of FL in these in vitro cytotoxicity experiments may result in a better representation of the potential therapeutic efficacy of in vivo FLT3-targeted therapy. It appears that in FLT3/WT and FLT3/PM samples that are at least moderately dependent on FLT3 signaling under standard culture conditions, the addition of FL further stimulates the FLT3 signaling cascade. This is likely to result in amplification of the antiapoptotic and proliferative signals initiated by activation of the FLT3 receptor and to an increase in the dependence on FLT3 signaling for the survival of the blasts. FLT3/WT and FLT3/PM blasts that demonstrated a lack of dependence on FLT3 signaling in the absence of FL, on the other hand, did not show an increase in FLT3 dependence with FL addition. These blasts presumably are more reliant on proliferative and survival pathways that do not involve FLT3. FLT3/ITD samples also did not demonstrate enhanced survival or cytotoxicity with exogenous FL. A possible explanation is that FLT3/ITD mutations already result in maximal stimulation of the FLT3 signaling cascade, so that additional FL stimulation does not result in an amplification of these signals. The finding that FLT3/ITD receptors heterodimerize with FLT3/WT receptors, leading to ligand-independent activation of the wild-type receptors, may explain why there is no need for FL to activate the wild-type receptors in heterozygotes.27 Thus, these findings suggest that FLT3 inhibition in vivo may be even more effective for a significant percentage of pediatric AML patients with FLT3/WT and FLT3/PM than suggested by in vitro assays lacking added FL, and that these patients may also benefit from the clinical use of FLT3 inhibitors.
While clinical trials of FLT3 inhibitors in children must first establish the safety, tolerability, and efficacy of these agents when given as monotherapy, it is likely that responses to FLT3 inhibitors as single agents will be transient, as has been observed in adult AML.43,44 Monotherapy with any molecularly targeted agent is unlikely to be curative in acute leukemia. The development of drug resistance is a ubiquitous problem in the treatment of cancer, which is why chemotherapy agents are usually given in combination. It is likely that resistance to FLT3 inhibitors given as monotherapy will develop by a variety of mechanisms, although we have not seen evidence of intrinsic resistance of activated FLT3 to CEP-701 in diagnostic AML samples (Figure 5).37 FLT3 inhibitors, therefore, are more likely to be effective if rationally incorporated into multi-agent combination chemotherapy treatment regimens. Furthermore, for FLT3/WT and FLT3/PM patients, inhibition of FLT3 signaling alone is, in most cases, insufficient to induce cytotoxicity. In many of these cases, the inhibition of FLT3 signaling may only partially withdraw the antiapoptotic and proliferative stimuli in the cell. By combining FLT3 inhibition with chemotherapy, additive or synergistic induction of cytotoxicity may be observed.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-03-1034.
Supported by grants from the National Cancer Institute (CA60441 [P.B], CA102624 [S.M.], CA095600 [M.L.], CA91177, CA90668, and CA70970 [D.S.]), Jose Carreras International Leukemia Foundation (S.M.), American Society of Clinical Oncology (M.L.), Sidney Kimmel Foundation (M.L.), Leukemia and Lymphoma Society (D.S.), and Burroughs Wellcome Fund (D.S.).
P.B. and S.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Donald Small is the Douglas Kroll Research Foundation Translational Researcher of the Leukemia and Lymphoma Society and the recipient of the Kyle Haydock Professorship in Oncology. The authors want to thank Bruce Ruggieri and Susan Jones-Bolin of Cephalon, Inc. for providing CEP-701, and Rosalyn Pham for assistance with preparation of figures.