Abstract
Angiotensin I–converting enzyme (ACE) inhibitors can affect hematopoiesis by several mechanisms including inhibition of angiotensin II formation and increasing plasma concentrations of AcSDKP (acetyl-N-Ser-Asp-Lys-Pro), an ACE substrate and a negative regulator of hematopoiesis. We tested whether ACE inhibition could decrease the hematopoietic toxicity of lethal or sublethal irradiation protocols. In all cases, short treatment with the ACE inhibitor perindopril protected against irradiation-induced death. ACE inhibition accelerated hematopoietic recovery and led to a significant increase in platelet and red cell counts. Pretreatment with perindopril increased bone marrow cellularity and the number of hematopoietic progenitors (granulocyte macrophage colony-forming unit [CFU-GM], erythroid burst-forming unit [BFU-E], and megakaryocyte colony-forming unit [CFU-MK]) from day 7 to 28 after irradiation. Perindopril also increased the number of hematopoietic stem cells with at least a short-term reconstitutive activity in animals that recovered from irradiation. To determine the mechanism of action involved, we evaluated the effects of increasing AcSDKP plasma concentrations and of an angiotensin II type 1 (AT1) receptor antagonist (telmisartan) on radioprotection. We found that the AT1-receptor antagonism mediated similar radioprotection as the ACE inhibitor. These results suggest that ACE inhibitors and AT1-receptor antagonists could be used to decrease the hematopoietic toxicity of irradiation.
Introduction
Irradiation and most chemotherapies result in marked hematopoietic toxicity by destroying hematopoietic progenitors, leading to bone marrow failure. The recent development of autografts from mobilized peripheral blood stem cells has led to more intensive therapy and reduced toxicity by providing cell support while awaiting marrow reconstitution. These procedures, however, are expensive and not always feasible.
Negative regulators of hematopoiesis may act as potent myelo-protectors by mediating G0/G1 arrest.1-6 In this way, they may prevent DNA breakage during the S phase and subsequent apoptosis. One of these negative regulators, the tetrapeptide AcSDKP (acetyl-N-Ser-Asp-Lys-Pro), has been evaluated in preclinical studies.7-12 The clinical application of this peptide has, however, been limited by its short half-life, due to its rapid degradation by an endogenous peptidase, angiotensin I–converting enzyme (ACE), the key molecule of the renin-angiotensin system.13-15
The renin-angiotensin system is an important determinant of vascular and fluid homeostasis as well as blood pressure regulation.16 In addition, it regulates cellular growth in a variety of tissues through its principal effector, angiotensin II, and its 2 receptors, AT1 and AT2.17 ACE is a tissue-bound enzyme with 2 catalytic domains. It converts angiotensin I into angiotensin II and can cleave other peptides such as bradykinin and AcSDKP.18 AcSDKP is almost exclusively degraded by the N-terminal domain of ACE,15 whereas the other substrates are almost equally cleaved by the 2 ACE domains.19 ACE inhibitors inhibit the formation of angiotensin II and prevent the degradation of AcSDKP thus increasing its concentration in plasma and urine.20,21 Thus, pharmacologic inhibition of ACE strongly affects the concentrations of AcSDKP and angiotensin II, 2 molecules involved in hematopoiesis. Indeed, angiotensin II not only synergizes the effects of erythropoietin on erythroid progenitors but also affects myeloid progenitor growth. Angiotensin II receptors (type AT1) have been detected on both erythroid and CD34+ cells.22,23
ACE inhibitors prevent the entry of murine hematopoietic stem cells into the cell cycle after irradiation24 and inhibit the proliferation of hematopoietic stem and progenitor cells in long-term bone marrow culture.25,26 Thus, pharmacologic inhibition of ACE may interfere with hematopoiesis.
To determine whether ACE inhibitors can prevent myelotoxicity, we evaluated the radioprotective effect of an ACE inhibitor, perindopril, on survival and on bone marrow hematopoiesis in a mouse model after lethal and sublethal doses of irradiation. To dissect the mechanisms by which ACE inhibition mediates this myeloprotection, we investigated the effects of 3 molecules on radioprotection: (1) AcSDKP; (2) RXP 407, a selective blocker of the N-terminal catalytic site of ACE involved in AcSDKP hydrolysis but with no or minimal effect on the conversion of angiotensin I into angiotensin II27 ; and (3) telmisartan, an antagonist of the AT1 receptor.
Materials and methods
Mice
Specific pathogen-free female (C57BL/6J × DBA2) F1 mice, weighing 20 to 22 g, were purchased from Janvier CERJ (Le Genest-St-Isle, France) and quarantined in our institute for at least 7 days prior to use. Mice received commercial rodent food and neutral pH water ad libitum. Mice were handled and housed in accordance with French laws for animal experimentation (Decree 87-848; October 19, 1987).
In vivo treatments
Mice were placed in ventilated plexiglas containers and exposed to an x-ray source at a dose rate of 0.43 Gy/minute. Mice were exposed (total body irradiation [TBI]) to a single dose of 5 or 7 Gy (nonlethal protocol) or to a single dose of 8.3, 8.7, 9.1, 9.5, or 10 Gy (a lethal protocol). Placebo (water) or perindopril was administered subcutaneously at 10, 30, or 90 mg/kg twice a day for 4 days starting 48 hours before the irradiation (Figure 1A). This protocol was based on that already reported for the protective effect of AcSDKP in a model of doxorubicin-induced myelotoxicity.12 To check the specificity of perindopril, we tested an inactive isomer of perindoprilate (the active metabolite of perindopril). The enantiomer (S-11579), kindly given by the Servier Institute (Suresnes, France), was administered at the dose of 30 mg/kg in the same conditions as perindopril. RXP 407, a selective blocker of the N-terminal ACE catalytic site, and telmisartan, an antagonist of AT1 receptors, were administered subcutaneously at 30 mg/kg twice a day for 4 days starting 48 hours before the irradiation (the same schedule as for perindopril). AcSDKP was administered subcutaneously, as previously described,12 at 7.2 μg/mouse, twice a day for 3 days starting 48 hours before irradiation.
Protocol used to assess survival and hematopoietic recovery. Water or perindopril (10, 30, or 90 mg/kg) was administered (↑) subcutaneously twice a day for 4 days beginning 48 hours before exposure to irradiation (gray arrow; single dose of either 7 Gy [nonlethal regimen] or 8.3, 8.7, or 9.1 Gy [lethal regimen]). (A) Peripheral blood (↓) and bone marrow (⊗) samples were collected every 2 or 3 days. Schematic representation of the experiments used to evaluate the short-term repopulating ability of bone marrow cells after reconstitution by survival and hematopoietic recovery (B). On day 46, bone marrow from 3 mice per group was grafted into 10 lethally irradiated mice.
Protocol used to assess survival and hematopoietic recovery. Water or perindopril (10, 30, or 90 mg/kg) was administered (↑) subcutaneously twice a day for 4 days beginning 48 hours before exposure to irradiation (gray arrow; single dose of either 7 Gy [nonlethal regimen] or 8.3, 8.7, or 9.1 Gy [lethal regimen]). (A) Peripheral blood (↓) and bone marrow (⊗) samples were collected every 2 or 3 days. Schematic representation of the experiments used to evaluate the short-term repopulating ability of bone marrow cells after reconstitution by survival and hematopoietic recovery (B). On day 46, bone marrow from 3 mice per group was grafted into 10 lethally irradiated mice.
Peripheral blood analysis
Each mouse was anesthetized with isoflurane and then bled by retro-orbital puncture from day 3 (d3) to day 38 (d38). Blood (90 μL) was collected in vials containing 10 μL of 3.8% trisodium citrate solution. Lymphocytes, neutrophils, red blood cells, and platelets were counted automatically on a MS-9 Vet hematology analyzer (Melet Schloesing, Cergy-Pontoise, France).
Bone marrow analysis
After isoflurane anesthesia, mice were killed by cervical dislocation and their femurs and tibias removed. Flushed bone marrow cells (5 × 104) were plated in 1 mL methyl-cellulose medium (MethoCult M3134; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with recombinant cytokines (interleukin 3 [IL-3] 10 ng/mL, thrombopoietin [TPO] 10 ng/mL, and erythropoietin [EPO] 2 U/mL) for colony assays of murine cells. Duplicate cultures were incubated for 7 days at 37° C in a humidified 5% CO2 atmosphere. Erythroid burst-forming unit (BFU-E) and megakaryocyte colony-forming unit (CFU-MK) assays were performed in a fibrinogen-based serum-free medium. Briefly, fibrinogen (F8630; Sigma-Aldrich, St-Louis, MO) was added (1 mg/mL) immediately before plating the cells with the serum-free medium consisting of Iscove modified Dulbecco medium (IMDM) plus deionized bovine serum albumin (BSA; 15 mg/mL), calcium chloride (370 ng/mL), l-glutamine (2 × 10–3 M), asparagine (20 μg/mL), β-mercaptoethanol (10–4 M), lipid mixture (40 ng/mL, including cholesterol, oleic acid, and phosphatidyl choline), human insulin (10 ng/mL; Sigma, St Louis, MO), and transferrin (300 μg/mL; Boehringer Mannheim, Mannheim, Germany). The serum-free medium was supplemented with a cytokine cocktail containing 10 ng/mL TPO, 1 U/mL EPO, 10 ng/mL IL-3, and 25 ng/mL stem cell factor (SCF) at final concentrations. After 7 days in a humidified atmosphere (37° C, 5% CO2 in air), cultures were dehydrated and fixed with 5% glutaraldehyde. Megakaryocytic and BFU-E colonies were counted after immunostaining with acetyl cholinest-erase and benzidine, respectively.
Short-term repopulating ability of bone marrow cells after reconstitution
To determine whether perindopril was able to protect the hematopoietic stem cell compartment, we compared the repopulating ability of bone marrow cells from perindopril-treated and irradiated mice with that of bone marrow cells from irradiated and untreated mice. Bone marrow cells were obtained from 3 donor mice 46 days after the beginning of irradiation (8.3 Gy) and injected intravenously at concentrations of 1 × 105, 3 × 105, and 1 × 106 cells into lethally irradiated recipient mice (n = 10 per group; Figure 1B). Recipient mice were exposed to a single dose of 10 Gy using an x-ray source at a dose rate of 0.43 Gy/minute.
Immunophenotyping by flow cytometry
Three months after grafting, the recipient mice were killed and Lin– Sca+ c-Kit+ cells were identified by indirect and direct immunofluorescence. To obtain the lineage-negative (Lin–) fraction, cell suspensions prepared from femurs and tibiae were indirectly stained for 30 minutes at 4° C in the dark by a cocktail of CD45/B220 (clone RA3-6B2), CD4 (clone GK.1.5), CD5 (clone 53-7.3), Ly-6/GR1 (clone RB6-8C5), CD11/Mac-1 (clone M1/70), and TER 119 monoclonal antibodies (Mabs; Pharmingen, San Diego, CA) and revealed by a sheep antirat kappa light-chain phycoerythrin-conjugated immunoglobulin G (IgG). After addition of rat IgG and washes, fluorescent isothiocyanate (FITC)–conjugated anti–Sca-1 and allophycocyanin (APC)–conjugated anti–c-Kit rat Mabs (Pharmingen) were added for 30 minutes at 4° C in the dark. After washing, cells were analyzed on a FACSort apparatus equipped with a laser and a diode set at 488 nM and 610 nM, respectively. Data were analyzed with Cellquest software (Becton Dickinson, San Jose, CA).
ACE assay
Plasma-ACE activity was measured using Hip-His-Leu (HHL) as a substrate, as previously described.19 The production of hippuric acid was detected after hydrolysis at 37° C for 30 minutes with 5 μL plasma. The reaction product was resolved and quantified by reverse-phase high-performance liquid chromatography (HPLC).
Statistical analysis
Results are reported as mean ± SD and expressed according to the postirradiation day. Survival was recorded daily for 48 days and survival data were analyzed by Kaplan-Meier plots and log-rank tests. Differences in the day-30 survival rate were evaluated by the chi-square test. Hematologic effect was assessed by studying the kinetics of blood cell and hematopoietic progenitor recovery. In each experiment (n = 5), blood samples (n = 3) were analyzed every 2 days. Differences between the mean values were analyzed by the Kruskal-Wallis test and multiple analysis of variance analysis (MANOVA).
Results
Effect of ACE inhibition on mice survival after irradiation
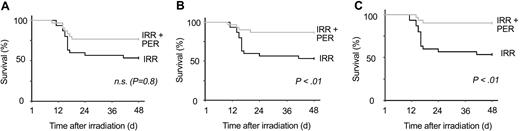
We first determined the sensitivity of B6D2F1 mice to radiation. Fifty percent of mice were still alive 30 days after receiving 8.3 Gy (LD50/30) and a LD100/30 was obtained at 9.1 Gy. We then determined the subcutaneous dose of perindopril necessary to inhibit plasma ACE activity fully. ACE was fully inhibited 1 hour after administration of 10, 30, and 90 mg/kg perindopril and returned to pretreatment level 12 hours after the last perindopril administration (Figure 2). We found that 10 mg/kg perindopril did not confer significant radioprotection (P < .08; Figure 3A), whereas the 2 higher doses (30 mg/kg and 90 mg/kg; Figure 3B-C) induced a marked and highly significant radioprotection with more than 85% of mice still alive 48 days after perindopril treatment (P < .01). The inactive isomer of perindoprilate had no radioprotective effect, showing that survival is dependent on ACE activity (data not shown).
Inhibition of plasma ACE activity by perindopril in mice irradiated with 8.3 Gy. Blood samples were collected from control and irradiated mice before administration of water or perindopril 1 hour after the first administration (A1), 1 hour after the injection on day 2 (A2), and on day 3, 12 hours after the last administration (A3). ACE activity was measured. Results are expressed as the percentage of ACE inhibition compared with water-treated controls. • indicates water-treated nonirradiated mice; ▴, water-treated irradiated mice; ▴, irradiated mice treated with 10 mg/kg perindopril; ▵, irradiated mice treated with 30 mg/kg perindopril; and ▪, irradiated mice treated with 90 mg/kg perindopril. All irradiated mice received a single dose of 7 Gy. Error bars indicate standard deviation.
Inhibition of plasma ACE activity by perindopril in mice irradiated with 8.3 Gy. Blood samples were collected from control and irradiated mice before administration of water or perindopril 1 hour after the first administration (A1), 1 hour after the injection on day 2 (A2), and on day 3, 12 hours after the last administration (A3). ACE activity was measured. Results are expressed as the percentage of ACE inhibition compared with water-treated controls. • indicates water-treated nonirradiated mice; ▴, water-treated irradiated mice; ▴, irradiated mice treated with 10 mg/kg perindopril; ▵, irradiated mice treated with 30 mg/kg perindopril; and ▪, irradiated mice treated with 90 mg/kg perindopril. All irradiated mice received a single dose of 7 Gy. Error bars indicate standard deviation.
Improvement of survival following treatment of irradiated mice with perindopril. B6D2F1 mice (30 per group) received a single dose of 8.3 Gy plus 10 mg/kg (A), 30 mg/kg (B), or 90 mg/kg (C) of subcutaneous perindopril twice a day for 4 days beginning 48 hours before exposure to irradiation. Survival was scored daily for 48 days. Log-rank tests and survival curves showed that irradiated mice given perindopril survived significantly (P = .08, P < .01, and P < .01 for the 3 doses, respectively) longer than irradiated mice that received water. IRR indicates irradiated; PER, perindopril; and NS, not significant.
Improvement of survival following treatment of irradiated mice with perindopril. B6D2F1 mice (30 per group) received a single dose of 8.3 Gy plus 10 mg/kg (A), 30 mg/kg (B), or 90 mg/kg (C) of subcutaneous perindopril twice a day for 4 days beginning 48 hours before exposure to irradiation. Survival was scored daily for 48 days. Log-rank tests and survival curves showed that irradiated mice given perindopril survived significantly (P = .08, P < .01, and P < .01 for the 3 doses, respectively) longer than irradiated mice that received water. IRR indicates irradiated; PER, perindopril; and NS, not significant.
To confirm these results, we performed 3 subsequent experiments with 30 mg/kg perindopril (Table 1 experiments 2-4). In all these experiments, perindopril had a significant radioprotective effect. To extend these results, we increased the irradiation dose to 8.7 Gy (Table 1 experiments 5-6) and 9.1 Gy (Table 1, experiment 7) and again found a statistically significant protective effect (P < .02 and P < .001, respectively). At 9.1 Gy, nearly no mice survived in the control group compared with 20% of those in the perindopril group.
The D0 (the dose that increases mouse survival along the exponential part of survival curve to 37% of the control value) was 7.8 Gy for irradiated mice compared with 8.9 Gy for perindopriltreated mice, yielding a DMF (dose-modifying factor, the ratio of the 2 D0 values) of 1.12. In a second set of experiments, we confirmed this DMF value (1.13) with an increase in D0 from 8.1 Gy for irradiated mice to 9.2 Gy for perindopril-treated mice.
Effects of ACE inhibition on hematopoietic recovery in irradiated mice
The lethality of irradiation doses of less than 10 Gy is related to the induction of profound and prolonged pancytopenia: animals usually die from infection or hemorrhage.28 To measure the beneficial effect of perindopril, we determined blood cell counts every 2 or 3 days from 3 to 30 days after irradiation. In these experiments, we used first a nonlethal dose of irradiation (7 Gy) to avoid a possible bias in the results induced by the fact that only some mice survived when a sublethal irradiation protocol is used. At 10 mg/kg of perindopril, no significant effect was observed on platelet or red blood cell counts. This concentration also had no effect on survival. However, 30 or 90 mg/kg perindopril significantly accelerated platelet recovery (Figure 4A). A slight but significant effect was also observed on red blood cells for perindopril-treated irradiated mice compared with placebo-treated mice nonirradiated mice (Figure 4B; P < .05). In contrast, none of the doses of perindopril had any effect on the polymorphonuclear blood cell (PMN) count (Figure 4C). However, as the number of PMNs in the blood of mice is low in steady-state conditions and as most PMNs are located in tissues, we cannot rule out the possibility that blood cell counts were unable to detect differences in PMN counts. Similar findings for platelets and red blood cells were obtained when mice were given a lethal dose (8.3 Gy) of irradiation (Tables 2-3).
Effects on blood cells following sublethal irradiation. Effects of perindopril on platelet (A), red blood cell (RBC) (B), and neutrophil (C) count following sublethal irradiation. B6D2F1 mice (n = 30) received a single dose of 7 Gy or 8.3 Gy plus either a placebo or 10, 30, or 90 mg/kg perindopril. Each data point represents the mean ± SD of 3 mice per experiment (n = 6). The Kruskal-Wallis test was used to compare irradiated and perindopril-treated mice. See Tables 2-3. RT indicates irradiation treated.
Effects on blood cells following sublethal irradiation. Effects of perindopril on platelet (A), red blood cell (RBC) (B), and neutrophil (C) count following sublethal irradiation. B6D2F1 mice (n = 30) received a single dose of 7 Gy or 8.3 Gy plus either a placebo or 10, 30, or 90 mg/kg perindopril. Each data point represents the mean ± SD of 3 mice per experiment (n = 6). The Kruskal-Wallis test was used to compare irradiated and perindopril-treated mice. See Tables 2-3. RT indicates irradiation treated.
We determined the number of bone marrow cells and hematopoietic progenitors (colony-forming cells [CFCs]) every 3 or 4 days from day 3 until day 28 after irradiation in mice that received 7 Gy with or without 30 mg/kg perindopril. In both cases, the numbers of bone marrow cells and hematopoietic progenitors were markedly reduced by irradiation (23 × 106 ± 2 × 106 bone marrow cells/leg in normal mice versus 0.74 × 106 ± 0.17 × 106 bone marrow cells/leg in mice 3 days after irradiation and 51 587 ± 7108 CFCs/leg in normal mice versus 367 ± 129 CFCs/leg in mice 3 days after irradiation). Perindopril induced a slight but significant increase in the total number of bone marrow cells (Figure 5A; P < .01). Results were also significant when all types of myeloid progenitors (Figures 5B-D) were included (2- to 3-fold increase; P < .01). Interestingly, under perindopril, the number of CFU-MKs (Figure 5D) increased to a greater extent than the number of other progenitor types. This was visible as early as day 7 after irradiation (P < .01). This increase in CFU-MKs following perindopril treatment preceded the increase in platelet count by about 4 or 5 days (Figures 4A and 5D).
Perindopril improves the recovery of bone marrow cells and hematopoietic progenitor cells following sublethal irradiation. B6D2F1 mice (n = 30) received a single dose of 7 Gy and received placebo or perindopril (30 or 90 mg/kg). In each experiment, mice (n = 3) were killed at different times and bone marrow was collected and pooled to perform colony assays. Results are expressed as the percentage of increase for these 2 parameters compared with irradiated mice. Cell counts in irradiated mice and perindopril-treated mice were compared using Student t test. Total cell counts (A; P < .001), CFU-GM counts (B; P < .001), CFU-MK counts (C; P < .01), and BFU-E counts (D; P < .02) were significantly higher in perindopril-treated irradiated mice than in placebo-treated irradiated mice. BMCs indicates bone marrow cells. Error bars represent SD.
Perindopril improves the recovery of bone marrow cells and hematopoietic progenitor cells following sublethal irradiation. B6D2F1 mice (n = 30) received a single dose of 7 Gy and received placebo or perindopril (30 or 90 mg/kg). In each experiment, mice (n = 3) were killed at different times and bone marrow was collected and pooled to perform colony assays. Results are expressed as the percentage of increase for these 2 parameters compared with irradiated mice. Cell counts in irradiated mice and perindopril-treated mice were compared using Student t test. Total cell counts (A; P < .001), CFU-GM counts (B; P < .001), CFU-MK counts (C; P < .01), and BFU-E counts (D; P < .02) were significantly higher in perindopril-treated irradiated mice than in placebo-treated irradiated mice. BMCs indicates bone marrow cells. Error bars represent SD.
ACE inhibition preserves short-term reconstituting cells (STRCs) following irradiation
As irradiation alters the hematopoietic stem cell (HSC) compartment, we evaluated the hematopoietic reconstituting activity of bone marrow cells from irradiated (8.3 Gy) mice treated or not with perindopril (30 mg/kg) in a radioprotective assay. Bone marrow cells from irradiated mice pretreated or not by perindopril were recovered after a complete normalization of their blood count (ie, 4 weeks after irradiation). Bone marrow cells from nonirradiated mice were used as control. Bone marrow cells (105, 3 × 105, and 106) from nonirradiated or irradiated mice treated with or without perindopril were injected into lethally irradiated mice (10 Gy). Complete radioprotection was observed when 3 × 105 cells were injected (Figure 6A). In contrast, 1 × 106 cells from non–perindopril-treated irradiated mice were necessary to obtain the same degree of radioprotection. The frequencies of competitive repopulating units (CRUs) in bone marrow from control mice, irradiated mice, and perindopril-treated mice were 1 per 19 000, 1 per 168 000, and 1 per 67 000, respectively (Figure 6B). This result clearly shows that perindopril preserves STRCs (2.5-fold more than in untreated irradiated mice).
Perindopril improves the short-term repopulating ability of bone marrow cells after reconstitution. Lethally irradiated (10 Gy) mice were intravenously injected with various numbers of BMCs (105, 3 × 105, 106 cells). The survival of the recipients (A) was evaluated until 3 months after grafting. Frequencies of CRUs in murine bone marrow (B) represent the mean of 3 experiments with lethally irradiated mice. Error bars represent SD.
Perindopril improves the short-term repopulating ability of bone marrow cells after reconstitution. Lethally irradiated (10 Gy) mice were intravenously injected with various numbers of BMCs (105, 3 × 105, 106 cells). The survival of the recipients (A) was evaluated until 3 months after grafting. Frequencies of CRUs in murine bone marrow (B) represent the mean of 3 experiments with lethally irradiated mice. Error bars represent SD.
To validate these data, we compared hematopoietic reconstitution in mice injected with 1 × 106 marrow cells from control mice, irradiated mice, or irradiated and perindopril-treated mice by analyzing the blood cell count at various times (Figure 7). Platelets, neutrophils, and red blood cells recovered more quickly in mice injected with bone marrow cells from perindopril-treated mice than in those that received bone marrow cells from irradiated mice. However, reconstitution was fastest with normal bone marrow cells.
Perindopril improves neutrophil, platelet, and red blood cell recovery following lethal irradiation of grafted mice. B6D2F1 mice (n = 30) were irradiated with a single dose of 10 Gy and received 106 bone marrow cells from perindopril-treated irradiated mice, irradiated mice, or untreated mice. Each data point represents the mean ± SD of 9 mice per experiments. The Kruskal-Wallis test was used to compare irradiated mice with perindopril-treated mice: lymphocytes (A; not significant [NS]; P = .08), neutrophils (B; P < .01), platelets (C; P < .001), and red blood cells (D; P < .03).
Perindopril improves neutrophil, platelet, and red blood cell recovery following lethal irradiation of grafted mice. B6D2F1 mice (n = 30) were irradiated with a single dose of 10 Gy and received 106 bone marrow cells from perindopril-treated irradiated mice, irradiated mice, or untreated mice. Each data point represents the mean ± SD of 9 mice per experiments. The Kruskal-Wallis test was used to compare irradiated mice with perindopril-treated mice: lymphocytes (A; not significant [NS]; P = .08), neutrophils (B; P < .01), platelets (C; P < .001), and red blood cells (D; P < .03).
Finally, we used flow cytometry to evaluate the reconstitution of primitive hematopoietic cells (Table 4). Mice injected with bone marrow cells derived from irradiated and perindopril-treated mice had significantly more Lin– cells than those injected with cells derived from irradiated untreated mice, although the percentage of Lin– cells remained lower than in normal mice. Mice grafted with marrow cells from perindopril-treated mice also appeared to contain slightly more cells with a more primitive phenotype, Lin– Sca+ c-kit+ (0.14%), than those grafted with bone marrow from irradiated mice (P < .07). However, the frequency of the Lin– Sca+ c-kit+ cells remained 2-fold lower in mice grafted with marrow cells from mice treated with perindopril than in those grafted with normal bone marrow (0.31%).
An antagonist of the AT1 receptor reproduces the ACE inhibitory effect
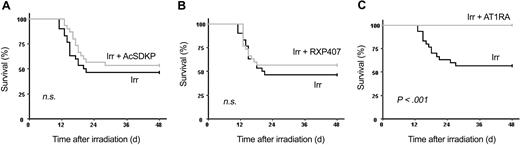
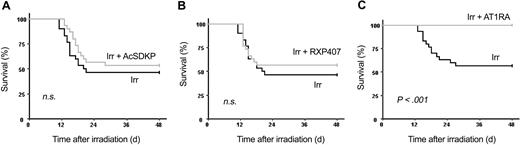
To understand the mechanism of perindopril action, we studied the effects of increasing plasma AcSDKP concentration by either AcSDKP infusion or by using RXP 407, an N-domain ACE inhibitor, to block AcSDKP degradation selectively. We also investigated the effect of telmisartan, an antagonist of the AT1 receptor. No significant differences in 30-day survival rate were observed between control irradiated mice (46.7% ± 9.05%) and irradiated mice treated with AcSDKP (53.3% ± 9.1%; Figure 8A) or RXP407 (56.6% ± 9.1%; Figure 8B).
AT1-receptor blockade reproduces the effects of ACE inhibition on mouse survival. B6D2F1 mice (30 per group) received a single dose of 8.3 Gy plus AcSDKP (A), RXP407 (B), or telmisartan (C). RXP407 and telmisartan were injected subcutaneously twice a day for 4 days beginning 48 hours before irradiation. AcSDKP was administered by subcutaneous route twice a day for 3 days beginning 48 hours before the single exposure. Survival was scored daily for 48 days. Log-rank tests with survival curves showed that telmisartan-treated irradiated mice survived significantly longer (P < .001) than untreated irradiated mice. Irr indicates irradiated.
AT1-receptor blockade reproduces the effects of ACE inhibition on mouse survival. B6D2F1 mice (30 per group) received a single dose of 8.3 Gy plus AcSDKP (A), RXP407 (B), or telmisartan (C). RXP407 and telmisartan were injected subcutaneously twice a day for 4 days beginning 48 hours before irradiation. AcSDKP was administered by subcutaneous route twice a day for 3 days beginning 48 hours before the single exposure. Survival was scored daily for 48 days. Log-rank tests with survival curves showed that telmisartan-treated irradiated mice survived significantly longer (P < .001) than untreated irradiated mice. Irr indicates irradiated.
Telmisartan had the same radioprotective effect as perindopril (Figure 8C): telmisartan and perindopril gave a 30-day survival rate of 100% and 96.3% ± 3.6%, respectively, versus 56.7% ± 9.1% for control irradiated mice. These results were confirmed in a second experiment with a 30-day survival rate of 100% for telmisartan, 93.1% ± 4.7% for perindopril, and 34.5% ± 8.8% for control irradiated mice.
Altogether, these results show that perindopril protects the STRCs from irradiation-related toxicity and that the increase in mice survival is mediated by inhibition of the angiotensin II pathway through the AT1 receptor and that the same effects can be obtained with an AT1 receptor antagonist.
Discussion
Myelosuppression is a major limiting factor in the treatment of cancers by chemotherapy and/or radiotherapy. In this study, we evaluated a novel approach to circumvent this problem by blocking the renin-angiotensin system in a model of ionizing radiation-induced myelotoxicity in mice. First, we tested the effect of perindopril, an ACE inhibitor. Irradiation was chosen for its severe and selective hematopoietic toxicity. Concentrations of perindopril, which totally inhibited plasma ACE activity, significantly protected mice from ionizing radiation toxicity and improved their survival at 3 lethal doses of irradiation (8.3, 8.7, and 9.1 Gy). Moreover, perindopril significantly and reproducibly improved mouse survival in a dose-dependent manner (60 mg/kg/d and 180 mg/kg/d). This improved survival was clearly correlated to reduced hematopoietic toxicity. At sublethal or nonlethal doses of irradiation, ACE inhibition significantly accelerated platelet recovery. The lowest platelet count was similar, but the platelet number was always higher during recovery from day 14 to day 28 in mice treated with perindopril than in irradiated mice. This difference may explain the improved survival because after a lethal irradiation regimen, mice essentially die from intracerebral microhemorrhage.29 In addition, the lowest red blood cell count was also higher in treated mice. No difference was observed for the blood neutrophil count, whereas perindopril had a marked effect on the number of bone marrow cells and the number of granulocyte macrophage colony-forming units (CFU-GMs). This suggests that the neutrophil count in murine peripheral blood is not representative of their total pool. This hypothesis requires further investigations.
In contrast to many types of chemotherapy, ionizing radiation has a major toxic effect on all hematopoietic compartments as shown here by the very low number of bone marrow progenitors following treatment. The rate of hematopoietic recovery for both peripheral blood cells and bone marrow progenitors may be related to protection of the HSC compartment. Indeed, hematopoietic recovery is related to the entry into the cell cycle of some HSCs that both self-renew and differentiate to rescue the different hematopoietic compartments. However, HSCs appear to have limited self-renewal capacities30 and it has been shown that the stem cell pool is never entirely reconstituted after a graft with a limited number of stem cells or after a toxic insult.31 This may explain the additive toxicity of successive chemotherapy regimens on hematopoiesis. To investigate whether ACE inhibition could also protect the HSC compartment, we evaluated the short-term bone marrow reconstitutive activity of progenitor cells from mice whose blood count had returned to normal after irradiation. We found that perindopril increased this reconstitutive activity by about 3-fold compared with the control irradiated mice. However, the activity remained lower than in normal mice. This result correlates with the number of cells with a primitive phenotype (Lin– Sca+ c-kit+) found in the bone marrow of recipient mice. In these experiments, bone marrow cells from perindopril-treated and irradiated mice had the greatest radioprotective effect. They also seem to have greater long-term reconstitutive activity as shown by the blood cell counts after reconstitution of irradiated mice. These results suggest that perindopril exerts a protective effect on HSCs and could diminish the toxicity induced by successive chemotherapy or radiotherapy regimens by preserving short-term reconstitutive activity.
Several mechanisms may account for the effect of perindopril. First, AcSDKP, whose plasma concentration is markedly increased by ACE inhibition,7,20 exerts a direct myeloprotective effect.12 This does not seem to be the case as neither administration of AcSDKP nor administration of RXP407, both of which selectively increase the plasma AcSDKP concentration without altering angiotensin II concentration, improved the survival of irradiated mice. It has already been demonstrated that AcSDKP is unable to decrease irradiation-induced mortality. In contrast, AcSDKP treatment is able to reduce doxorubicin-induced lethality. However, doxorubicin has a milder hematopoietic toxicity than irradiation. Thus, mortality following doxorubicin treatment may be mediated by nonhematopoietic toxicity.1 Therefore, in our irradiation protocol, ACE inhibition exerted its hemoprotective effect through a mechanism other than an increase in AcSDKP concentration. The main physiologic role of ACE is the conversion of angiotensin I into angiotensin II, which apart from its effects on blood pressure, behaves as a cell growth factor. There is also increasing evidence that angiotensin II plays a role in the regulation of hematopoiesis. It has been clearly demonstrated that angiotensin II acts synergistically with erythropoietin on erythroid progenitors23 and may also affect other hematopoietic lineages.22,32 To check whether the renin-angiotensin system itself is involved in ACE inhibition, we inhibited the effect of angiotensin II by using an AT1-receptor antagonist, telmisartan. AT1-receptor blockade induced a marked increase in mice survival similar to that observed with perindopril. Therefore, the radioprotective effect induced by ACE inhibition seems to be essentially mediated by the inhibition of angiotensin II production. Further experiments will be required to determine the precise mechanisms by which this angiotensin II inhibition mediates its myeloprotective effect. Angiotensin II may directly act on the stem cell compartment by promoting entry into the cell-cycle entry.24-26 Alternatively, angiotensin II may indirectly regulate the stem cell compartment by acting on the hematopoietic niche. For example, inhibition of the angiotensin II signaling pathway reduces the production of several cytokines such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor β1 (TGFβ1).33,34
In conclusion, this study shows that a short-term ACE inhibition induces marked hematopoietic radioprotection mediated by the angiotensin II pathway through AT1 receptors. It suggests that optimization of this treatment may be a way of reducing the hematopoietic toxicity of chemotherapy and radiotherapy in humans. It also opens new avenues of investigations on the role of angiotensin II on hematopoiesis.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2003-11-3828.
Supported by a grant from Servier (Suresnes, France).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Patrice Ardouin and Annie Rouchès for taking care of the mice, Françoise Le Pesteur for her technical assistance, Christopher McNamara for improving the English of the manuscript, and Ferdinand Le Noble for critical reading of the manuscript. Enantiomer (S-11579) and perindopril were kindly provided by Servier Institute, and telmisatan was provided by Boehringer Ingelheim, France. RXP407 was given by J. Cotton and V. Dive (CEA, Saclay, France) and A. Yiotakis (University of Athens, Greece).

![Figure 1. Protocol used to assess survival and hematopoietic recovery. Water or perindopril (10, 30, or 90 mg/kg) was administered (↑) subcutaneously twice a day for 4 days beginning 48 hours before exposure to irradiation (gray arrow; single dose of either 7 Gy [nonlethal regimen] or 8.3, 8.7, or 9.1 Gy [lethal regimen]). (A) Peripheral blood (↓) and bone marrow (⊗) samples were collected every 2 or 3 days. Schematic representation of the experiments used to evaluate the short-term repopulating ability of bone marrow cells after reconstitution by survival and hematopoietic recovery (B). On day 46, bone marrow from 3 mice per group was grafted into 10 lethally irradiated mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-11-3828/6/m_zh80160465220001.jpeg?Expires=1765215309&Signature=xJz6CT23bP19xqT9ypTMKha7KVRGjpd5KnKw08Awg26OXmWbdw-aIUCQj8lIyCZCJRglvlZ6SalGyUc3pzpxE4E2zEkuFIqZMqhy8~p1qqFE7SGmKGYwhwkKru8l~biL7FjamZDwcIfcKM-45NsZrJjSBtst8JpDI7u-7krnuEN~buKTUPOpnS1aJ0yh12ECiWJ95AWsYX0YV5hZsN6t-pAr06OIvVB08wB-FVX40MCkidkSth37L1hiy7LFxY~uMje315Tym7vLm5I5S5rFTK2GEGC92FI94ES3D4umXWRFY4GheFY5KD2eaWTBMU8DkxwrrXsEBf-rNmTxHmXACA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 7. Perindopril improves neutrophil, platelet, and red blood cell recovery following lethal irradiation of grafted mice. B6D2F1 mice (n = 30) were irradiated with a single dose of 10 Gy and received 106 bone marrow cells from perindopril-treated irradiated mice, irradiated mice, or untreated mice. Each data point represents the mean ± SD of 9 mice per experiments. The Kruskal-Wallis test was used to compare irradiated mice with perindopril-treated mice: lymphocytes (A; not significant [NS]; P = .08), neutrophils (B; P < .01), platelets (C; P < .001), and red blood cells (D; P < .03).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-11-3828/6/m_zh80160465220007.jpeg?Expires=1765215309&Signature=iZY6dtaJrLAGyTI3rghZMXLqzLFOyY82uOgBsgbCgISmJoMBgDrbmTdzTx4oBLFK1LRIq40fSD8UtkvQWIv5Kwc1yIZGezHSZzNZhVmmsN5IbYwlpr6P73sEv3Vu2494PFh38g-hpYkoZbbI8KbfkOplEpb-WC7KGkHoRpl-oXusrsyiE23kebiku530gX0a0kQuVloMgFbXEOZc9Lb492swbOcMVBci2~nZkubg690HiSWn~ZlywpWxcsO35vEYKkHeBLx167ows-aG7qQJ1X2PHELxY3doIbwUR62FehaEFaOaAX8dTYYqnXP9lOZpKBI2IIgLE0JeW8HD50875w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 7. Perindopril improves neutrophil, platelet, and red blood cell recovery following lethal irradiation of grafted mice. B6D2F1 mice (n = 30) were irradiated with a single dose of 10 Gy and received 106 bone marrow cells from perindopril-treated irradiated mice, irradiated mice, or untreated mice. Each data point represents the mean ± SD of 9 mice per experiments. The Kruskal-Wallis test was used to compare irradiated mice with perindopril-treated mice: lymphocytes (A; not significant [NS]; P = .08), neutrophils (B; P < .01), platelets (C; P < .001), and red blood cells (D; P < .03).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-11-3828/6/m_zh80160465220007.jpeg?Expires=1765215310&Signature=S6cXRuYNIy0slNpH7WWq0KcIc03vPC~Zfh3cJfcHzqP4llAyR5In7WIdVTC-5hihbZzLrHWhmiOFyIqR3~x33IwKot~ADONvZrWIfDeD2LP6tHuzpH06a3--k-GAwbzvulbuqO9c17HZqLrY3TEJ5WUTKIM7oMOFxhNN~lknadh98QfyS9HQRJTj187-FMPmqghn7bLaecD~kF6Fj4JkYG5EVls9VqF8Lbwd9gOxitADPEvYzr6wHzA8am~bJr1-g1z5aRT8Nxc6tzNwpCheZXIMj-EMzX0f9OgjeniMZZaibV4D-zMxUfHwDXlMW457PLmOxov-V3g7yfxGM85geA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)