Abstract
Interleukin-10 (IL-10) has potent immunoregulatory effects on the maturation and the antigen-presenting cell (APC) function of dendritic cells (DCs). The molecular basis underlying these effects in DCs, however, is ill defined. It is well established that the transcription factor NF-κB is a key regulator of DC development, maturation, and APC function. This study was initiated to determine the effects of IL-10 on the NF-κB signaling pathway in immature DCs. IL-10 pretreatment of myeloid DCs cultured from bone marrow resulted in reduced DNA binding and nuclear translocation of NF-κB after anti-CD40 antibody or lipopolysaccharide (LPS) stimulation. Furthermore, inhibited NF-κB activation was characterized by reduced degradation, phosphorylation, or both of IκBα and IκBϵ but not IκBβ and by reduced phosphorylation of Ser536, located in the trans-activation domain of p65. Notably, IL-10–mediated inhibition of NF-κB coincided with suppressed IκB kinase (IKK) activity in vitro. Furthermore, IL-10 blocked inducible Akt phosphorylation, and inhibitors of phosphatidylinositol 3-kinase (PI3K) effectively suppressed the activation of Akt, IKK, and NF-κB. These findings demonstrate that IL-10 targets IKK activation in immature DCs and that suppressing the PI3K pathway in part mediates blockade of the pathway.
Introduction
Dendritic cells (DCs) play a key role in regulating immune responses to foreign and self-antigens.1,2 On the uptake of antigen, DCs process and present peptides to naive T cells and secrete proinflammatory or anti-inflammatory cytokines that influence the nature of the response. The capacity of DCs to promote immunity compared with tolerance within a responding population of T cells is believed to be a function of the subset, maturation, or activation state of the DCs.1,2 For example, T-cell tolerance is induced on antigen presentation by inactivated, immature DCs characterized by low expression of the costimulatory molecules CD80 and CD86. Interleukin-10 (IL-10) has also been reported to be a potent regulator of DC maturation and effector function. Up-regulation of costimulatory molecule expression, secretion of proinflammatory cytokines such as IL-12 and tumor necrosis factor-α (TNF-α), and DC capacity to stimulate T cells are effectively suppressed by IL-10.3-9 Furthermore, APC function can be influenced by IL-10 secreted by DCs in an autocrine manner.10,11
The NF-κB family of transcription factors has a major role in mediating inflammatory responses,12-14 which includes regulating DC development, maturation, and function.15-23 Notably, NF-κB activity controls the expression of many genes involved in the APC function of DCs, such as IL-12, TNF-α, major histocompatibility complex (MHC) 2, and costimulatory molecules. The NF-κB complex consists of homodimers or heterodimers of the structurally related proteins p50, p52, p65 (RelA), c-Rel, and RelB. In most resting cells, NF-κB is sequestered in the cytoplasm complexed with the inhibitory molecules IκBα, IκBβ, and IκBϵ.12-14 In response to a variety of stimuli such as lipopolysaccharide (LPS), TNF-α, IL-12, and CD40 engagement, IκB proteins are phosphorylated, poly-ubiquitinated, and degraded by the 26S proteosome. Releasing NF-κB from IκB proteins facilitates nuclear translocation and subsequent binding to consensus sequences and induction of gene transcription. Reduced numbers of myeloid DCs in mice in which the relB gene has been disrupted provide evidence that NF-κB has a role in DC development.16-18 Furthermore, we and others have shown that hyperactivation or inhibition of NF-κB activity can enhance or block, respectively, the capacity of DCs to secrete proinflammatory cytokines and to stimulate naive CD4+ and CD8+ T cells.21-24
Phosphorylation of the IκB inhibitory molecules is catalyzed by IκB kinase (IKK), a multisubunit complex consisting of IKK1/IKKα, IKK2/IKKβ, and IKKγ/NEMO.14,25 Kinase activity is contained in the IKK1 and IKK2 subunits, whereas IKKγ serves a scaffold/regulatory function. Biochemical analyses and targeted gene deletions have demonstrated that IKK2 is essential for NF-κB activation in response to proinflammatory mediators such as IL-1, TNF-α, and LPS.26-28 Activation of the complex is achieved in part by the phosphorylation of IKK catalyzed by diverse kinases, namely, NF-κB–inducing kinase (NIK), MEKK1, MEKK3, Cot, NAK/TBK, and protein kinase C-ζ (PKC-ζ).14,25 Included in this group is the serine-threonine kinase Akt, found in the phosphatidylinositol 3-kinase (PI3K) pathway.29-31 We and others have demonstrated that the PI3K/Akt pathway can regulate NF-κB activation, though different mechanisms have been described.31-39
Studies have demonstrated that IL-10 inhibits the activity of NF-κB in different cell types, such as human and mouse macrophages and T cells.40-42 Furthermore, we previously demonstrated that IL-10 blocked NF-κB activity through the inhibition of IKK in a human monocyte cell line.43 Nevertheless, the mechanism by which IL-10 inhibits NF-κB transcriptional activity varies depending on the cell type. Moreover, IL-10–mediated suppression has been reported to have no effect on NF-κB activity in human macrophages.44 The current study was initiated to determine the mechanism by which IL-10 regulates the APC function of DCs. Evidence is provided that NF-κB activation is blocked in myeloid DCs after pretreatment with IL-10 and that this inhibition correlates with suppressed Akt and downstream IKK activity.
Materials and methods
Mice
NOD/LtJ, BALB/cJ, and NOR/Lt mice were maintained and bred under specific pathogen-free conditions. The NOD.CL4 mouse line expresses transgenes encoding the CL4 clonotypic T-cell receptor (TCR).23 The CL4-clonotypic TCR is H2Kd restricted and specific for an influenza hemagglutinin (HA) peptide spanning amino acid residues 512 to 520.
Preparation of primary DCs
Femurs of male or female mice 8 to 12 weeks of age were used to isolate bone marrow–derived DCs, as previously described.22,23 Bone marrow was depleted of CD4 (GK1.5)–, CD8 (HO2.2)–, MHC class 2 (M1/42.3.9.8 HLK)–, and B220 (RA3-3A1/6.1)–expressing cells through complement-mediated lysis. DC precursors were plated on 6-well, ultra-low–cluster tissue culture plates (Corning Inc, Corning, NY) in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and penicillin/streptomycin (base medium), 10 ng/mL murine granulocyte macrophage–colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ), and 10 ng/mL murine IL-4 (PeproTech). On day 2, nonadherent cells were harvested and cultured as described for 8 days. Culture medium was added on days 4 and 7. For all experiments, DCs were harvested on day 10 of the cultures. We used 7-amino-actinomycin D (7-AAD) (BD PharMingen, San Diego, CA) staining to access the viability of DCs.
DCs were isolated from the spleen by preparing a single-cell suspension depleted of red blood cells (RBCs) that was incubated with 0.5 mg/mL collagenase A (Roche, Indianapolis, IN) and 5 μg/mL DNase I (Roche) for 20 minutes at 37° C. Cells were washed and DCs purified using an EasySep Murine CD11c Positive Selection Kit (Stem Cell Technologies, Vancouver, BC, Canada). Flow cytometric analysis demonstrated more than 90% purity based on CD11c expression.
EMSA and Western blotting
DCs cultured in base medium at 5 × 105 cells/well in 6-well, low-cluster plates (Corning) were stimulated with 500 ng/mL LPS, 10 μg/mL anti-CD40 antibody (HM40-3; BD PharMingen), or 10 μg/mL purified hamster immunoglobulin M (IgM) isotype control (clone G235-11; BD PharMingen) for specified times. Nuclear and cytoplasmic extracts were prepared as described.22 Electrophoretic mobility shift assay (EMSA) was performed as described using NF-κB–binding, 32P-labeled double-stranded DNA probes from the MHC class 1 H2K promoter, 5'-CAGGCTGGGGATTCCCATCTCCACAGTTTCACTTC-3',22 and the murine IL-12(p40) promoter, 5'-CTTCTTAAAATTCCCCCAGA-3.45 A double-stranded OCT1 DNA probe, 5'-TGTCGAATGCAAATCACTAGAA-3' (Santa Cruz Biotechnology, Santa Cruz, CA), was used as control. Bands were visualized using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). For supershift experiments, 2 μg each anti-p50 (sc-114X), anti-p52 (sc-298), anti-p65 (sc-8008X), anti-cRel (sc-70X), and anti-RelB (sc-226X) (Santa Cruz Biotechnology) was added to lysates and incubated for 1 hour at room temperature before addition of the 32P-labeled probe.
For Western blot analysis, 80 μg cytoplasmic extract was resolved through sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using an 8% to 10% separating gel. Proteins were transferred to Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ) using a semi-dry transfer system and blocked with 5% dried milk in phosphate-buffered saline (PBS) and 0.1% Tween-20. Blots were probed with anti-IκBα (sc-371), anti-IκBβ (sc-969), anti-IκBϵ (sc-7156), anti-IKK1 (sc-7182) (Santa Cruz Biotechnology); anti-IKK2 (2684), antiphospho-IκBα (9246), antiphospho-p65 (3031), antiphospho-IKK1/IKK2 (2681) antiphospho Akt (9271), anti-Akt (9272) (Cell Signaling Technology, Beverly, MA); and anti-β actin (A2066; Sigma, St Louis, MO) antibodies. Binding of horseradish peroxidase (HRP)–labeled goat antirabbit antibody (sc-2004) or goat antimouse antibody (sc-2005) (Santa Cruz Biotechnology) was determined using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockland, IL).
IKK assay
DCs (5 × 106) were stimulated, and whole-cell lysates prepared. IKK signalosome was immunoprecipitated using the Catch and Release kit (Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal anti-IKK1, and anti-IKK2. In vitro kinase experiments were performed as described22 by incubating 0.25 μg immunoprecipitated protein with 0.5 μg glutathione-S-transferase (GST)–IκBα substrate. Reaction mixtures were incubated for 60 minutes at 30° C with 5 μCi (0.185 MBq) [γ-32P] adenosine triphosphate (ATP) in kinase buffer. Kinase reactions were terminated on the addition of SDS-PAGE sample buffer and analyzed using SDS-PAGE. Bands were visualized using autoradiography.
Akt assay
DCs (4 × 106) were stimulated as described, and whole-cell lysates prepared. Akt kinase activity was determined using an Akt Kinase Assay Kit (Cell Signaling Technology). Briefly, Akt was immunoprecipitated according to the manufacturer's instructions, and kinase activity was determined by measuring phosphorylation of a GSK-3 substrate through an immunoblot probed with a phospho-GSK-3α/β (Ser21/9)–specific antibody.
Measuring DC secretion of IL-12(p70) and TNF-α
DCs were harvested on day 10 of culture and plated at 106 cells/mL in a 24-well plate with 1 mL base medium and were treated accordingly. Cells were washed and stimulated with LPS (500 ng/mL), anti-CD40 (10 μg/mL), or isotype-matched control antibody (10 μg/mL) for 48 hours. Supernatants were collected and assayed using enzyme-linked immunosorbent assay (ELISA) for IL-12(p70) and TNF-α according to the manufacturer's instructions (BD PharMingen).
DC/T-cell assays
CD8+ T cells were purified from the spleens of NOD.CL4 mice using anti-CD8 magnetic beads (Miltenyi Biotech, Auburn, CA). T cells were CD62hiCD44loCD69lo and more than 90% CD8+. DCs were pretreated with murine IL-10 for 24 hours, washed 3 times, and incubated with 5 μg/mL HA peptide in 1.0 mL base medium supplemented with 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 1 × nonessential amino acids, and 1 mM glutamine (complete medium) in a 24-well plate. After 30 minutes, DCs were washed 4 times, and 3.5 × 105 T cells were added to each well in 1.0 mL complete medium. At 48 hours, culture supernatants were harvested, and levels of IL-2 were determined using ELISA, according to the manufacturer's instructions (BD PharMingen).
Results
IL-10 pretreatment suppresses the APC function of DCs
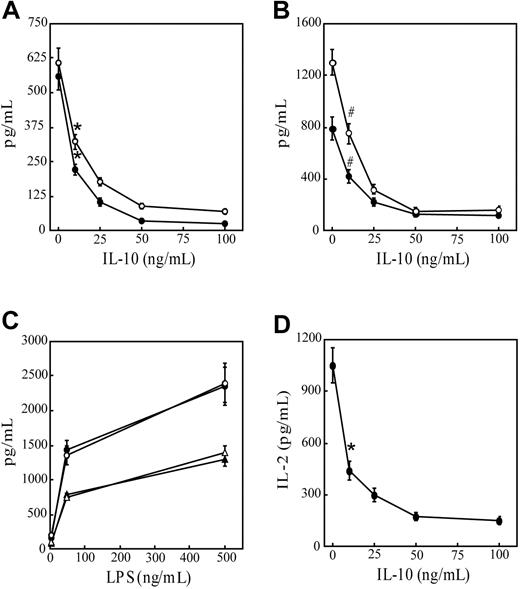
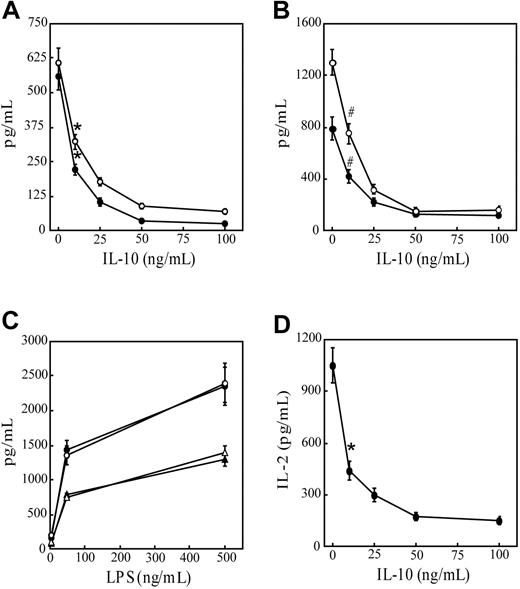
To confirm the immunoregulatory effects of IL-10, immature DCs were pretreated with varying concentrations of IL-10 and examined for their capacity to secrete proinflammatory cytokines after anti-CD40 antibody or LPS stimulation and to stimulate T cells. As reported,22,23 DCs cultured from the bone marrow of NOD mice in GM-CSF and IL-4 for 10 days were more than 98% CD11c+CD11b+CD8α–, and they exhibited an immature phenotype (Table 1). DC cultures pretreated with increasing concentrations of IL-10 exhibited, in a dose-dependent manner, reduced secretion IL-12p70 and TNF-α after stimulation with anti-CD40 antibody (Figure 1A) or LPS (Figure 1B). IL-10 concentrations of 50 ng/mL or more resulted in maximum cytokine suppression. DCs exhibited more than 96% viability in the treatment groups based on flow cytometry (data not shown). No significant effect on IL-12p70 or TNF-α secretion was detected in cultures, however, when DCs were cotreated with 50 ng/mL IL-10 and LPS (Figure 1C) or with anti-CD40 antibody (data not shown). Pretreatment with IL-10 for 24 hours also inhibited the capacity of peptide-pulsed DCs to stimulate naive CD8+ T cells. IL-2 secretion by CD8+ T cells prepared from NOD.CL4 mice transgenic for an H2Kd-restricted TCR specific for HA was reduced in a dose-dependent manner after pretreatment of DCs with increasing concentrations of IL-10 (Figure 1D). Finally, IL-10 pretreatment inhibited the LPS-induced up-regulation of CD40, CD86, and IAg7 cell surface expression (Table 1). In agreement with findings reported by others,3-9 these results demonstrate that pretreatment with IL-10 inhibits the APC function of DCs, while costimulation does not.
IL-10 pretreatment suppresses secretion of proinflammatory cytokines and APC function of DCs. Bone marrow–derived DCs were pretreated with varying doses of IL-10 for 24 hours, stimulated with (A) 10 μg/mL anti-CD40 antibody or (B) 500 ng/mL LPS for 48 hours. IL-12p70 (○) and TNF-α (•) levels were measured in culture supernatants using ELISA. Levels of pIL-12p40 and TNF-α were lower than 25 pg/mL in the absence of (A) anti-CD40 antibody or (B) LPS stimulation. (C) DCs were stimulated with varying concentrations of LPS alone (• and ▴) or cotreated with 50 ng/mL IL-10 for 48 hours (○ and ▵), and the amounts of IL-12p70 (• and ○) and TNF-α (▴ and ▵) were measured in culture supernatants. (D) DCs were pretreated with varying concentrations of IL-10 for 24 hours, pulsed with 5 μg/mL HA peptide, and cultured with 3.5 × 105 NOD.CL4 T cells for 48 hours. Levels of IL-2 secretion in culture supernatants were measured using ELISA. Less than 20 pg/mL IL-2 was detected in cultures lacking HA peptide. Data represent mean ± SD of 4 individual cultures and are representative of 3 independent experiments. *P < 10–4 compared with no IL-10 pretreatment. #P < 10–3 compared with no IL-10 pretreatment (Student t test).
IL-10 pretreatment suppresses secretion of proinflammatory cytokines and APC function of DCs. Bone marrow–derived DCs were pretreated with varying doses of IL-10 for 24 hours, stimulated with (A) 10 μg/mL anti-CD40 antibody or (B) 500 ng/mL LPS for 48 hours. IL-12p70 (○) and TNF-α (•) levels were measured in culture supernatants using ELISA. Levels of pIL-12p40 and TNF-α were lower than 25 pg/mL in the absence of (A) anti-CD40 antibody or (B) LPS stimulation. (C) DCs were stimulated with varying concentrations of LPS alone (• and ▴) or cotreated with 50 ng/mL IL-10 for 48 hours (○ and ▵), and the amounts of IL-12p70 (• and ○) and TNF-α (▴ and ▵) were measured in culture supernatants. (D) DCs were pretreated with varying concentrations of IL-10 for 24 hours, pulsed with 5 μg/mL HA peptide, and cultured with 3.5 × 105 NOD.CL4 T cells for 48 hours. Levels of IL-2 secretion in culture supernatants were measured using ELISA. Less than 20 pg/mL IL-2 was detected in cultures lacking HA peptide. Data represent mean ± SD of 4 individual cultures and are representative of 3 independent experiments. *P < 10–4 compared with no IL-10 pretreatment. #P < 10–3 compared with no IL-10 pretreatment (Student t test).
IL-10 pretreatment inhibits nuclear DNA binding activity of NF-κB
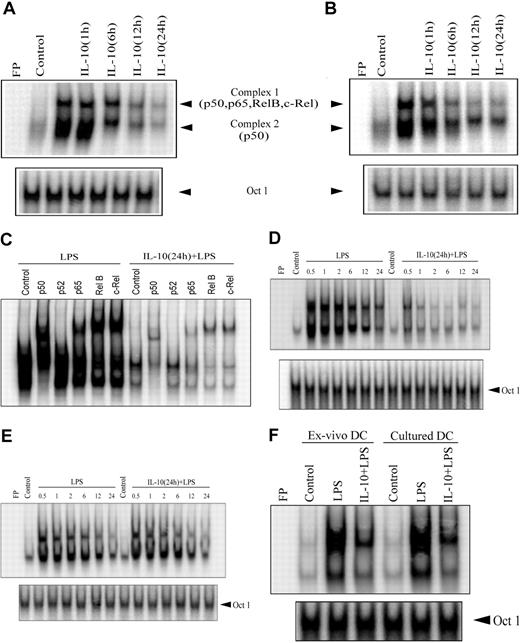
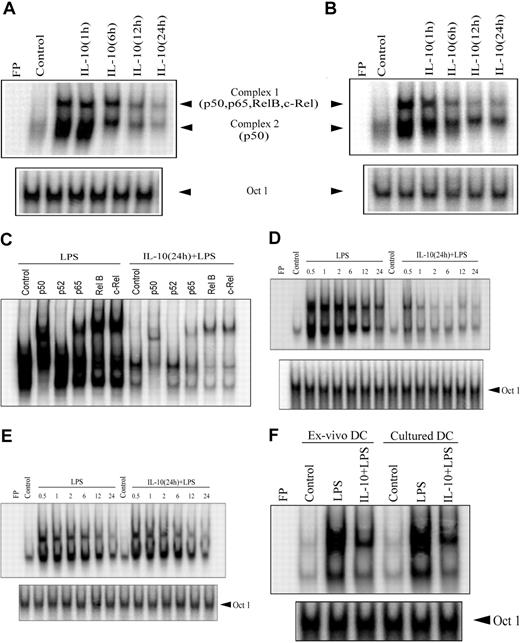
Given that NF-κB plays a key role in regulating the APC function of DCs, the effect of IL-10 pretreatment on NF-κB activation was investigated. EMSA was performed on nuclear extracts prepared from DCs pretreated with 50 ng/mL IL-10 for varying periods of time and then stimulated with anti-CD40 antibody or LPS for 30 minutes. Because maximum suppression of cytokine secretion and T-cell stimulation was detected in DCs pretreated with 50 ng/mL IL-10 (Figure 1), this concentration was used here and in subsequent experiments. IL-10 pretreatment inhibited nuclear DNA binding activity of NF-κB in a time-dependent manner (Figure 2). Significant inhibition of DNA binding by nuclear NF-κB, represented by 2 sets of complexes, was detected between 12 and 24 hours of IL-10 pretreatment for LPS or anti-CD40 antibody stimulation (Figure 2A-B). For example, LPS-induced NF-κB DNA binding was reduced 16- and 22-fold on IL-10 pretreatment for 12 and 24 hours, respectively, compared with LPS stimulation alone (Figure 2A). Similar levels of OCT1 DNA binding were observed regardless of the duration of IL-10 pretreatment (Figure 2), demonstrating that suppression of nuclear DNA binding was NF-κB specific.
IL-10 pretreatment of DC inhibits the DNA-binding activity of nuclear NF-κB. DCs were pretreated with 50 ng/mL IL-10 for the indicated times or were left untreated for 24 hours and then were stimulated with (A) 500 ng/mL LPS or (B) 10 μg/mL anti-CD40 antibody for 30 minutes. Nuclear extracts were prepared, and DNA binding to the H2K-specific oligonucleotide probe was analyzed using EMSA. (C) Components of the different NF-κB complexes binding to the H2K probe were determined through supershift analysis. (D), For the kinetics study, DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with 500 ng/mL LPS for the indicated times (in hours). DNA binding was analyzed using EMSA. (E) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times (in hours). (F) Isolated (ex vivo) splenic or cultured bone marrow– derived DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with 500 ng/mL LPS for 30 minutes. Nuclear extracts were prepared, and DNA binding to H2K-specific oligonucleotide probes was measured using EMSA. A double-stranded OCT1 DNA probe was used as an internal control. Data are representative of at least 3 independent experiments. FP indicates free probe.
IL-10 pretreatment of DC inhibits the DNA-binding activity of nuclear NF-κB. DCs were pretreated with 50 ng/mL IL-10 for the indicated times or were left untreated for 24 hours and then were stimulated with (A) 500 ng/mL LPS or (B) 10 μg/mL anti-CD40 antibody for 30 minutes. Nuclear extracts were prepared, and DNA binding to the H2K-specific oligonucleotide probe was analyzed using EMSA. (C) Components of the different NF-κB complexes binding to the H2K probe were determined through supershift analysis. (D), For the kinetics study, DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with 500 ng/mL LPS for the indicated times (in hours). DNA binding was analyzed using EMSA. (E) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times (in hours). (F) Isolated (ex vivo) splenic or cultured bone marrow– derived DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with 500 ng/mL LPS for 30 minutes. Nuclear extracts were prepared, and DNA binding to H2K-specific oligonucleotide probes was measured using EMSA. A double-stranded OCT1 DNA probe was used as an internal control. Data are representative of at least 3 independent experiments. FP indicates free probe.
Using antibodies specific for each Rel family member, supershift analysis demonstrated that complex 1 contained heterodimeric complexes of p50, p65, c-Rel, and RelB, whereas complex 2 was composed of p50 homodimers (Figure 2C). Interestingly, LPS-induced DNA binding of p50/p50 homodimer complexes (complex 2) was significantly inhibited after only 6 hours of IL-10 pretreatment relative to the heterodimeric complexes (Figure 2A). At this point, a 5.7-fold reduction of p50/p50 DNA binding was detected compared with extracts prepared from DCs stimulated with LPS alone.
Further analysis of nuclear extracts prepared from DCs pretreated with IL-10 for 24 hours demonstrated early and persistent inhibition of NF-κB DNA binding after anti-CD40 antibody or LPS stimulation (Figure 2D). Reduced DNA binding was detected with either H2K- (Figure 2D) or IL-12p40–specific oligonucleotide probes (data not shown). For example, IL-10 pretreatment reduced NF-κB DNA binding to the H2K probe from 7.3- to 15.8-fold within 30 minutes to 2 hours of LPS stimulation, relative to extracts prepared from DCs treated with LPS only (Figure 2D). Supershift analysis indicated that binding of the different NF-κB complexes to the H2K probe was uniformly suppressed after 24 hours of IL-10 pretreatment (Figure 2C). In contrast to the suppression observed by IL-10 pretreatment, coincubation with IL-10 and LPS had no significant effect on the level or kinetics of NF-κB DNA binding compared with LPS treatment alone (Figure 2E). Similarly, NF-κB DNA binding induced through anti-CD40 antibody was unaltered by IL-10 cotreatment (data not shown).
To ensure that the effects of IL-10 pretreatment were not intrinsic to bone marrow–derived DCs, DCs were directly isolated from the spleen, and NF-κB DNA binding was assessed after LPS stimulation. Splenic DCs exhibited a typical myeloid-like phenotype characterized by a CD11b+CD11c+CD8α– profile. IL-10 pretreatment of splenic DCs reduced the nuclear DNA binding activity of NF-κB by 7-fold, comparable to that observed for bone marrow–derived DCs (Figure 2F).
These results demonstrate that IL-10 pretreatment of cultured or ex vivo DCs suppresses nuclear DNA binding of NF-κB independent of the type of stimulus or DNA probe. However, NF-κB DNA binding is unaltered when DCs are cotreated with IL-10 and anti-CD40 antibody or LPS. The latter result is consistent with the inability of IL-10 to suppress cytokine secretion on costimulation with anti-CD40 antibody or LPS (Figure 1C).
IL-10 pretreatment inhibits IκB degradation and p65 phosphorylation
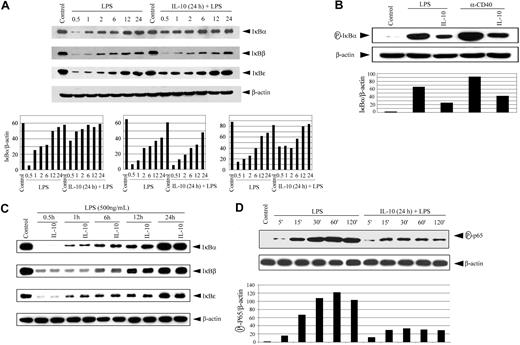
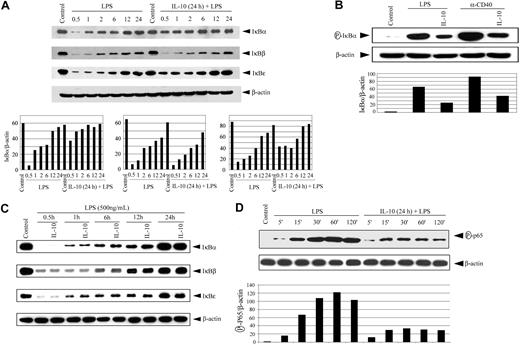
The lack of nuclear DNA binding by NF-κB suggested that IL-10 pretreatment was blocking degradation of the IκB proteins. Accordingly, the status of IκBα, IκBβ, and IκBϵ in cytoplasmic extracts was determined by Western blot analysis using antibodies specific for the respective inhibitory proteins. Degradation of the 3 IκB proteins was readily detected in cytoplasmic extracts prepared from DCs stimulated with LPS (Figure 3A). However, pretreatment with IL-10 for 24 hours significantly inhibited degradation of IκBα and, to a lesser extent, IκBϵ after LPS stimulation. This was most evident during the early kinetics of LPS stimulation (Figure 3A). After 30 minutes of LPS treatment at which maximum IκBα and IκBϵ degradation was observed, IL-10 pretreatment reduced degradation by 7- and 3-fold, respectively. IL-10 pretreatment also inhibited the phosphorylation of IκBα approximately 3-fold after LPS or anti-CD40 antibody stimulation (Figure 3B). Interestingly, LPS-induced degradation of IκBβ was not significantly affected by IL-10 pretreatment (Figure 3A). Furthermore, IκB protein degradation was unaffected when DCs were cotreated with IL-10 and LPS (Figure 3C). These results demonstrate that IL-10 pretreatment of DCs suppresses the degradation, phosphorylation, or both of IκBα and IκBϵ but not of IκBβ.
IL-10 pretreatment of DC inhibits IκB protein degradation, phosphorylation, and p65 phosphorylation. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then were stimulated with 500 ng/mL LPS for the indicated times (in hours). Cytoplasmic extracts were resolved through SDS-PAGE and analyzed by Western blot using an anti-IκBα antibody or on stripping the membrane, antibody specific for IκBβ,IκBϵ,or β-actin. (bottom panels) Corresponding densitometric analyses were determined by measuring the ratio of intensity of the respective IκB proteins to β-actin expression per unit area for a given time and were presented as an arbitrary unit. (B) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then were stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 30 minutes. Cytoplasmic extracts were analyzed by Western blot using an antiphospho-IκBα antibody. The same membrane was reprobed with antiβ-actin antibody (bottom panel). Corresponding densitometric analyses determined as a ratio of intensity of phospho-IκBα to β-actin expression per unit area. (C) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times (in hours). Cytoplasmic extracts were analyzed for IκBα,IκBβ,IκBϵ,or β-actin expression. (D) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for the indicated times. Nuclear extracts were examined through Western blot using antibodies specific for phospho-p65 and on stripping p65 protein or β actin. Densitometric analyses represent the ratio of intensity of phospho-p65 to β-actin expression per unit area and intensity of p65 compared with β-actin. The circled P indicates phospho.
IL-10 pretreatment of DC inhibits IκB protein degradation, phosphorylation, and p65 phosphorylation. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then were stimulated with 500 ng/mL LPS for the indicated times (in hours). Cytoplasmic extracts were resolved through SDS-PAGE and analyzed by Western blot using an anti-IκBα antibody or on stripping the membrane, antibody specific for IκBβ,IκBϵ,or β-actin. (bottom panels) Corresponding densitometric analyses were determined by measuring the ratio of intensity of the respective IκB proteins to β-actin expression per unit area for a given time and were presented as an arbitrary unit. (B) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then were stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 30 minutes. Cytoplasmic extracts were analyzed by Western blot using an antiphospho-IκBα antibody. The same membrane was reprobed with antiβ-actin antibody (bottom panel). Corresponding densitometric analyses determined as a ratio of intensity of phospho-IκBα to β-actin expression per unit area. (C) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times (in hours). Cytoplasmic extracts were analyzed for IκBα,IκBβ,IκBϵ,or β-actin expression. (D) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for the indicated times. Nuclear extracts were examined through Western blot using antibodies specific for phospho-p65 and on stripping p65 protein or β actin. Densitometric analyses represent the ratio of intensity of phospho-p65 to β-actin expression per unit area and intensity of p65 compared with β-actin. The circled P indicates phospho.
LPS has been reported to induce phosphorylation of the p65 Rel homology and trans-activation domains, thereby increasing p65 transcription activity.46-49 To determine whether IL-10 pretreatment also inhibited p65 phosphorylation, nuclear extracts were prepared from LPS-stimulated DCs and were analyzed using Western blot. The antibody was specific for phosphorylated Ser536, found in the trans-activation domain of p65. LPS treatment induced increased phosphorylation of nuclear p65 in a time-dependent manner (Figure 3D). In contrast, IL-10 pretreatment for 24 hours reduced nuclear p65 phosphorylation up to 3.5-fold (Figure 3D). Importantly, reduced phospho-p65 correlated with up to a 3.0-fold decrease in nuclear p65 protein compared with DCs stimulated with LPS only (Figure 3D). These results indicate that IL-10 pretreatment inhibits the phosphorylation and nuclear translocation of p65 in DCs.
IL-10 pretreatment inhibits IKK activity
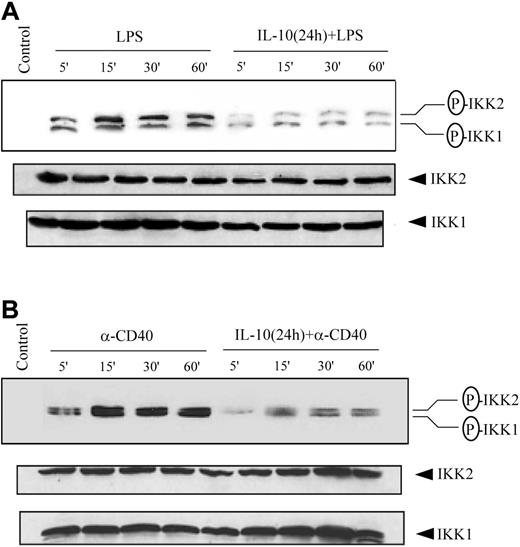
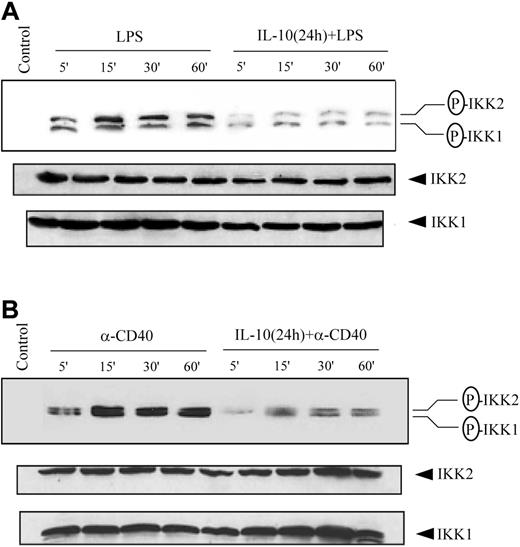
Phosphorylation and subsequent degradation of the IκB proteins is initiated by the IKK complex on appropriate cellular stimulation.12-14,25 Therefore, we investigated the possibility that IL-10 pretreatment inhibited IKK activity. Initially, IKK activation status was assessed through measurements of complex phosphorylation. The IKK complex is activated in part by the phosphorylation of serine residues located in the activation loops of IKK1 (Ser176 and Ser180)50,51 and IKK2 (Ser177 and Ser181).26 Cytoplasmic extracts prepared from DCs pretreated with IL-10 and stimulated with LPS or anti-CD40 antibody were examined through Western blot using antibodies specific for phosphorylated IKK1 Ser180 and IKK2 Ser181. Treatment with either LPS (Figure 4A) or anti-CD40 antibody (Figure 4B) alone induced IKK1 and IKK2 phosphorylation. In contrast, IL-10 pretreatment reduced IKK1 and IKK2 phosphorylation after stimulation with LPS (Figure 4A) or anti-CD40 antibody (Figure 4B). A comparative study with DCs isolated from NOD, NOR, and BALB/c demonstrated comparable inhibition of IKK1 and IKK2 phosphorylation, indicating that suppression of IKK activity in DCs through IL-10 pretreatment was not mouse strain specific (data not shown).
IL-10 pretreatment of DC inhibits phosphorylation of IKK1 and IKK2. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with (A) 500 ng/mL LPS or (B) 10 μg/mL anti-CD40 antibody for the indicated times. Cytoplasmic extracts were prepared, and the expression of phosphorylated IKK was analyzed using specific antiphospho-IKK1 (Ser180) or IKK2 (Ser181) antibody. The same membrane was reprobed with anti-IKK1 and anti-IKK2 antibodies. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
IL-10 pretreatment of DC inhibits phosphorylation of IKK1 and IKK2. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours and then stimulated with (A) 500 ng/mL LPS or (B) 10 μg/mL anti-CD40 antibody for the indicated times. Cytoplasmic extracts were prepared, and the expression of phosphorylated IKK was analyzed using specific antiphospho-IKK1 (Ser180) or IKK2 (Ser181) antibody. The same membrane was reprobed with anti-IKK1 and anti-IKK2 antibodies. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
We also determined whether reduced IKK activation after IL-10 pretreatment also reflected diminished kinase activity. Cultured DCs were incubated with IL-10 or were left untreated, stimulated with anti-CD40 antibody or LPS for varying times, and IKK1 or IKK2 immunoprecipitated from cytoplasmic extracts using corresponding antibodies. IKK activity was assessed by measuring phosphorylation of a GST-IκBα substrate in vitro. IL-10 pretreatment suppressed IKK activity in a time-dependent manner (Figure 5A). For example, IKK activity induced by LPS or anti-CD40 antibody stimulation was inhibited approximately 4- to 5-fold by 12 and 24 hours of IL-10 pretreatment (Figure 5A). Furthermore, IL-10 pretreatment for 24 hours inhibited IKK1 and IKK2 activity (3- to 6-fold) at all times examined after stimulation with LPS (Figure 5B) or with anti-CD40 antibody (Figure 5C). In contrast, IL-10 and LPS cotreatment of DCs had no significant effect on IKK activity compared with treatment with LPS only (Figure 5D). Together these results demonstrate that IL-10 pretreatment influences the NF-κB signaling pathway in DCs by reducing phosphorylation and subsequent activation of IKK1 and IKK2 activity.
IL-10 pretreatment of DCs inhibits IKK activity. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for the indicated times, and then stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 15 minutes. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with (B) 500 ng/mL LPS or (C) 10 μg/mL anti-CD40 antibody for the indicated times. (D) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times. IKK signalosome was immunoprecipitated using anti-IKK1 or anti-IKK2 antibodies. In vitro kinase reactions were performed by preincubating immunoprecipitated protein with GST-IκBα and [γ-32P] ATP. Kinase reactions were analyzed using SDS-PAGE visualized by autoradiography. In all experiments, IKK1 and IKK2 expression was analyzed using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
IL-10 pretreatment of DCs inhibits IKK activity. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for the indicated times, and then stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 15 minutes. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with (B) 500 ng/mL LPS or (C) 10 μg/mL anti-CD40 antibody for the indicated times. (D) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times. IKK signalosome was immunoprecipitated using anti-IKK1 or anti-IKK2 antibodies. In vitro kinase reactions were performed by preincubating immunoprecipitated protein with GST-IκBα and [γ-32P] ATP. Kinase reactions were analyzed using SDS-PAGE visualized by autoradiography. In all experiments, IKK1 and IKK2 expression was analyzed using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
IL-10 pretreatment inhibits Akt activation
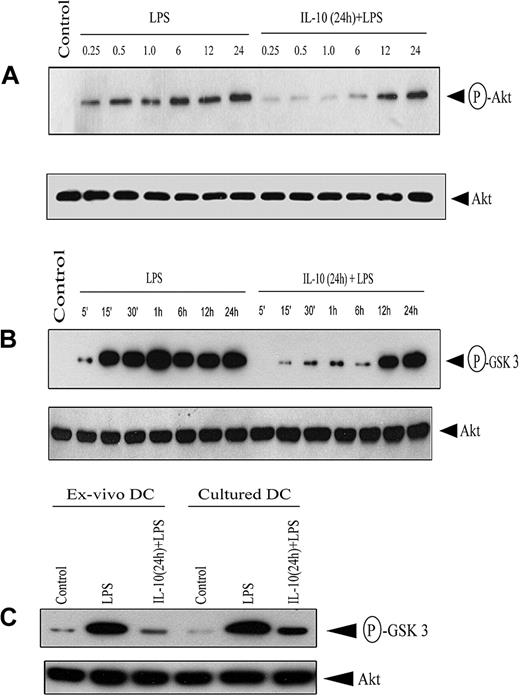
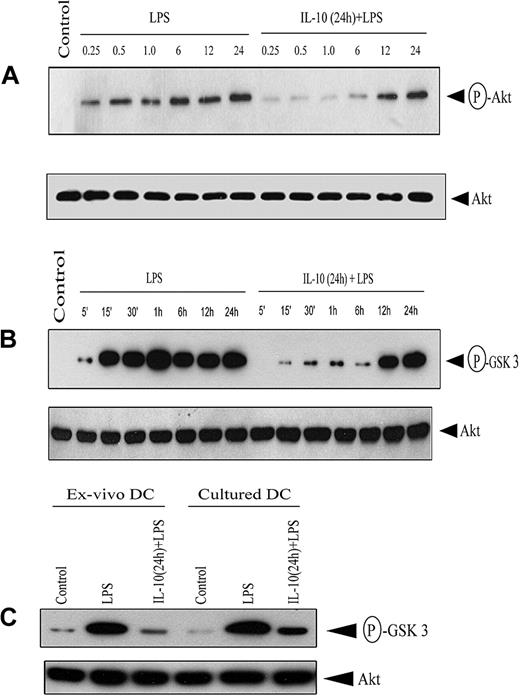
Akt regulates NF-κB in an inducer and a cell-specific manner.33-37 The effect of IL-10 pretreatment on Akt activation was assessed by measuring phosphorylation of the C-terminus Ser473. Western blot analysis using antibody specific for phospho-Ser473 demonstrated that LPS stimulation induced Akt phosphorylation (Figure 6A). In contrast, IL-10 pretreatment markedly inhibited LPS-induced phosphorylation 3- to 5-fold, though phosphorylated Akt was detected after 12 hours of LPS stimulation (Figure 6A). Notably, Akt protein expression was comparable between the 2 treatment groups (Figure 6A).
IL-10 pretreatment of DC inhibits Akt phosphorylation. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for the indicated times (in hours). Cytoplasmic extracts were resolved through SDS-PAGE and analyzed by Western blot using an antiphospho-Akt (Ser473) antibody or, on stripping the membrane, antibody specific for Akt. (B) Under similar conditions, DCs were stimulated with 500 ng/mL LPS for the indicated times, and in vitro Akt kinase activity was measured by assessing phosphorylation of the GSK-3 fusion protein after SDS-PAGE resolution and visualization by autoradiography. (C) Ex vivo splenic DCs and cultured bone marrow–derived DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for 30 minutes. In vitro Akt kinase activity was measured. In all experiments, Akt protein expression was determined using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
IL-10 pretreatment of DC inhibits Akt phosphorylation. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for the indicated times (in hours). Cytoplasmic extracts were resolved through SDS-PAGE and analyzed by Western blot using an antiphospho-Akt (Ser473) antibody or, on stripping the membrane, antibody specific for Akt. (B) Under similar conditions, DCs were stimulated with 500 ng/mL LPS for the indicated times, and in vitro Akt kinase activity was measured by assessing phosphorylation of the GSK-3 fusion protein after SDS-PAGE resolution and visualization by autoradiography. (C) Ex vivo splenic DCs and cultured bone marrow–derived DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with 500 ng/mL LPS for 30 minutes. In vitro Akt kinase activity was measured. In all experiments, Akt protein expression was determined using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
Next, Akt kinase activity was measured in DC lysates. LPS stimulation alone induced persistent Akt kinase activity based on in vitro phosphorylation of a GSK-3 substrate (Figure 6B). In contrast, IL-10 pretreatment significantly reduced Akt kinase activity up to 6 hours after LPS stimulation (Figure 6B), consistent with Akt phosphorylation (Figure 6A). Densitometric analysis indicated 6- to 8-fold inhibition of Akt kinase activity in IL-10–pretreated DCs, whereas Akt protein expression was comparable between the 2 treatment groups (Figure 6B). Notably, IL-10 pretreatment of isolated, splenic DCs also resulted in an 8-fold reduction of LPS-induced Akt kinase activity (Figure 6C). These results indicate that Akt activation and kinase activity are suppressed early after IL-10 pretreatment in cultured and isolated DCs.
PI3K inhibition suppresses the activation of Akt, IKK, and NF-κB and inhibits the APC function of DCs
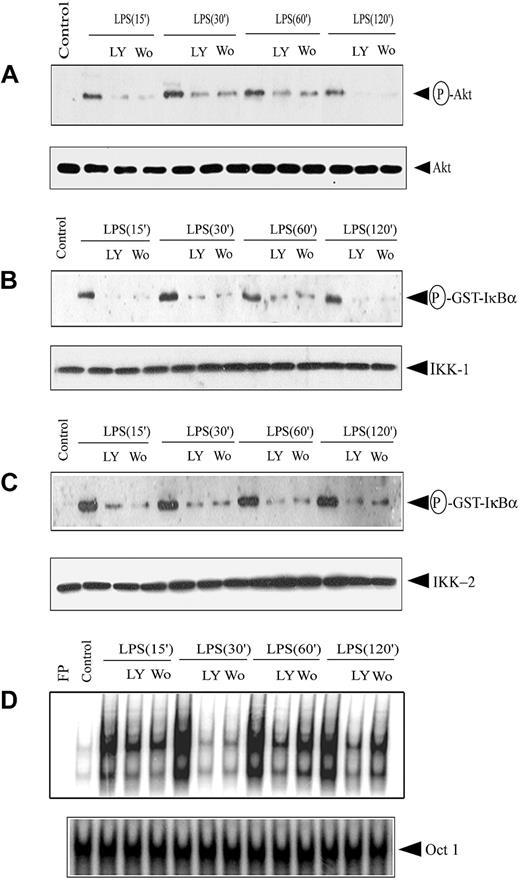
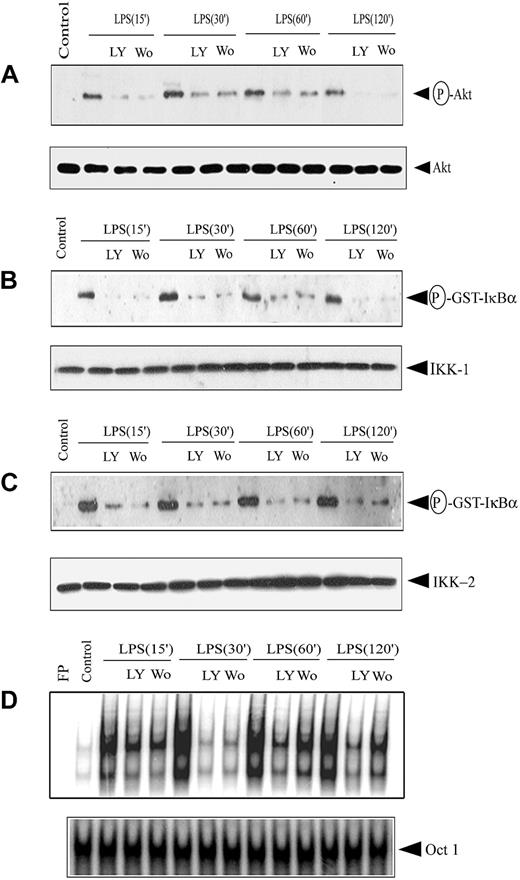
Because upstream PI3K activation is required for Akt phosphorylation, PI3K inhibitor effects on Akt and subsequent IKK and NF-κB activation were investigated. DCs were treated 1 hour before LPS stimulation with PI3K inhibitor LY294002 (50 mM) or wortmannin (200 nM). Incubation with the PI3K inhibitors resulted in marked inhibition of Akt phosphorylation (3- to 9-fold) at all time points examined after LPS stimulation (Figure 7A). No significant difference in Akt protein expression, however, was observed between the 2 treatment groups (Figure 7A). Using 200 nM wortmannin and 50 mM LY294002, maximum inhibition of Akt phosphorylation was obtained with more than 94% viability of DCs, as determined by flow cytometry.
PI3K inhibitors block IKK activity and DNA binding of nuclear NF-κB. DCs were pretreated with either 200 nM wortmannin (Wo) or 50 μM LY294002 (LY) for 1 hour or left untreated, and then stimulated with 500 ng/mL LPS for the indicated times. (A) Cytoplasmic extracts were prepared, and phosphorylated Akt expression was analyzed using an antiphospho-Akt (Ser473)–specific antibody. The same membrane was reprobed with an anti-Akt antibody. IKK signalosome was immunoprecipitated using (B) anti-IKK1 or (C) anti-IKK2 antibodies, and in vitro kinase activity was measured as described. In all experiments, protein expression of IKK1 and IKK2 was analyzed through Western blot. (D) Nuclear extracts were prepared, and DNA binding to the H2K-specific oligonucleotide probe was measured using EMSA. A double-stranded OCT1 DNA probe was used as an internal control. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
PI3K inhibitors block IKK activity and DNA binding of nuclear NF-κB. DCs were pretreated with either 200 nM wortmannin (Wo) or 50 μM LY294002 (LY) for 1 hour or left untreated, and then stimulated with 500 ng/mL LPS for the indicated times. (A) Cytoplasmic extracts were prepared, and phosphorylated Akt expression was analyzed using an antiphospho-Akt (Ser473)–specific antibody. The same membrane was reprobed with an anti-Akt antibody. IKK signalosome was immunoprecipitated using (B) anti-IKK1 or (C) anti-IKK2 antibodies, and in vitro kinase activity was measured as described. In all experiments, protein expression of IKK1 and IKK2 was analyzed through Western blot. (D) Nuclear extracts were prepared, and DNA binding to the H2K-specific oligonucleotide probe was measured using EMSA. A double-stranded OCT1 DNA probe was used as an internal control. Data are representative of at least 3 independent experiments. The circled P indicates phospho.
We investigated effects of the PI3K inhibitors on IKK activity and NF-κB activation. In vitro IKK1 and IKK2 activity was reduced 3- to 7-fold and 4- to 8-fold, respectively, by treatment with wortmannin or LY294002 before LPS stimulation (Figure 7B-C). No significant difference was observed in the level of IKK1 or IKK2 protein in extracts used to measure kinase activity (Figure 7B-C). Kinetic analysis of LPS stimulation indicated reduced NF-κB DNA binding activity at all time points tested when DCs were pretreated with wortmannin or LY294002 (Figure 7D). DNA binding of NF-κB was reduced 2- to 6-fold under the respective conditions (Figure 7D). Similar levels of OCT1 DNA binding were observed, demonstrating that suppression of nuclear DNA binding was NF-κB specific (Figure 7D). Collectively, these results indicate that inhibited PI3K activity suppresses downstream activation of Akt, IKK, and NF-κB.
Finally, we determined the effects of PI3K inhibition on the capacity of cultured DCs to secrete proinflammatory cytokines after LPS stimulation and to stimulate T cells. Pretreatment with 50 mM LY294002 reduced the levels of LPS-induced IL-12p70 and TNF-α secretion by 6- and 4-fold, respectively (Table 2). Similarly, pretreating DCs with 200 nM wortmannin inhibited the secretion of IL-12p70 and TNF-α by 4-fold after LPS stimulation (Table 2). Pretreatment with LY294002 or wortmannin also significantly suppressed the stimulatory capacity of peptide-pulsed DCs to activate NOD.CL4 CD8+ T cells, as determined by IL-2 secretion (Table 3). These results demonstrate that pretreatment with PI3K inhibitors significantly suppresses the APC function of DCs.
Discussion
A potent inhibitor, IL-10 affects the expression of proinflammatory molecules and the function of a number of cell types, such as T cells, monocytes, macrophages, and nonimmune cells.52 In addition, IL-10 effectively blocks the maturation and APC function of myeloid-derived DCs.3-11 In agreement with these findings, our pretreatment of immature DCs with IL-10 effectively suppressed IL-12p70 and TNF-α secretion after stimulation with LPS or anti-CD40 antibody (Figure 1A-B) and the capacity to stimulate CD8+ T cells (Figure 1D). The latter correlated with a limited increase of surface CD40 and CD86 (and IAg7) expression by IL-10–pretreated DCs stimulated with LPS (Table 1).
The key molecular events involved in the immunoregulation of DCs by IL-10 have yet to be delineated. Different mechanisms associated with IL-10–mediated immunoregulation have been reported that are dependent on the cell type or the signaling pathway engaged. For example, IL-10 suppresses TNF-α gene expression in LPS-stimulated human macrophages through transcriptional and posttranscriptional events independently of NF-κB.44 Furthermore, IL-10 inhibits LPS-induced activation of macrophages by eliciting expression of suppressor of cytokine signaling 3.53 On the other hand, IL-10–mediated suppression of NF-κB activation has been observed in a variety of cell types, and different mechanisms modulating the pathway have been reported.40-43 For instance, IL-10 has been shown to selectively up-regulate the expression of IκBα in brain astrocytes to block NF-κB–dependent expression of the nitric oxide synthase-2 gene.54 Evidence that the NF-κB pathway is also targeted in DCs comes from 2 studies showing that IL-10 prevents nuclear translocation and DNA binding of RelB in human DC precursors or immature DCs, respectively.9,10 We and others have reported that blockade of NF-κB activation in general significantly affects the maturation and APC function of DCs.19,21-23 NOD DCs transfected with a vector encoding a modified IκBα molecule that inhibits NF-κB activation exhibit a phenotype analogous to NOD DCs treated with IL-10, namely, reduced levels of IL-12p70 and TNF-α secretion on stimulation and impaired ability to stimulate naive T cells.22,23 The current study provides insight into the molecular basis for IL-10–mediated immunoregulation of immature DCs by demonstrating that the NF-κB pathway is inhibited through the suppression of IKK activity.
Consistent with the suppression of IKK activity, various downstream events associated with NF-κB activation were inhibited by IL-10 pretreatment. DNA binding of nuclear NF-κB was blocked independently of the stimulus or of the oligonucleotide probe used for EMSA through IL-10 pretreatment (Figure 2). Notably, the kinetics of IL-10–mediated suppression of NF-κB DNA binding (Figure 2A-B) and IKK activity (Figure 5A) coincided. For example, maximum inhibition of each event was detected after 12 to 24 hours of IL-10 pretreatment. Supershift analysis also demonstrated that under optimal conditions of IL-10 pretreatment, consisting of 24-hour incubation with 50 ng/mL IL-10, DNA binding of the different NF-κB components was uniformly suppressed (Figure 2C). However, DNA binding of p50/p50 homodimers compared with heterodimeric complexes was markedly reduced after 6 hours of IL-10 pretreatment (Figure 2A), a pretreatment time found to be suboptimal for all other parameters examined. This latter observation indicates that sensitivity to IL-10 and its effects on DNA binding can vary among the NF-κB complexes. Wang et al40 demonstrated that IL-10 may inhibit proteasomal processing of the p100 and p105 subunits of NF-κB, thereby blocking synthesis of p52 and p50, respectively. Accordingly, IL-10 may also affect NF-κB signaling by blocking p105 phosphorylation and subsequent processing.
The lack of DNA binding by nuclear NF-κB correlated with diminished nuclear translocation of p65 (Figure 3D). This result is compatible with reduced IKK-mediated phosphorylation of the IκB proteins, resulting in persistent retention of NF-κB complexes in the cytoplasm. Indeed, IL-10 pretreatment reduced phosphorylation and degradation of IκBα and IκBϵ after anti-CD40 antibody or LPS stimulation (Figure 3A-B). Surprisingly, IL-10 pretreatment failed to prevent the degradation of IκBβ after LPS (or anti-CD40 antibody) treatment (Figure 3A), suggesting that IL-10 differentially affects IκB protein degradation. Furthermore, LPS-induced phosphorylation of p65 Ser536 was effectively suppressed by IL-10 pretreatment (Figure 3D). Recently, Yang et al49 reported that phosphorylation of Ser536, located in the trans-activation domain of p65, was dependent on IKK2 activity. Therefore, IL-10–mediated suppression of IKK may directly reduce the transcription function of p65 containing NF-κB complexes.
The current finding that IL-10 pretreatment inhibited IKK phosphorylation and activity agrees with earlier work by our group.43 IL-10 treatment was found to suppress TNFα–induced IKK activity in vitro in the human monocytic cell line THP-1. Interestingly, the suppressive effect was observed in THP-1 cells with either no or a brief (5-minute) pretreatment period with IL-10. In contrast, IKK activity (Figure 5A) and subsequent NF-κB activation (Figure 2A-B) were effectively suppressed in immature DCs only after 12- to 24-hour pretreatment with IL-10. This result is consistent with reports demonstrating that the effects of IL-10 on the maturation of immature DCs and on APC function are typically observed after 24 hours of pretreatment.3-11 These observations indicate that in immature DCs, inhibition of the NF-κB pathway by IL-10 requires de novo protein synthesis. Moreover, the difference between THP-1 cells and DCs regarding the need for IL-10 pretreatment suggests that cell-specific mechanisms are involved in the suppression of IKK activity, even for the same signaling pathway. In view of its dominant role activating NF-κB in response to proinflammatory signals,26-28 a block in IKK2 activity likely accounts for the suppressive effects of IL-10, though a recent study24 reports that NF-κB activation in DCs by CD40 signaling is IKK2 dependent, whereas LPS stimulation is not.
The effects of IL-10 on IKK activity are likely to be mediated by multiple mechanisms. The transient effect of IL-10 on Akt activation (Figure 6A-B) supports this hypothesis. IL-10 pretreatment, for example, may interfere with the assembly or maintenance of an active IKK complex. Oligomerization of IKK-γ has been shown to be essential for proper assembly and autophosphorylation of the IKK complex.55,56 IL-10 may inhibit the function or expression of proteins such as chaperones, which are involved in assembling the heteromeric complex and maintaining the conformation between IKK2 and the IKK-γ scaffold necessary for efficient IKK2 autophosphorylation.
As an initial effort to define the mechanism(s) by which IL-10 inhibits IKK activity in immature DCs, a role for the PI3K signaling pathway was investigated. We and others have demonstrated that NF-κB activation can be mediated through the PI3K/Akt pathway.31-39 Furthermore, a recent study57 reported that the survival of LPS-stimulated, monocyte-derived human DCs is markedly reduced by the inhibition of PI3K and the downstream phosphorylation of Akt. Accordingly, LPS-mediated phosphorylation (Figure 6A) and in vitro kinase activity of Akt (Figure 6B) were significantly inhibited by IL-10 pretreatment, suggesting that the PI3K pathway in DCs is indeed a target of IL-10. Consistent with this conclusion are findings that pretreatment with wortmannin or LY294002 inhibited Akt phosphorylation (Figure 7A), in vitro IKK1 and IKK2 activity (Figure 7B-C), and NF-κB DNA binding induced by LPS (Figure 7D). Moreover, the PI3K inhibitors significantly suppressed LPS-induced secretion of IL-12p70 and TNF-α (Table 2) and the capacity of DCs to stimulate naive CD8+ T cells (Table 3). Collectively, these results demonstrate that the PI3K signaling pathway has a key role in regulating the activation and APC function of DCs. Interestingly, the effects of IL-10 on the PI3K pathway and, in turn, the role of PI3K in NF-κB activation appear to be dependent on cell type. In contrast to our results, IL-10 stimulates PI3K and Akt activation in murine progenitor myeloid cells.58 Furthermore, PI3K suppression enhances LPS-induced NF-κB activation in human monocytes.39 Ongoing efforts focus on defining the mechanism(s) by which the PI3K pathway is suppressed by IL-10 in DCs.
The effects of IL-10 on bone marrow–derived DCs were also evident for myeloid-like DCs isolated directly from the spleen. Ex vivo IL-10 pretreatment of splenic DCs effectively inhibited NF-κB DNA binding (Figure 2F) and Akt kinase activity (Figure 6C) induced by LPS stimulation to an extent similar to that observed in cultured DCs. These findings indicate that the inhibitory effects of IL-10 are applicable to myeloid-like DCs in general.
In summary, this work demonstrates that IL-10 effectively immunoregulates the APC function of DCs through suppression of IKK activity and subsequent NF-κB activation. Inhibition of NF-κB activation is in part the consequence of IL-10 targeting the PI3K pathway.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2003-12-4302.
Supported by National Institute of Dental and Craniofacial Research grant 1-P60-DE 13079.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 5. IL-10 pretreatment of DCs inhibits IKK activity. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for the indicated times, and then stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 15 minutes. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with (B) 500 ng/mL LPS or (C) 10 μg/mL anti-CD40 antibody for the indicated times. (D) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times. IKK signalosome was immunoprecipitated using anti-IKK1 or anti-IKK2 antibodies. In vitro kinase reactions were performed by preincubating immunoprecipitated protein with GST-IκBα and [γ-32P] ATP. Kinase reactions were analyzed using SDS-PAGE visualized by autoradiography. In all experiments, IKK1 and IKK2 expression was analyzed using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-12-4302/6/m_zh80160465210005.jpeg?Expires=1770461046&Signature=p7EBVpM~Y-JoXVVi~pawwO9Z7vOdbKvm52y~d-mbvNyroL9~hSrmEABcXOJyP3Zy-dNwEqFoweSCMfUeNGMCJmkzc2v0709EzkgMeegMKBvhfRAiv2dtOBiJTnuIexu9wGGtWGyHSmGGmJDwTaVSDjsicFgwmBMv5nsyCsNuCp8tMM6PexJh5Q~xz3doTvruYJscR6Rsi1-nfub-LqPo~1V6aFBBOzr-RpPeeGAWfoAznDHntrDhOBFKgpoJFdji0d4c7w5JyDY-xMeyG~LHqCr8KHcI-4yjMjjl3adgLnBvxmX7j~2ujKGCIDrQqoUr6HajMJaPBl3UsMMLs8-92g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. IL-10 pretreatment of DCs inhibits IKK activity. (A) DCs were pretreated with 50 ng/mL IL-10 or left untreated for the indicated times, and then stimulated with 500 ng/mL LPS or 10 μg/mL anti-CD40 antibody for 15 minutes. DCs were pretreated with 50 ng/mL IL-10 or left untreated for 24 hours, and then stimulated with (B) 500 ng/mL LPS or (C) 10 μg/mL anti-CD40 antibody for the indicated times. (D) DCs were costimulated with IL-10 (50 ng/mL) and LPS (500 ng/mL) or treated with LPS alone for the indicated times. IKK signalosome was immunoprecipitated using anti-IKK1 or anti-IKK2 antibodies. In vitro kinase reactions were performed by preincubating immunoprecipitated protein with GST-IκBα and [γ-32P] ATP. Kinase reactions were analyzed using SDS-PAGE visualized by autoradiography. In all experiments, IKK1 and IKK2 expression was analyzed using Western blot. Data are representative of at least 3 independent experiments. The circled P indicates phospho.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-12-4302/6/m_zh80160465210005.jpeg?Expires=1770509508&Signature=oh9mJvmZapmrydlnPx6Arn-Tz2q-hsF2ahvxr9110PMHUcqU6tQRy36NaAzPbCv3ZPiIBGG9-hAApzFIyAm4E4UskyGe0K2cDTKml0KB83ZpNnPbOpvxJKshZ8c-cF~UbOrI3YNTAvKVQAenLK2fpBzmW9GNEcR1MwRXWW35pvyOBvDEkztIYinS2XxNSXp5htSsQt2Qn4x94XbzvX~mxVFf8jalKkYcbh~ipr8RCYPvjTYmFIiTcRfoaPD3a5nC6rPjbGIcnInL2KbysOuqwNT1sGnpm520NQsHnQMiXGgt7AkEPUoWpkFx~02OuyLvCqOImO8npi9CTOhh9ecV-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)