Abstract
Imatinib mesylate (STI571, imatinib) inhibited DNA synthesis in primary human T cells stimulated with allogeneic mature dendritic cells or phytohemagglutinin (PHA) but did not induce apoptosis. The values for the concentration that inhibits 50% (IC50) of T-cell proliferation stimulated by dendritic cells and PHA were 3.9 μM and 2.9 μM, respectively, that is, within the concentration range found in patients treated with imatinib mesylate. Interestingly, imatinib mesylate did not inhibit expression of T-cell activation markers CD25 and CD69, although it reduced the levels of activated nuclear factor-κB (NF-κB) and changed phosphorylation or protein levels of Lck, ERK1/2, retinoblastoma protein, and cyclin D3. When T cells were washed free of imatinib mesylate, they proliferated in response to PHA, demonstrating that inhibition is reversible. Treatment with imatinib mesylate led to accumulation of the cells in G0/G1 phase of the cell cycle. The in vitro observations were confirmed in vivo in a murine model of delayed-type hypersensitivity (DTH). In mice treated with imatinib mesylate, DTH was reduced in comparison to sham-injected controls. However, the number of splenic T cells was not reduced showing that, similarly to in vitro observations, imatinib mesylate inhibited T-cell response, but did not cause apoptosis. These findings indicate that long-term administration of high-dose imatinib mesylate might affect immunity.
Introduction
Imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) is a reversible tyrosine kinase inhibitor effective in treatment of chronic myelogenous leukemia1 (CML), gastrointestinal stromal tumors,2,3 eosinophilic disorders,4 and systemic mast cell disease5 and has been tried in several other diseases.6,7 The drug binds preferentially to adenosine triphosphate (ATP)–binding sites of the c-kit protooncogene product, platelet-derived growth factor receptor (PDGF-R), and Abelson kinase (c-ABL)8-10 impeding the ensuing signal transduction (for a review, see Savage and Antman11 ). Inhibition of the ABL portion of the BCR-ABL fusion protein, pathognomonic of CML,12 is the basis of imatinib mesylate efficacy in inducing hematologic and cytogenetic remissions in CML.1,13 Inhibition of c-KIT is considered critical for imatinib mesylate effects in gastrointestinal stromal tumors14 and mast cell disease.5
In our efforts to develop immunotherapy for CML,15,16 we studied the effects of imatinib mesylate on the development of CML-specific immunity on administration of autologous mature dendritic cells (DCs). Consequently, we measured the effects of imatinib mesylate on in vitro correlates of immunity.17 At concentrations of 5 μM, achieved in the serum of patients on the standard therapeutic dose of 400 mg/d,1 the drug was cytostatic, it arrested transit into the S phase of the cell cycle, and it impeded in vitro proliferation of human normal and leukemic T cells. We analyzed the effects of imatinib mesylate on the activity of intracellular signal transduction molecules associated with the control of T-cell function. The drug diminished phosphorylation of Lck, ERK 1/2, and Rb proteins and reduced the levels of cyclin D3 and of activated nuclear transcription factor κB (NF-κB). In addition, imatinib mesylate inhibited delayed-type hypersensitivity (DTH) in mice without affecting T-cell numbers, indicating that the drug inhibits T-cell response but does not induce apoptosis.
Materials and methods
Cells and reagents
T cells were isolated by negative immunoadsorption (Pan T kit; Miltenyi Biotec, Auburn, CA), pooled from 3 or more normal donors and cryopreserved until use. On thawing, the cells were incubated in X-VIVO 15 medium (BioWhittaker, Walkersville, MD) supplemented with 1.0% human AB serum (Sigma, St Louis, MO), in a humidified atmosphere of 5.0% CO2 at 37°C. For experiments longer than 1 day, the medium contained 1.0% penicillin/streptomycin solution (Sigma) as well. Human acute T-cell leukemia cells Jurkat (ATCC TIB-152), acute T lymphoblastic leukemia cells CCRF-CEM (ATCC CCL-119), and hematopoietic malignant K-562 cells (ATCC CCL-243), were obtained from American Type Culture Collection (Manassas, VA) and cultured under the same conditions. Allogeneic DCs were derived from CD14+ cells and cultured with T cells as previously described.16 Imatinib mesylate (Novartis) was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 mM. The stock solution was stored at –20°C until use, diluted to the final concentration in X-VIVO 15 medium, and added to cells immediately.
Cell proliferation assays
T cells, 1 × 105 in 200 μL/well of 96-well microtiter plates (Corning, Corning, NY), were stimulated with DCs or phytohemagglutinin M (PHA-M; 10 μg/mL, Sigma) or PDGF or both (R&D Systems, Minneapolis, MN) in a final volume of 0.2 mL/well. The cells were incubated with graded concentrations of imatinib mesylate, incubated for 4 days, pulsed with 2.0 μCi (0.074 MBq) tritiated thymidine (Amersham, Arlington Heights, IL) per well, and incubated for further 12 hours when they were harvested, and incorporated radioactivity was then quantified.16
Flow cytometry
We stained T cells with CD25-specific monoclonal antibody conjugated to fluorescein (clone M-A251; PharMingen, San Diego, CA) or CD69-specific monoclonal antibody conjugated to phycoerythrin (clone HP-4B3; Ancell, Bayport, MN) and analyzed them by flow cytometry. Cell cycle distribution was measured by flow cytometry in T cells stained with propidium iodide18 and analyzed by ModFit LT software (Verity Software, Topsham, ME). Apoptosis was quantified by annexin V binding (Annexin-V-FLUOS staining kit; Roche Diagnostics, Indianapolis, IN).
Caspase 3 activity
The cells were suspended at 1.0 × 106 cells/mL in 10.0% fetal bovine serum (FBS; Cellgro, Herndon, VA) and 1.0% penicillin/streptomycin (Sigma). Aliquots of 1 mL were placed in 24-well flat-bottom cell culture plates (Corning) and incubated at 37° C in humidified 5.0% CO2 in the presence or absence of PHA (10.0 μg/mL) plus interleukin 2 (IL-2; 500 U/mL; R&D Systems) and imatinib mesylate. After a 3-day incubation, cells were collected by centrifugation, washed, and treated with the fluorogenic caspase 3 substrate PhiPhiLux-G1D2 (OncoImmunin, Gaithersburg, MD) according to the manufacturer's instructions. Fluorescence was quantified by flow cytometry (excitation at 488 nm) and the relative amounts of activated caspase 3 determined from a calibration curve.
Protein phosphorylation analysis by Western blotting
T cells (2 × 106/mL in X-VIVO 15 medium with 1.0% human AB serum) were incubated with imatinib mesylate, 10 μM, or an equivalent volume of DMSO at 37° C for 1 hour. Then PHAwas added to a final concentration of 10 μg/mL and 6 mL of the cell suspension per well was plated in 6-well tissue culture plates. At various intervals following plating, cells from one well were collected, centrifuged, and lysed in 0.5 mL Laemmli sample buffer followed by heating at 100° C for 5 minutes.19 Lysate aliquots, each equivalent to 0.6 × 106 cells, were resolved by electrophoresis on 7.5% or 10% polyacrylamide gels. Electrophoresed proteins were electroblotted onto Trans-Blot nitrocellulose transfer membranes (Bio-Rad Laboratories, Hercules, CA) in 25 mM Tris (tris(hydroxymethyl)aminomethane), 192 mM glycine buffer, pH 8.3, at 125 V and 4° C for 2 hours. The membrane was incubated in 5.0% nonfat powdered milk in 0.1 M NaCl, 20 mM Tris, 0.1% Tween-20, pH 7.4, (TBS/T) for 1 hour. Following incubation, the membrane was washed with TBS/T and incubated with the primary antibody at 4° C overnight. (All washes included triplicate 5-minute incubations in TBS/T with agitation. All antibodies were used at dilutions recommended by the manufacturers.) The membrane was washed, incubated with the secondary antibody for 1 hour at room temperature, and washed again. Thereafter, the membrane was incubated in the SuperSignal West Pico chemiluminescent horseradish peroxidase (HRP) substrate (Pierce Biotechnology, Rockford, IL) for 5 minutes, exposed to X-OMAT AR film (Eastman Kodak, Rochester, NY) that was developed by an X-OMAT developer (Eastman Kodak). Optical density of individual bands on the film was registered by a ChemiDoc image acquisition system (Bio-Rad Laboratories) and quantified by Quantity One 1-D analysis software (Bio-Rad Laboratories).
For detection of proteins and phosphoproteins we used monoclonal antibodies specific for β-actin (as control of protein loading; Novus Biologicals, Littleton, CO), cyclin D3 (Cell Signaling Technology, Beverly, MA), and Abelson kinase (BD Biosciences, San Diego, CA). In addition, from Cell Signaling Technology we obtained immunopurified polyclonal rabbit antibodies specific for c-Kit, c-Kit Y719-PO4, Lck, Lck Y505-PO4, Erk1/2 T202-PO4/Y204-PO4, Rb S780, and Rb S807/811-PO4. Polyclonal goat antibodies specific for rabbit IgG and mouse IgG, both coupled to HRP, were from Santa Cruz Biotechnology (Santa Cruz, CA).
Measuring NF-κB activation by ELISA
T cells were suspended in serum-free medium at 6.0 × 106 cells/mL, plated in 6-well plates, and rested overnight. Following a 1-hour incubation at 37° C, imatinib mesylate was added followed by the addition of PHA (to the final concentration of 10 μg/mL) 1 hour later. Thereafter, the cells were incubated for an additional 4 hours, resuspended by pipetting, and collected by centrifugation. Nuclear extracts were prepared according to Pettit et al.20 The level of activated NF-κB in extracts was assessed in a chemiluminescence-detected enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's directions (Oxford Biomedical Research, Oxford, MI). Luminescence was quantified by a ChemiDoc system (Bio-Rad Laboratories). Protein concentration was measured by the Bio-Rad DC Assay (Bio-Rad Laboratories).
DTH
The effect of imatinib mesylate on DTH was quantified with the permission of the Mayo Clinic Institutional Animal Care and Use Committee and performed as described by Sunday et al21,22 and Luo and Dorf.23 Groups of 5 female B6AF1 mice (Jackson Laboratories, Bar Harbor, ME), 8 to 12 weeks old, received intraperitoneal injections of imatinib mesylate, 50 mg/kg in 10% DMSO, for 21 consecutive days. In addition, groups of similar mice received an equivalent amount of DMSO alone (sham injected). Mice were primed on day 14 by a subcutaneous injection in the flank of 50 μL 4-hydroxy-3-nitrophenyl acetyl O-succinimide ester (NP-O-Su; Biosearch Technologies, Novato, CA) dissolved in phosphate-buffered saline (PBS), pH 7.8, at 7.0 g/100 mL. Six days after priming, mice received injections of the challenge dose of 25 μL NP-O-Su (2.0 g/100 mL) into the right footpad and of 25 μL PBS into the left. Footpad thickness was measured 24 hours later by a digital micrometer.
We prepared single-cell suspensions from spleens of these mice by meshing individual spleens through metal sieves and lysing red blood cells in lysis buffer (1.0 mL/spleen; Sigma) for 5 minutes at room temperature. Thereafter, cells were incubated with anti-CD3 and anti-CD8 antibody (BD Biosciences, Mountain View, CA) for 20 minutes at 4° C, washed, and fixed with 1.0% paraformaldehyde. Flow cytometry was performed with a FACScan flow cytometer and CellQuest software (both BD Biosciences).
Statistics
All experiments were repeated 2 to 5 times with qualitatively the same outcome. The probability that the mean values of 2 experimental groups were identical was tested by 2-tailed Student t test for paired samples. The level of significance was set at a P value of .05. Where applicable, data are reported as the mean ± SD.
Results
Imatinib mesylate inhibits proliferation of human primary T cells and T-cell lines
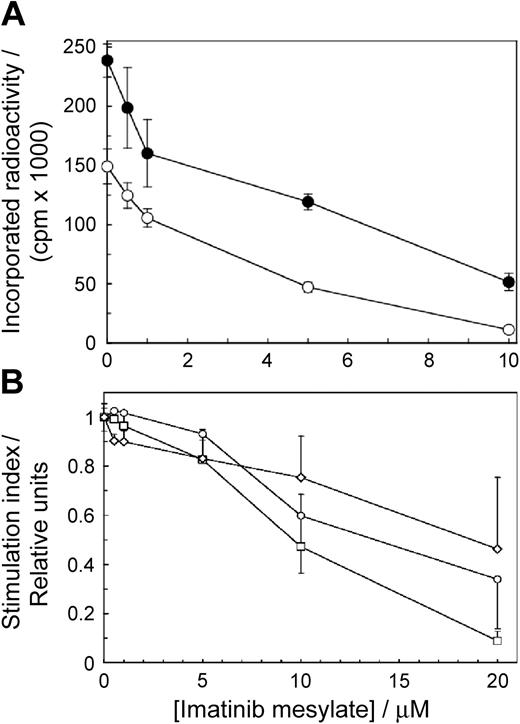
To determine if imatinib mesylate affects T-cell proliferation, we stimulated the cells with allogeneic mature DCs or PHA in the presence of imatinib mesylate. The drug inhibited T-cell proliferation as a function of concentration (Figure 1A). The effects were significant at 0.5 μM imatinib mesylate for the cells stimulated by DCs (P = .002) and at 1.0 μM imatinib mesylate for the cells stimulated with PHA (P = .00004). The IC50 values for imatinib mesylate–inhibited T-cell proliferation stimulated by DCs and PHA were 3.9 μM and 2.9 μM, respectively. Thus, imatinib mesylate arrested T-cell proliferation in a dose-dependent manner at concentrations akin to those achieved in the serum of patients receiving standard imatinib mesylate therapy of 400 mg daily.1 Because the effects of PHA and DCs were similar, for further experiments we selected PHA to simplify interpretation of the results obtained by electrophoresis and Western blotting.
Imatinib mesylate inhibits proliferation of primary human T cells and T-cell lines. (A) Proliferation of 1.5 × 107 T cells/2.5 mL was stimulated with allogeneic mature DCs (○) or PHA (•) and measured as a function of imatinib mesylate concentration. The cells were incubated with imatinib mesylate for 96 hours. Then tritiated thymidine was added for 12 hours when the cells were harvested and incorporated radioactivity was measured. Differences between positive controls and imatinib mesylate–treated cells were apparent at 0.5 μM for the cells stimulated with DCs (P = .002) and at 1.0 μM for cells stimulated with PHA (P = .00004). (B) Human T-cell lines CCRF-CEM (○) and Jurkat (□) and CML cell line K562 (⋄) were incubated in graded concentrations of imatinib mesylate for 18 hours and evaluated as in panel A. The results are expressed as stimulation indices (mean value ± SD of radioactivity incorporated in the presence of the drug divided by the mean value ± SD of radioactivity incorporated in the absence of the drug).
Imatinib mesylate inhibits proliferation of primary human T cells and T-cell lines. (A) Proliferation of 1.5 × 107 T cells/2.5 mL was stimulated with allogeneic mature DCs (○) or PHA (•) and measured as a function of imatinib mesylate concentration. The cells were incubated with imatinib mesylate for 96 hours. Then tritiated thymidine was added for 12 hours when the cells were harvested and incorporated radioactivity was measured. Differences between positive controls and imatinib mesylate–treated cells were apparent at 0.5 μM for the cells stimulated with DCs (P = .002) and at 1.0 μM for cells stimulated with PHA (P = .00004). (B) Human T-cell lines CCRF-CEM (○) and Jurkat (□) and CML cell line K562 (⋄) were incubated in graded concentrations of imatinib mesylate for 18 hours and evaluated as in panel A. The results are expressed as stimulation indices (mean value ± SD of radioactivity incorporated in the presence of the drug divided by the mean value ± SD of radioactivity incorporated in the absence of the drug).
We quantified the effects of imatinib mesylate on proliferation of transformed human CD3+ T cell–derived lymphoblastoid line CCL119, T-cell leukemia Jurkat line, and the BCR-ABL+ CML cell line K562. We incubated the cells with imatinib mesylate for 18 hours when we measured the extent of thymidine incorporation. Imatinib mesylate inhibited DNA synthesis of all 3 transformed cell lines in a dose-dependent fashion but was less potent than in normal cells (Figure 1B). After 18 hours with imatinib mesylate none of the cell lines lost viability (as determined by trypan blue exclusion) and increased binding of annexin V (data not shown). After 48 hours, however, at 1.0 μM imatinib mesylate only 28% ± 4 % of K562 cells (P = .005 relative to imatinib mesylate–free control) were viable compared to 86% ± 4% for CCL-119 cells (P = .44) and 99% ± 8% for Jurkat cells (P = .92). A quantitative comparison of imatinib mesylate effects in normal T cells and cell lines must await a detailed kinetic analysis of the cell cycle, but apparently imatinib mesylate does not affect primary T cells only.
Imatinib mesylate neither reduces T-cell viability nor stimulates their apoptosis
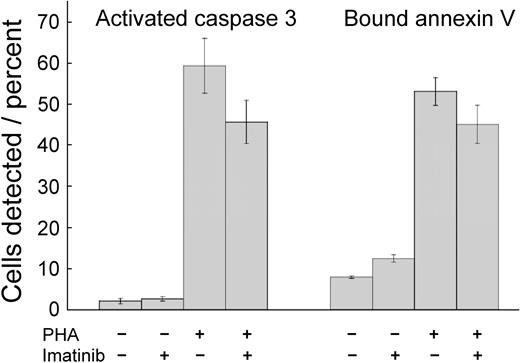
Imatinib mesylate toxicity might provide an explanation for inhibited T-cell proliferation. Therefore, we incubated the cells with imatinib mesylate for 72 hours and quantified apoptosis by determining the fraction of cells demonstrating activated caspase 324 and the fraction that bound annexin V.25,26 Frequency of cells positive for activated caspase 3 and binding of annexin V were low in the absence of PHA (Figure 2). Stimulation by PHA increased the frequency of cells exhibiting either marker (P < .001 for both). Imatinib mesylate–treated and PHA-activated T cells did not change their annexin V binding (P = .10), indicating that the drug did not induce apoptosis under these conditions. Caspase 3 activation can be used as a marker for apoptosis, but the levels of enzyme are increased in T-cell stimulation.27,28 PHA treatment activated caspase 3, but imatinib mesylate inhibited this effect (P = .03), similarly as it inhibited T-cell proliferation. Thus, the changes induced by PHA and imatinib mesylate in caspase 3 activation may reflect changes in T-cell stimulation, rather than enhanced apoptosis. Consequently, under these conditions imatinib mesylate was cytostatic, but not cytotoxic.
Imatinib mesylate neither reduces viability nor stimulates apoptosis in T cells. Unstimulated T cells and T cells stimulated with PHA were cultured for 72 hours without or with imatinib mesylate (5.0 μM). Then the cells were assayed for activated caspase 3 and for binding of annexin V. Shown are mean values ± SD. PHA stimulation increased the frequency of cells with activated caspase 3 and cells binding annexin V (P < .001 for both). Imatinib mesylate induced a decrease of activated caspase 3 levels (P = .03) but did not change the frequency of cells binding annexin V (P = .10).
Imatinib mesylate neither reduces viability nor stimulates apoptosis in T cells. Unstimulated T cells and T cells stimulated with PHA were cultured for 72 hours without or with imatinib mesylate (5.0 μM). Then the cells were assayed for activated caspase 3 and for binding of annexin V. Shown are mean values ± SD. PHA stimulation increased the frequency of cells with activated caspase 3 and cells binding annexin V (P < .001 for both). Imatinib mesylate induced a decrease of activated caspase 3 levels (P = .03) but did not change the frequency of cells binding annexin V (P = .10).
Imatinib mesylate–treated T cells accumulate in the G0/G1 phase of the cell cycle
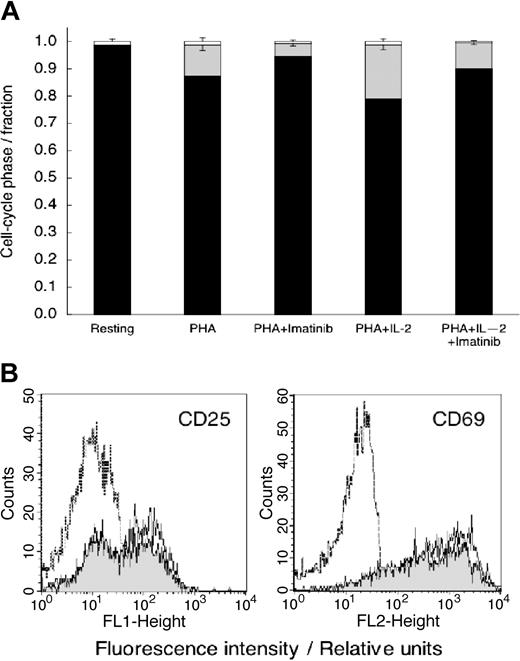
Because imatinib mesylate was not cytotoxic, we postulated that the decrease in DNA synthesis (Figure 1) results from the effects of imatinib mesylate on cell cycle progression. To test this hypothesis, we stimulated imatinib mesylate–treated cells with PHA alone or with the combination of PHA (10 μg/mL) and IL-2 (500 U/mL) that powerfully stimulates T-cell proliferation.29 We measured DNA content 72 hours later. Data in Figure 3A show that essentially all unstimulated (control) T cells were in the G0/G1 phase of the cycle. PHA stimulated DNA synthesis and progression into the S phase (P < .001 relative to resting cells), an effect inhibited by imatinib mesylate (P < .001 relative to PHA-stimulated cells). Imatinib mesylate inhibited T-cell cycle progression even in the presence of PHA plus IL-2 (P < .01 relative to the cells stimulated by both PHA and IL-2).
Imatinib mesylate treatment results in T-cell accumulation in the G0/G1 phase of the cell cycle but does not inhibit T-cell activation. (A) Cell cycle distribution of unstimulated T cells and T cells stimulated with PHA, or the combination of PHA- and IL-2–stimulated T cells with and without imatinib mesylate. The cells were stained with propidium iodide 24 hours after initiation of experiment and analyzed by flow cytometry. ▪ represents the fraction of cells in G0/G1; ▦, the fraction in S phase; and □, the fraction in G2/M phase. Shown are mean values ± SD. (B) Untreated T cells (dashed line) and T cells activated with PHA (10 μg/mL) in imatinib mesylate absence (solid line) or presence (10 μM; shaded area) were analyzed for expression of CD25 or CD69 24 hours after initiation of culture.
Imatinib mesylate treatment results in T-cell accumulation in the G0/G1 phase of the cell cycle but does not inhibit T-cell activation. (A) Cell cycle distribution of unstimulated T cells and T cells stimulated with PHA, or the combination of PHA- and IL-2–stimulated T cells with and without imatinib mesylate. The cells were stained with propidium iodide 24 hours after initiation of experiment and analyzed by flow cytometry. ▪ represents the fraction of cells in G0/G1; ▦, the fraction in S phase; and □, the fraction in G2/M phase. Shown are mean values ± SD. (B) Untreated T cells (dashed line) and T cells activated with PHA (10 μg/mL) in imatinib mesylate absence (solid line) or presence (10 μM; shaded area) were analyzed for expression of CD25 or CD69 24 hours after initiation of culture.
Aprerequisite for entry of cells into the S phase is phosphorylation of the retinoblastoma protein (Rb) that is regulated by the cyclin D3/cdk4 complex30,31 (also reviewed by Olashaw and Pledger32 ). We quantified the levels of cyclin D3 protein and Rb phosphorylation in the presence of imatinib mesylate by Western blotting. At 12, 16, and 24 hours after initiation of a typical experiment the amount of Rb phosphoprotein was reduced to 19%, 39%, and 77% of control, respectively; the amount of cyclin D was reduced to 28%, 32%, and 59% of control, respectively (data not shown; effects at later times might have been artificially reduced by the film saturation at the darker control bands). These effects are consistent with imatinib mesylate–induced arrest of the cell cycle before the S phase.
Imatinib mesylate does not inhibit expression of T-cell activation markers CD25 and CD69
Membrane molecules CD25 and CD69 are expressed as a result of T-cell stimulation, but their expression is not coupled to proliferation.33 Hence, these molecules can be viewed as evidence of early T cell activation.33 We measured the levels of CD25 and CD69 in imatinib mesylate–treated T cells activated by PHA. After an 18-hour incubation with PHA, the frequency of cells expressing CD25 and CD69 markedly increased (Figure 3B). Imatinib mesylate–treated T cells demonstrated a similar increase in CD25 and CD 69 levels characteristic of activated T cells, rather than of resting T cells. Thus, the combined data in Figures 1, 2 and 3 show that imatinib mesylate selectively inhibits cellular targets leading to proliferation without effecting T cell activation or apoptosis.
Imatinib mesylate can terminate already initiated proliferation signals, but its effects are reversible
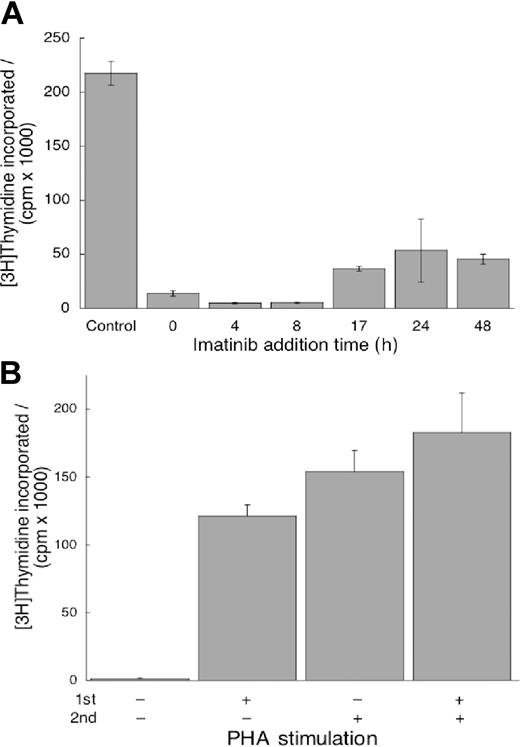
Because the mechanisms initiating T-cell proliferation and activation are uncoupled,33 in agreement with the data in Figure 3A-B, it is of interest to determine if imatinib mesylate can terminate proliferation signals after they have been initiated. We plated cells without imatinib mesylate in the presence of PHA as in Figure 2. Imatinib mesylate was added at increasing intervals from initiation of culture (Figure 4A) and DNA synthesis was determined. We found that imatinib mesylate could inhibit proliferation of T cells stimulated even 48 hours before introduction of the drug (Figure 4A). Thus, imatinib mesylate could terminate already initiated intracellular signaling in the pathways leading to proliferation.
Imatinib mesylate can terminate proliferation signals already initiated, but its effects on T cells are reversible. (A) Imatinib mesylate (10 μM) was added 4 to 48 hours after PHA stimulation. DNA synthesis was quantified as described in Figure 1. Values measured in the presence of imatinib mesylate at all time points were different from values in imatinib mesylate–free cells (P < .05). (B) First, the cells were treated with imatinib mesylate, one group stimulated by PHA and the other unstimulated. The cells were incubated for 24 hours, washed free of imatinib mesylate, and replated without it. Subsequently, one half of each group was stimulated with PHA and the other half remained unstimulated. After incubation for an additional 96 hours, DNA synthesis was measured as described in Figure 1. Cells treated with imatinib mesylate and without PHA in the first incubation did not synthesize DNA without PHA in the second, whereas those treated with PHA in the first incubation did resume proliferation without stimulation in the second. However, in the presence of PHA in the second incubation the cells proliferated similarly (P = .22) irrespective of whether pretreatment with imatinib mesylate occurred in the absence or presence of PHA. Both panels display mean values ± SD.
Imatinib mesylate can terminate proliferation signals already initiated, but its effects on T cells are reversible. (A) Imatinib mesylate (10 μM) was added 4 to 48 hours after PHA stimulation. DNA synthesis was quantified as described in Figure 1. Values measured in the presence of imatinib mesylate at all time points were different from values in imatinib mesylate–free cells (P < .05). (B) First, the cells were treated with imatinib mesylate, one group stimulated by PHA and the other unstimulated. The cells were incubated for 24 hours, washed free of imatinib mesylate, and replated without it. Subsequently, one half of each group was stimulated with PHA and the other half remained unstimulated. After incubation for an additional 96 hours, DNA synthesis was measured as described in Figure 1. Cells treated with imatinib mesylate and without PHA in the first incubation did not synthesize DNA without PHA in the second, whereas those treated with PHA in the first incubation did resume proliferation without stimulation in the second. However, in the presence of PHA in the second incubation the cells proliferated similarly (P = .22) irrespective of whether pretreatment with imatinib mesylate occurred in the absence or presence of PHA. Both panels display mean values ± SD.
If imatinib mesylate inhibits proliferation but does not induce apoptosis, the cells will resume proliferation on removal of the drug. To test this hypothesis, we incubated T cells with imatinib mesylate, stimulated one half of the wells with PHA, and incubated all cells for 24 hours. Then we washed the cells, treated one half of the wells from each sample with PHA and incubated all for additional 96 hours when we measured thymidine incorporation. We found the cells unstimulated in both incubations did not proliferate (Figure 4B). The cells stimulated by PHA in the first incubation proliferated without it in the second. The cells stimulated in the second incubation proliferated to the same extent irrespective of whether they were stimulated in the first. These observations demonstrate that imatinib mesylate did not affect the proliferation potential of T cells and that the effects of the drug are reversible.
Imatinib mesylate reduces levels of phosphorylated Lck and ERK1/2 and of activated NF-κB
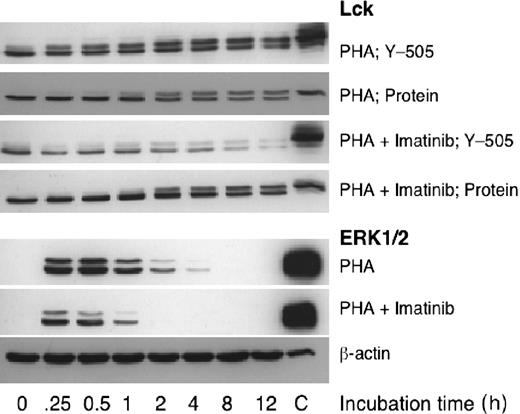
Abelson kinase (and its constitutively activated mutant BCR-ABL), c-KIT protein, and PDGF-R are well-documented cellular targets of imatinib mesylate.10 PDGF, common in human serum, suppresses T-cell activation.34 Monoclonal antibodies specific for the extracellular component of c-KIT did not recognize T cells (data not shown), suggesting that the full-length c-KIT is absent in T cells. Indeed, we detected no c-KIT and no imatinib mesylate–associated change in the levels of c-ABL phosphorylation by Western blotting (data not shown). Similarly, by functional assays we found that exogenous PDGF did not affect T cells (data not shown). Apparently c-ABL, c-KIT, and PDGF-R do not play a manifest role in inhibition of T-cell proliferation by imatinib mesylate. Thus, T cells must contain other imatinib mesylate targets. In an attempt to identify some imatinib mesylate–sensitive intracellular signaling pathways we measured the levels of phosphorylation of Lck molecule and ERK1/2, both associated with T-cell receptor (TCR)–mediated signaling35 and of activated NF-κB, a transcription factor activated by numerous pathways including the one initiated at the TCR.35
We exposed PHA-stimulated T cells to imatinib mesylate and determined the relative levels of Lck protein and phosphorylation at its Tyr505 as a function of time. We found no apparent difference between drug-treated and control cells in the levels of Lck protein, but the drug reduced Tyr505 phosphorylation (Figure 5). This effect was apparent at the first time point measured at 15 minutes after stimulation. Similarly, we noticed that imatinib mesylate inhibited phosphorylation of ERK1/2 (Figure 5). In addition, we determined the level of activated NF-κB in nuclear extracts of T cells activated by PHAin the presence of increasing imatinib mesylate concentrations and expressed the results as percentage of PHA-stimulated increase in levels of activated NK-κB. At 4.4 μM imatinib mesylate, the amount of activated NK-κB was reduced to 58.7% ± 1.1% (P = .003) and decreased to 38.4% ± 0.9% at 17.6 μM. These effects of imatinib mesylate on phosphorylation of Lck and ERK1/2 and activation of NF-κB are compatible with the concentration dependence of the observed inhibition of T-cell proliferation and function.
Imatinib mesylate inhibits phosphorylation of molecules participating in cellular signaling. To identify imatinib mesylate–sensitive intracellular signaling pathways we measured the levels of phosphorylation of Lck and ERK1/2, both associated with TCR-mediated signaling. We exposed PHA-stimulated T cells to imatinib mesylate (10 μM) and found that the drug did not affect the levels of Lck protein, but it inhibited Tyr505 phosphorylation. Similarly, imatinib mesylate inhibited phosphorylation of ERK1/2.
Imatinib mesylate inhibits phosphorylation of molecules participating in cellular signaling. To identify imatinib mesylate–sensitive intracellular signaling pathways we measured the levels of phosphorylation of Lck and ERK1/2, both associated with TCR-mediated signaling. We exposed PHA-stimulated T cells to imatinib mesylate (10 μM) and found that the drug did not affect the levels of Lck protein, but it inhibited Tyr505 phosphorylation. Similarly, imatinib mesylate inhibited phosphorylation of ERK1/2.
Imatinib mesylate inhibits DTH in mice
To determine if imatinib mesylate affects T cells in vivo similarly to the effects in vitro, we treated mice with the drug daily throughout the experiment.36,37 After 10 days of treatment, we immunized mice with NP-O-Su and challenged 6 days later with a subcutaneous injection of the same agent into one footpad and of PBS into the other. Twenty-four hours later we quantified the extent of DTH by measuring the thickness of the footpads. Footpads of control animals (no imatinib mesylate) thickened considerably on the challenge with NP-O-Su (Table 1). Systemic treatment with imatinib mesylate abolished this effect (Table 1). In contrast, imatinib mesylate had no effect on the total splenocyte number (P = .25) and numbers of CD3+ cells (P = .52) and CD8+ cells (P = .16; data not shown). Taken together, these data show that the reduced DTH might result from a systemic inhibition of the T-cell response, rather than from the diminished number of T cells.
Discussion
We found that imatinib mesylate inhibits T-cell proliferation at concentrations similar to those found in patients treated for CML and gastrointestinal stromal tumors.3 The effect is manifest both in T cells nonspecifically stimulated by PHA and in those stimulated by DCs. Imatinib mesylate neither prevented activation of T cells nor killed them, but it inhibited cell cycle progression. In an attempt to identify the pertinent intracellular pathways targeted by imatinib mesylate, we found reduced phosphorylation or protein levels of all molecules we selected for study. This observation could explain the attenuation of T-cell function, but it was not helpful in the identification of critical imatinib mesylate targets. Possibly, T-cell function is impaired by the cumulative effect of partial inhibition of numerous phosphonucleotide-binding molecules rather than by the definitive inhibition of few key molecules (eg, BCR-ABL or c-KIT). A similar conclusion has been reached recently in a study of differentiation of CD34+ hematopoietic cells into DCs where imatinib mesylate suppressed phosphorylation of molecules participating in numerous intracellular signaling pathways that are independent of c-ABL, c-KIT, and PDGF-R.38
We did detect the presence of c-ABL in T cells and cannot thus completely rule out its role in imatinib mesylate effects. However, there is little evidence for such a role of c-ABL in signal transduction leading to T-cell cycle triggering, although the molecule generally does participate in regulation of later stages of cell cycle progression.39 Our data are compatible with such a role for c-ABL because imatinib mesylate inhibited T-cell cycle progression, but did not affect T-cell activation. This observation is fully in line with the recent evidence for such uncoupling of activation and cell cycle progression in human T cells.33 In addition, these data demonstrate that imatinib mesylate can be used as a tool in studies of (un)coupling of T-cell activation and proliferation.
We found that imatinib mesylate was immunosuppressive in an in vivo model of DTH. The clinical relevance of this finding is unclear. Because the effects of the drug are rapidly reversed on its removal, these effects are likely to be sensitive to imatinib mesylate pharmacokinetics. It is possible, in fact, that the key determinant of the extent of immunosuppression is the nadir in fluctuations of levels of imatinib mesylate. Thus, sustaining therapeutically adequate imatinib mesylate levels may impede acute inflammatory responses or undermine control of subclinical infections. Nonetheless, information about the effects on imatinib mesylate on immunity is scarce and circumstantial. Several mostly anecdotal observations of secondary effects of imatinib mesylate in patients with CML support the notion that imatinib mesylate suppresses immunity in vivo. For example, patients treated with imatinib mesylate experienced a higher incidence of herpes zoster outbursts, which diminished on treatment with antiviral drugs.40 One patient developed a B-cell lymphoma positive for the Epstein-Barr virus41 and another developed pulmonary alveolar proteinosis42 ; both complications receded after reduction in the imatinib mesylate dose or complete discontinuation of the drug. Similarly, imatinib mesylate–borne immunosuppression could explain the surprising remission of rheumatoid arthritis in a patient with CML 2 months after initiation of imatinib mesylate therapy.43 Recent attempts to overcome resistance to imatinib mesylate by higher doses of the drug may provide data for further unraveling of the relationship of imatinib mesylate and immunity. A more definite establishment of immunosuppressive effects of imatinib mesylate may add this well-tolerated drug to the list of clinically useful agents for control of T-cell malignancies and autoimmunity.
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2003-12-4266.
Supported by National Institutes of Health grant R01CA-84368 and Mayo Clinic Comprehensive Cancer Center Support grant CA-15083. Stem Cell Laboratory has been supported by Mrs Adelyn L. Luther, Singer Island, FL; and Commonwealth Cancer Foundation for Research, Richmond, VA. L.S. is a scholar of the Glen and Florence Voyles Foundation, Terre Haute, IN.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Troy Voeltz for technical help and Dr Frank Prendergast for continuing interest and support.