Abstract
When a blood clot is formed, vitronectin (VN) is incorporated. Here we studied the consequence of VN incorporation for platelet interactions under flow. Perfusion of whole blood over a fibrin network, formed from purified fibrinogen, resulted in approximately 20% surface coverage with blood platelets. Incorporation of purified multimeric VN into the fibrin network resulted in a 2-fold increase in surface coverage with platelets and in enhancement of platelet aggregate formation. A human monoclonal antibody (huMab VN18), directed against the multimeric form of VN, inhibited platelet adhesion to the combined fibrin/VN matrix to the level of adhesion on fibrin alone. This inhibition was also shown when whole blood was perfused over a plasma-derived clot. Surprisingly, the inhibitory action of the antibody was not directed toward VN incorporated into the fibrin network but toward VN released from the platelets. We conclude that VN-potentiated platelet-clot interaction requires VN in the clot and multimeric VN bound to the platelet surface. Our results provide evidence that homotypic VN interactions contribute to platelet adhesion and aggregation to a blood clot. This report demonstrates for the first time that self-assembly of VN may provide a physiologically relevant contribution to platelet aggregation on a blood clot.
Introduction
Vitronectin (VN) is a multifunctional 75-kDa glycoprotein present in plasma, extracellular matrix, and in the α-granules of blood platelets.1-3 VN plays a role in complement-mediated cell lysis; it is involved in hemostasis; it mediates cell adhesion and migration; and it stabilizes plasminogen activator inhibitor type-1 (reviewed by Tomasini and Mosher4 and Hess et al5 ). In plasma, VN circulates in a folded form, in which most ligand-binding domains are hidden. Activation of VN coincides with most of its biologic activities. In the extracellular matrix and in blood platelets, VN is present in an activated, multimeric form.6 When blood platelets become activated, they secrete multimeric VN, of which half remains platelet bound.7 The presence of VN has been demonstrated on the surface of platelets7,8 and has been implicated in platelet adhesion9-11 and aggregation.12,13
Recently, codistribution of VN and fibrin polymers was demonstrated.3,14,15 Moreover, a role for VN in the stabilization of thrombi was demonstrated in vivo.16 Although a role for VN in platelet adhesion, platelet aggregation, and thrombus formation is evident, the mechanism that underlies this phenomenon remained elusive. We found that a newly developed human antibody directed against multimeric VN (huMab VN18) inhibited platelet-platelet interactions in an aggregation assay in whole plasma. This antibody also inhibited platelet-clot interactions under flow. We established that VN mediates platelet-clot interactions through homotypic association between platelet-bound VN and fibrin-incorporated VN. Our results provide a novel insight into the molecular mechanism of VN-mediated platelet adhesion/aggregation and, ultimately, stabilization of a thrombus.
Materials and methods
Proteins and chemical compounds
Human multimeric VN was purified from plasma as described.17 Human albumin was obtained from Behring (Marburg, Germany). Human fibrinogen (Fbg) was from American Diagnostica (Greenwich, CT; 98% pure) and purified by size exclusion chromatography on Sepharose 4B,18 which yields VN-free Fbg. Human thrombin was a gift from Dr Kisiel (University of New Mexico, Albuquerque, NM). Plasminogen activator inhibitor-1 (PAI-1) was provided by Dr Reilly (Du Pont Pharma, Wilmington, DE). H-D-Phe-Pro-Arg-chloromethylketone (PPACK) was from Bachem (Bubendorf, Germany), unfractionated heparin from Organon Technika (Oss, the Netherlands), and adenodiphosphate (ADP) from Boehringer (Mannheim, Germany). Thrombin receptor activating peptide (TRAP, amino acid residues SFLLRN) was synthesized with a semiautomatic peptide synthesizer (Labortec, Bubendorf, Switzerland).
Antibodies
Polyclonal antibody (Poab) against VN (Ra#60) was a kind gift from Dr Preissner (Institute of Biochemistry, Giessen, Germany). Poab A0080 against fibrin(ogen) was obtained from Dako (Glostrup, Denmark). Monoclonal antibodies (Moabs) against VN (VN7, 16A7, 19G8, and 12B5) were a gift from Dr DeClerck (University of Leuven, Belgium). Moab M4 was donated by Dr Hayashi (Ochanomizu University, Tokyo, Japan). Moab C17, directed against glycoprotein (GP) IIIa and inhibiting ADP-induced platelet aggregation,19 was a gift from Dr von dem Borne (Central Laboratory Bloodtransfusion, Amsterdam, the Netherlands). Moab against P-selectin (Wasp12.2) was isolated from the supernatant of a hybridoma cell line obtained from the American Type Culture Collection (Rockville, MD).
Human monoclonal phage antibodies
The selection protocol for huMab VN18 using phage display technology, generation of human immunoglobulin G (IgG), and characterization of its specificity were described by Bloemendal et al.20 Multimeric VN or plasma-derived VN incubated with urea was recognized equally by huMab VN18, whereas VN in plasma or purified from plasma was hardly recognized.20
Biotinylation of compounds
HuMab VN18, control huMab, purified VN, and unfractionated heparin were dialyzed against bicarbonate buffer (0.1 M NaHCO3, 0.5 M NaCl, pH 8.3). Biotin label (n-hydroxysuccinimidobiotin; Sigma, Steinheim, Germany) was freshly dissolved in dimethyl sulfoxide (1 mg/mL) (Merck, Darmstadt, Germany), and 200 μL was added to 1 mL compound solution, incubated for 4 hours at room temperature, and dialyzed against phosphate-buffered saline (PBS) to remove nonbound biotin.
Platelet aggregation studies in whole plasma
Platelet-rich plasma (PRP) (10 minutes at 160g at room temperature, no brake) and platelet-free plasma (PFP) (15 minutes at 1200g at room temperature) was prepared from citrate-anticoagulated blood withdrawn from an aspirin-free donor. The number of platelets in PRP was determined using a cell counter (model 871; Mölab, Hilden, Germany) and was adjusted to about 200 × 109/L (200 000/μL) by dilution with PFP. This platelet suspension (450 μL) was used in the aggregation assay (Chronolog, Kordia, the Netherlands) in which the change of light transmission was measured for 8 minutes. Platelet-free plasma was used as reference. Aggregation was induced by addition of ADP (50 μM), TRAP (10 μM), or collagen (Horm, 1 μg/mL; Nycomed Pharma, Breda, the Netherlands) in the absence or presence of huMab VN18 or control huMab (0, 1, 10 and 50 μg/mL).
Preparation of adhesive surfaces on coverslips
Glass coverslips (Menzel Gläser, Braunschweig, Germany) were cleaned overnight using 2% chromium trioxide solution and rinsed with distilled water. Multimeric VN (1.43 μM) was coated overnight at room temperature, washed, and blocked for 15 minutes with 1% human albumin solution (HAS) dissolved in PBS to prevent nonspecific protein binding. Fibrin was formed by adding 0.5 U/mL thrombin (final concentration) to a Fbg solution dissolved in PBS (1 mg/mL), which was sprayed on glass coverslips using a retouching airbrush (model 100; Badger Brush, Franklin Park, IL).21 The combined surface of fibrin and VN was made by the same procedure from a mixture of Fbg (1 mg/mL), multimeric VN (10 to 143 nM), and thrombin (0.5 U/mL). After spraying, all surfaces were incubated for 15 minutes with 100 nM PPACK in PBS to block residual thrombin activity and subsequently blocked for 15 minutes with 1% HAS.
The plasma-derived clots were generated according to Collet et al.22 Briefly, blood from healthy volunteers was anticoagulated with trisodium citrate. PFP was obtained by centrifugation, and plasma was recalcified up to a final concentration of 20 mM. After 1 minute, thrombin was added to a final concentration of 1 U/mL. Immediately after the addition of thrombin, the plasma was sprayed on coverslips using the retouching airbrush. Then the coverslips were left to form the fibrin network, incubated for 15 minutes with 100 nM PPACK in PBS to block residual thrombin activity, and blocked for 15 minutes with 1% HAS. After each step, the coverslips were washed 3 times with PBS.
Perfusion studies
Perfusion studies were carried out in a specially devised vacuum perfusion chamber with well-defined rheologic characteristics.23 Whole blood was anticoagulated with 80 μM PPACK.
In a separate series of experiments reconstituted blood was used, prepared as follows: 1 vol Krebs-Ringer buffer (4 mM KCl, 107 mM NaCl, 20 mM NaHCO3, 2 mM Na2SO4, 4.76 mM citric acid, 14 mM Na citrate, and 5 mM d-glucose, pH 5.0) was added to 1 vol PRP. A platelet pellet was obtained by centrifugation (10 minutes at 500g, 20° C) and washed twice in Krebs-Ringer buffer (pH 6.0). Platelet concentration was adjusted to 200 × 109/L (200 000/μL) in 4% HAS (4% human albumin, 4 mM KCl, 124 mM NaCl, 20 mM NaHCO3, 2 mM Na2SO4, 2.5 mM CaCl2, 1.5 mM MgCl2, 5 mM d-glucose, and 20 U/mL low-molecular-weight heparin [LMWH], pH 7.35). Red cells were washed 3 times with 154 mM NaCl and 5 mM d-glucose by centrifugation at 2000g at 20° C for 5 minutes and packed by centrifugation for 12 minutes at 2000g. Packed red cells were added to the perfusate to obtain a hematocrit of 0.40 (40%) 5 minutes before perfusion.24 Whole blood or reconstituted blood was prewarmed to 37° C for 5 minutes and then drawn from a container through the perfusion chamber over the coated coverslips during 5 minutes. Wall shear rates of 500 s–1 were maintained with an automated syringe pump (Harvard Apparatus, South Natick, MA). Coverslips were rinsed in HEPES buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.15 M NaCl, pH 7.4) fixed in 0.5% glutaraldehyde in PBS, dehydrated in methanol, and stained with May-Grünwald Giemsa.25 All perfusions were performed in triplicate and results are presented as the mean coverage (%) ± standard error of the mean.
Evaluation of platelet deposition by light microscopy
Perfused coverslips were mounted in Entellon (Merck) and platelet deposition was evaluated with a light microscope (Leitz, Wetzlar, Germany) using a × 40 objective lens (Leitz) and a charge-coupled device (CCD) camera (Jai, Copenhagen, Denmark) coupled to a frame grabber (Matrox Electronic Systems, Quebec, QC, Canada) using Optimas 6.0 software (DVS, Breda, the Netherlands) for image analysis. Platelet deposition was expressed as percentage of the surface covered with platelets. Statistical 1-way analysis was performed by analysis of variance (ANOVA), with a value of P less than .05 indicating statistical significance.
Evaluation of platelet deposition by confocal microscopy
Platelet adhesion to the fibrin or fibrin/VN surface was studied by confocal laser scanning microscopy (CLSM). Coverslips were fixed for 30 minutes in 3% paraformaldehyde and 0.025% glutaraldehyde in PBS, blocked by 0.15 M glycine and 1% bovine serum albumin (BSA) in PBS (30 minutes), and permeabilized with 0.5% Triton X-100 in PBS (5 minutes). The coverslips were incubated with 100 μL BODIPY-fluorophore–conjugated phallacidin (0.13 μM in PBS containing 1% BSA; Molecular Probes, Eugene, OR) and Poab Ra#60 directed against VN. Coverslips were incubated with goat antirabbit IgG conjugated with fluorescein isothiocyanate (FITC) (Molecular Probes). Between steps, coverslips were washed 3 times with PBS. Finally, the coverslips were mounted in VECTASHIELD (H-1000; Vector Laboratories, Burlingame, CA), and pictures were made using CLSM (Leica TCS 4D; Leica Microsystems, Heidelberg, Germany).
Orientation of multimeric VN in the fibrin network
Recognition of multimeric VN, either directly coated (3 μg/mL) or incorporated into a fibrin network, was analyzed in enzyme-linked immunosorbent assay (ELISA) using different antibodies. The combined fibrin/VN network was formed by preincubation of 100 μg/mL Fbg, 3 μg/mL multimeric VN, and 0.5 U/mL thrombin. The clots were allowed to form overnight. The next day, the plate was centrifuged (15 minutes, 2500g) to remove clot-trapped liquid. Plates were blocked with PBS containing 3% BSA and 0.1% Tween 20 and incubated with a concentration range of antibodies (0.01 to 10 μg/mL Moabs, Poabs, or huMabs) or biotinylated heparin (0.3-330 mU/mL). Binding of antibodies or biotinylated heparin was detected with peroxidase-conjugated secondary antibodies rabbit–anti-Moab, swine–anti-Poab, rabbit–antihuman κ light chain, or with peroxidase-conjugated streptavidin (all from Dako). Staining was performed with the peroxidase substrate diaminobenzidine (DAB; Sigma). The concentration of antibodies or biotinylated heparin that resulted in half-maximal binding to plastic-coated VN was determined. Using this concentration, binding to VN and fibrin/VN was measured. The relative binding, expressed in percentage, was calculated by dividing binding to fibrin/VN by binding to VN alone. The ratio for Poab Ra#60 was set as 100%. The experiment was performed in triplicate, and representative results are shown.
Flow cytometric analysis of platelets
Platelets were isolated as described above for reconstituted blood in “Perfusion studies.” Platelets resuspended in 4% HAS in Krebs-Ringer buffer (pH 6.0) were stimulated for 1 hour at 37° C with ADP (50 μM) or for 5 minutes with thrombin (0.5 U/mL). Platelets were washed, resuspended in 4% HAS in PBS to a concentration of 500 000 in 10 μL, and incubated for 30 minutes at room temperature with biotinylated huMab VN18 (10 μg/mL), control huMab (10 μg/mL), or with Moab Wasp12.2 directed against P-selectin (10 μg/mL). Platelets were washed with 4% HAS solution, and binding of the biotinylated antibodies was detected with phycoerythrin (PE)–labeled streptavidin (1:100; Dako). Binding of Wasp12.2 was detected with FITC-labeled rabbit-antimouse IgG (Southern Biotechnology Associates, Birmingham, AL). Cytometric analysis (Becton Dickinson, San Jose, CA) was performed on platelets that were identified by their dot plots of forward light scatter versus right-angle side light scatter. Cytometric data were analyzed using WinMDI software. The FITC-fluorescent signals, representing P-selectin reactivity, were plotted on the x-axis, whereas the PE-fluorescent signals, representing binding of huMabs, were plotted on the y-axis.
Homotypic VN interaction
Multimeric VN (71 nM) was coated overnight in a 96-well plate. Plates were blocked with PBS containing 3% BSA and 0.1% Tween 20. Biotinylated VN was incubated in a concentration range of 0 to 143 nM for 1 hour at room temperature. Binding of biotinylated VN was detected with peroxidase-conjugated streptavidin, followed by DAB. Plates were washed extensively between each step. Next, a competition assay was performed. Biotinylated VN was used at a concentration that gave half-maximal binding (28 nM) to immobilized VN. Increasing amounts of a peptide consisting of the heparin-binding domain of VN (residues 348 to 361, 0 to 46 μM), a peptide consisting of residues 47 to 64 of VN (0 to 406 μM), a control peptide derived from α-1-proteinase inhibitor (105Y, 0 to 444 μM),26 unfractionated heparin (0 to 330 mU/mL), or PAI-1 (0-1.9 μM) were used. Results are expressed as percentage binding in the absence of any compound.
Results
HuMab VN18 inhibits ADP-stimulated platelet aggregation in whole plasma
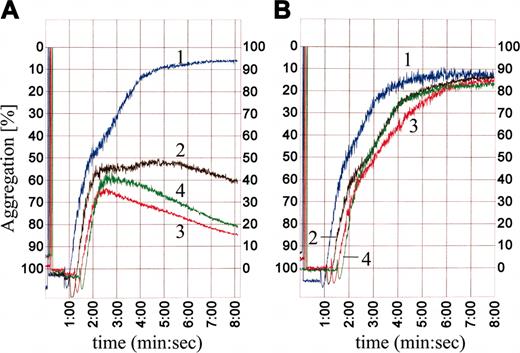
We have developed a fully human monoclonal antibody, huMab VN18, that specifically recognizes multimeric VN20 and is directed against a region in the hemopexin II domain (H.J.B., H.C.d.B., E.E.V., manuscript in preparation). We investigated whether platelet aggregation induced by ADP (50 μM), TRAP (10 μM), and collagen (1 μg/mL) was affected by huMab VN18. Figure 1 shows that ADP-induced aggregation occurred in a standard biphasic mode. The second phase of aggregation was inhibited dose dependently by huMab VN18. In the presence of 10 μg/mL huMab VN18, aggregation decreased to 20% of maximal aggregation. Higher amounts of huMab VN18 (50 μg/mL) had no additional inhibitory effect (Figure 1A). At 10 and 50 μg/mL huMab VN18, ADP-stimulated platelets showed a decline in light transmittance after 3 minutes, indicative of disaggregation (Figure 1A). A control huMab did not affect the ADP-induced platelet aggregation (Figure 1B), and huMab VN18 had no effect on nonstimulated platelets (data not shown). HuMab VN18 also inhibited TRAP-induced platelet aggregation but had no effect on collagen-induced platelet aggregation (data not shown). These data indicate that at low concentration, huMab VN18 prevented aggregate formation in whole plasma during the second, release-dependent phase and caused disaggregation at higher concentrations.
HuMab VN18 inhibits platelet aggregation in platelet-rich plasma. (A) Addition of increasing amounts of huMab VN18 to platelet-rich plasma inhibited ADP-induced platelet aggregation dose dependently, whereas a control huMab (B) had no effect. 1 indicates no antibody; 2, 1 μg/mL; 3, 10 μg/mL; 4, 50 μg/mL.
HuMab VN18 inhibits platelet aggregation in platelet-rich plasma. (A) Addition of increasing amounts of huMab VN18 to platelet-rich plasma inhibited ADP-induced platelet aggregation dose dependently, whereas a control huMab (B) had no effect. 1 indicates no antibody; 2, 1 μg/mL; 3, 10 μg/mL; 4, 50 μg/mL.
HuMab VN18 inhibits whole blood–derived platelet deposition onto a plasma-derived clot
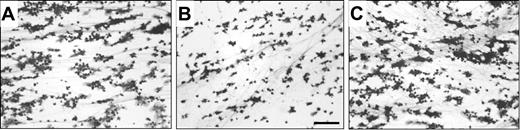
The role of VN in platelet adhesion to a plasma-derived clot was studied under flow. Immunofluorescent staining using an FITC-labeled Poab against fibrin(ogen) and a TRITC-labeled Moab against multimeric VN revealed codistribution of multimeric VN and fibrin in the plasma-derived clot (not shown). Whole blood perfusions (5 minutes at shear rate 500 s–1) were performed, and platelet deposition was monitored. Platelets covered 34% ± 7% of the clot surface (Figure 2A). Addition of huMab VN18 (16 μg/mL, Figure 2B) to the whole blood resulted in a significant decrease (50%) of platelet coverage (17% ± 2%). Increasing concentrations of huMab VN18 had no additional inhibiting effect on platelet adhesion (data not shown). A control huMab had no effect (31% ± 7%, 16 μg/mL, Figure 2C). Addition of huMab VN18 resulted in predominantly nonspread adhered platelets and decreased amounts of aggregates (Figure 2B). These results indicate that multimeric VN promotes platelet-clot interactions under flow.
HuMab VN18 inhibits platelet adhesion from whole blood to a plasma-derived clot under flow. Perfusion was performed for 5 minutes at a shear rate of 500 s–1. Coverslips were processed for light microscopic evaluation (original magnification × 250) and quantified. (A) Image of platelet adhesion on a plasma clot. The surface is covered for 34% ± 7% with platelets. (B) Inhibition of platelet adhesion in the presence of huMab VN18 (16 μg/mL) to 17% ± 2% surface coverage. (C) A control huMab did not affect platelet adhesion. Bar represents 25 μm.
HuMab VN18 inhibits platelet adhesion from whole blood to a plasma-derived clot under flow. Perfusion was performed for 5 minutes at a shear rate of 500 s–1. Coverslips were processed for light microscopic evaluation (original magnification × 250) and quantified. (A) Image of platelet adhesion on a plasma clot. The surface is covered for 34% ± 7% with platelets. (B) Inhibition of platelet adhesion in the presence of huMab VN18 (16 μg/mL) to 17% ± 2% surface coverage. (C) A control huMab did not affect platelet adhesion. Bar represents 25 μm.
Fibrin is essential for the VN-mediated platelet-clot interaction
To investigate the contribution of multimeric VN on platelet-clot interactions obtained from flowing whole blood in more detail, we prepared matrices from purified components. We first tested whether platelets adhere to multimeric VN. It has already been demonstrated that isolated platelets adhere to VN under static conditions. Under flow conditions (5 minutes at shear 500 s–1), the platelets from whole blood did not adhere to VN at all (Figure 3D). Next, a fibrin clot was formed in the absence or presence of increasing amounts of multimeric VN (0 to 143 nM). Platelet coverage to fibrin alone accounted for 25.5% and increased to a maximal platelet coverage of 52% in the presence of 142 nM VN (Figure 3A). In the next set of experiments, the combined fibrin/VN clot was formed with 143 nM multimeric VN. To evaluate the degree of spreading, single platelets adherent to fibrin or to fibrin/VN were compared. In the absence of VN, single platelets were small with some protrusions (Figure 3B), whereas single platelets on the fibrin/VN matrix showed a spread morphology (Figure 3C).
Fibrin-associated multimeric VN potentiates platelet adhesion and aggregation. Whole-blood perfusions were performed for 5 minutes at a shear rate of 500 s–1 on (A) fibrin in the absence or presence of increasing amounts of VN (0 to 143 nM). Platelet coverage shows a VN-dependent increase. A detail of single platelets adherent to fibrin (Ai; B) or fibrin plus VN (Aii; C)(143 nM) is shown (original magnification, × 1000). In a duplicate set of experiments, perfusions were performed on (D) immobilized VN or on clots composed of fibrin or fibrin with 143 nM VN in the absence or presence of control huMab, huMab VN18 directed against multimeric VN, Moab C17 against the βIIIa subunit of the VN-receptor, Poab A0080 against fibrin(ogen), and after preincubation of the fibrin/VN network with PAI-1 (100 μg/mL). Perfusion results are shown as mean coverage (%) ± SEM.
Fibrin-associated multimeric VN potentiates platelet adhesion and aggregation. Whole-blood perfusions were performed for 5 minutes at a shear rate of 500 s–1 on (A) fibrin in the absence or presence of increasing amounts of VN (0 to 143 nM). Platelet coverage shows a VN-dependent increase. A detail of single platelets adherent to fibrin (Ai; B) or fibrin plus VN (Aii; C)(143 nM) is shown (original magnification, × 1000). In a duplicate set of experiments, perfusions were performed on (D) immobilized VN or on clots composed of fibrin or fibrin with 143 nM VN in the absence or presence of control huMab, huMab VN18 directed against multimeric VN, Moab C17 against the βIIIa subunit of the VN-receptor, Poab A0080 against fibrin(ogen), and after preincubation of the fibrin/VN network with PAI-1 (100 μg/mL). Perfusion results are shown as mean coverage (%) ± SEM.
Next, platelet adhesion was studied in the presence of huMab VN18. Whole blood perfusion showed 21% ± 5% platelet coverage on a fibrin network (Figure 3D). Again, a 2-fold increase in platelet adhesion was found on a combined fibrin/VN network (42% ± 1%, P < .01 versus fibrin alone, Figure 3D). Addition of huMab VN18 (16 μg/mL) resulted in inhibition of platelet adhesion to the combined fibrin/VN network (20% ± 5%, P < .01 versus control huMab) to the level of adhesion to fibrin alone (21% ± 5%, Figure 3D). Increasing concentrations of huMab VN18 had no additional inhibiting effect on platelet adhesion (not shown). A control huMab had no effect on platelet adhesion. On a surface of fibrin alone, huMab VN18 had no effect (Figure 3D). Preincubation of both fibrin and the combined fibrin/VN network with an antibody against fibrin(ogen) resulted in more than 90% inhibition of surface coverage (in both cases P < .01 versus control huMab, Figure 3D). Blockage of the platelet integrin βIIIa subunit with Moab C17 blocked platelet adhesion completely to both the fibrin and the fibrin/VN network (in both cases P < .01 versus control huMab, Figure 3D). These results indicate that fibrin is a requisite for the VN-potentiating platelet interaction with a combined fibrin/VN network.
PAI-1 has been shown to bind to fibrin through VN27 primarily through the amino-terminal somatomedin B domain.28-32 To investigate the contribution of the amino-terminal domain of VN in platelet adhesion, the combined fibrin/VN network was preincubated with PAI-1 (100 μg/mL). This resulted in reduced platelet adhesion comparable to the level on fibrin alone (22% ± 5%, P < .05 versus absence of PAI-1, Figure 3D).
Plasma-VN does not contribute to the VN-potentiating platelet adhesion
To investigate a possible contribution of plasma-VN in the platelet-clot interaction, perfusion studies on fibrin and the combined fibrin/VN matrix were performed with reconstituted blood containing HAS instead of plasma. The fibrin/VN network showed twice as much platelet coverage as fibrin alone (data not shown), and this increase in platelet adhesion was abolished in the presence of huMab VN18 (16 μg/mL), similar to the results obtained with whole blood (Figure 3). These results indicate that plasma-VN did not contribute to the VN-potentiating effect on platelet-clot interactions.
The localization of VN after perfusion was studied using CLSM using a green fluorescently labeled Poab directed against VN and red fluorescently labeled platelets. In the combined matrix, multimeric VN colocalized with the network of fibrin fibers (Figure 4A,C,E). When fibrin was made from fibrinogen alone, VN was not detected on the fibrin network but was associated with the platelets (Figure 4B,D,F). Because the perfusate did not contain plasma and therefore no plasma-VN, this finding implies that during perfusion on fibrin, VN is secreted from platelets and becomes associated with the platelet surface.
Isolated platelets in plasma-deficient reconstituted blood express VN on their surface after perfusion over a fibrin network. Perfusion studies were performed for 5 minutes at a shear rate of 500 s–1 with reconstituted blood containing human albumin solution instead of plasma on fibrin plus multimeric VN (A, C, E) or fibrin (B, D, F) fibers. After perfusion, coverslips were processed for confocal laser scanning microscopy (original magnification × 250): VN was visualized with an FITC-labeled Poab (green, A-B), and platelets were labeled with TRITC-phallacidin (red, C-D). The merged signal is shown in yellow (E-F). White bar represents 25 μm.
Isolated platelets in plasma-deficient reconstituted blood express VN on their surface after perfusion over a fibrin network. Perfusion studies were performed for 5 minutes at a shear rate of 500 s–1 with reconstituted blood containing human albumin solution instead of plasma on fibrin plus multimeric VN (A, C, E) or fibrin (B, D, F) fibers. After perfusion, coverslips were processed for confocal laser scanning microscopy (original magnification × 250): VN was visualized with an FITC-labeled Poab (green, A-B), and platelets were labeled with TRITC-phallacidin (red, C-D). The merged signal is shown in yellow (E-F). White bar represents 25 μm.
Multimeric VN is associated with fibrin mainly through the carboxyl-terminal region
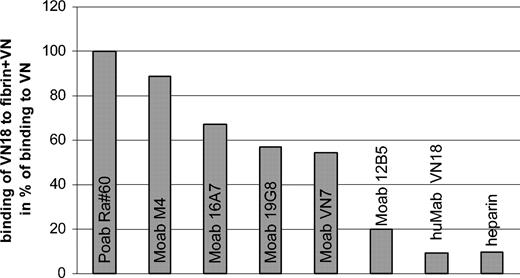
Plasma-VN did not contribute to the VN-mediated platelet-clot interaction; therefore, the inhibitory effect of huMab VN18 must be directed toward multimeric VN, either incorporated into the fibrin clot or bound to the platelets. HuMab VN18 hardly recognized multimeric VN when incorporated into a fibrin network (9% compared with directly coated VN) (Figure 5). We subsequently tested the recognition profile of other Moabs directed against VN, of which the epitopes have been mapped (de Boer et al33 and data not shown). The antibody recognition of fibrin-incorporated multimeric VN decreased when the epitope of the Moab was localized more carboxyl-terminally (Figure 5). Interestingly, the binding site of huMab VN18 has been mapped to a region between residues 268 to 336 (H.J.B., H.C.d.B., E.E.V., manuscript in preparation), which partially overlaps the binding site of Moab 12B5 (residues 239 to 310). Taken together, these results indicate that the carboxyl-terminal region of VN becomes inaccessible upon incorporation of multimeric VN into the fibrin clot and suggests involvement of this region in the interaction of VN with fibrin. Because the heparin-binding domain is also located in the carboxyl-terminal region (residues 348 to 361), we tested whether binding of heparin to fibrin-incorporated VN was affected. Indeed, binding of biotinylated heparin to fibrin-associated multimeric VN accounted for only 10% of the binding of heparin to directly coated VN.
The carboxyl terminus of multimeric VN is masked when VN is incorporated into a fibrin network. The concentration of antibody or biotinylated heparin was determined at which half-maximal binding to plastic-coated multimeric VN was observed. Using this concentration, binding to multimeric VN incorporated into a fibrin network was measured. Relative binding was calculated as percentage binding of antibodies to fibrin/VN divided by binding to VN alone, taking Poab Ra#60 as 100%. This was done for Moabs directed against the amino-terminus (M4, 16A7), the middle portion (19G8, VN7), and the carboxyl terminus (12B5, huMab VN18) of VN and for heparin.
The carboxyl terminus of multimeric VN is masked when VN is incorporated into a fibrin network. The concentration of antibody or biotinylated heparin was determined at which half-maximal binding to plastic-coated multimeric VN was observed. Using this concentration, binding to multimeric VN incorporated into a fibrin network was measured. Relative binding was calculated as percentage binding of antibodies to fibrin/VN divided by binding to VN alone, taking Poab Ra#60 as 100%. This was done for Moabs directed against the amino-terminus (M4, 16A7), the middle portion (19G8, VN7), and the carboxyl terminus (12B5, huMab VN18) of VN and for heparin.
These results support the idea that incorporation of multimeric VN into a fibrin network occurs mainly through interaction with the carboxyl-terminal portion, resulting in the loss of the huMab VN18 epitope and the heparin-binding capacity. Furthermore, this indicates that the inhibiting effect of huMab VN18 on platelet adhesion must be directed toward multimeric VN present on the platelet surface.
Activated platelets express the epitope for huMab VN18 on their surface
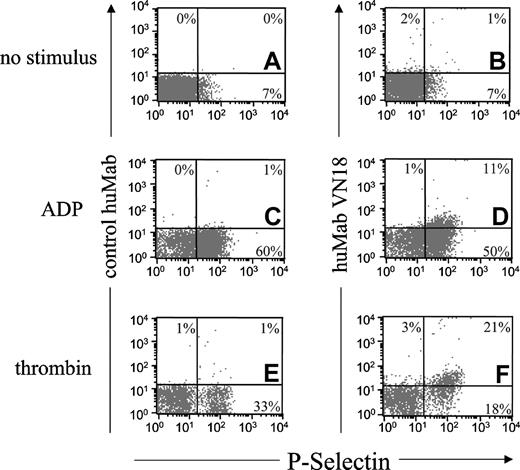
The results in Figure 4 imply that the epitope for huMab VN18 is present on the surface of activated platelets. Therefore, we performed fluorescence-activated cell sorter (FACS) analysis on isolated platelets, either resting or activated. The activation of the platelets was monitored using a Moab against P-selectin. After the isolation procedure, a small amount of the platelets already expressed P-selectin (7%, Figure 6A-B), but expression of the huMab VN18 epitope was not observed (Figure 6B). ADP stimulation (50 μM) resulted in abundant P-selectin expression, as was evidenced by a shift of the platelet population along the x-axis (Figure 6C-D). The P-selectin–positive platelets also expressed the huMab VN18 epitope (Figure 6D). Thrombin stimulation (0.5 U/mL) caused P-selectin expression on a subpopulation of the platelets, leading to 30% P-selectin–positive platelets (Figure 6E-F), which simultaneously expressed the huMab VN18 epitope (Figure 6F). In all cases, the control huMab showed no binding to the platelets (Figure 6A,C,E). These FACS data indicate that expression of the activation marker P-selectin coincides with expression of the epitope for huMab VN18. Thus, these results support our conclusion that huMab VN18 affects platelet adhesion through an interaction with platelet-associated multimeric VN.
Stimulated platelets express the huMab VN18 epitope after stimulation. Isolated platelets were not stimulated (A-B) or stimulated with ADP (50 μM; C-D) or thrombin (0.5 U/mL; E-F). Stimulation of platelets was monitored using the activation marker P-selectin, expressed as fluorescent signal on the x-axis. Binding of control huMab (10 μg/mL; A, C, E) or huMab VN18 (10 μg/mL; B, D, F) was expressed as fluorescent signal on the y-axis. The percentage of platelets localized in the rectangles of the plots is shown.
Stimulated platelets express the huMab VN18 epitope after stimulation. Isolated platelets were not stimulated (A-B) or stimulated with ADP (50 μM; C-D) or thrombin (0.5 U/mL; E-F). Stimulation of platelets was monitored using the activation marker P-selectin, expressed as fluorescent signal on the x-axis. Binding of control huMab (10 μg/mL; A, C, E) or huMab VN18 (10 μg/mL; B, D, F) was expressed as fluorescent signal on the y-axis. The percentage of platelets localized in the rectangles of the plots is shown.
Homotypic VN interactions occur through the amino-terminal and carboxyl-terminal region
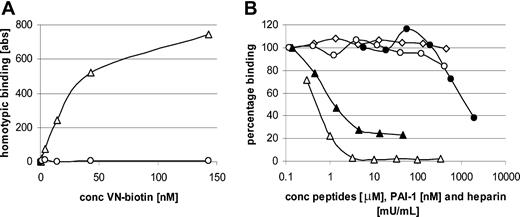
Our results indicate that fibrin-incorporated VN and platelet-bound VN contribute to platelet-clot interactions. The only possible explanation for this observation would be the occurrence of homotypic VN interactions. We therefore tested whether purified biotinylated VN (0 to 143 nM) was able to bind directly to purified VN (71 nM) coated to plastic wells. Biotinylated, multimeric VN bound to immobilized VN dose dependently (Figure 7A).
Homotypic VN interactions occur through the amino-terminal and carboxyl-terminal portions. (A) Homotypic VN interaction was measured after incubation of increasing amounts of biotinylated multimeric VN on immobilized multimeric VN (▵) or bovine serum albumin (○). Half-maximal binding occurred at 28 nM. (B) Homotypic VN binding was characterized using a competition ELISA in which increasing amounts of unfractionated heparin (▵), VN peptide containing residues 348 to 361 (▴) and residues 47 to 64 (○), or control peptide 105Y (⋄) or PAI-1 (•) was added to a fixed concentration of biotinylated VN (28 nM). Results are shown as percentage of binding in the absence of competitors.
Homotypic VN interactions occur through the amino-terminal and carboxyl-terminal portions. (A) Homotypic VN interaction was measured after incubation of increasing amounts of biotinylated multimeric VN on immobilized multimeric VN (▵) or bovine serum albumin (○). Half-maximal binding occurred at 28 nM. (B) Homotypic VN binding was characterized using a competition ELISA in which increasing amounts of unfractionated heparin (▵), VN peptide containing residues 348 to 361 (▴) and residues 47 to 64 (○), or control peptide 105Y (⋄) or PAI-1 (•) was added to a fixed concentration of biotinylated VN (28 nM). Results are shown as percentage of binding in the absence of competitors.
To characterize this homotypic interaction in more detail, binding of biotinylated VN to immobilized VN was tested in the presence of increasing amounts of a positively charged peptide containing the heparin-binding domain of VN (residues 348 to 361, 0to46 μM), a negatively charged peptide containing residues 47 to 64 of VN (0 to 406 μM), a control peptide derived from α1-proteinase inhibitor (105Y, 0 to 444 μM), and the negatively charged compound heparin (0 to 330 mU/mL). VN peptide 348-361 and heparin inhibited homotypic VN interactions by 80% and 100%, respectively (Figure 7B). Peptide 47-64 showed a maximal inhibition of 20% at the highest concentration, and the control peptide derived from α1-proteinase inhibitor showed no inhibition (Figure 7B).
Because PAI-1 inhibited binding of platelets from whole blood to the combined fibrin/VN network, PAI-1 was tested for its ability to inhibit homotypic VN interactions. At the highest PAI-1 concentration that we could add (1.9 μM), 60% inhibition was observed (Figure 7B).
Our results demonstrate that homotypic interactions between VN molecules exist, most probably through oppositely charged domains.
Discussion
In this paper, we established 2 important new features of VN. (1) When incorporated into a fibrin clot, VN promotes platelet adhesion and aggregate formation. (2) VN-dependent platelet adhesion and aggregation on a fibrin clot is mediated by homotypic interactions of VN molecules bound to the platelet surface and VN molecules associated with fibrin fibers.
Our conclusions are in part deduced from results obtained with huMab VN18. To clarify our conclusions, the different features of huMab VN18 are discussed here.
HuMab VN18 is directed against an epitope in hemopexin domain II (data not shown), which is cryptic in the native form and becomes exposed when VN is activated.20 As a result, huMab VN18 exclusively recognizes multimeric VN and not the plasma form (Table 1). Using FACS analysis, we have shown that multimeric VN is expressed on the platelet surface once the platelets are activated with either ADP or thrombin (Table 1). Multimeric VN on the platelet surface actually contributed to platelet-platelet interactions induced by ADP (Table 1), because huMab VN18 inhibited ADP-induced aggregation in platelet-rich plasma. Platelet aggregation occurs in 2 phases: In the first phase, single platelets associate after activation of integrins; the second phase is started when the α-granules release their content and secrete adhesive proteins like von Willebrand factor, fibrinogen, fibronectin, and VN. This release reaction coincides with the formation of large aggregates. During the second phase, about half of the platelet-derived, secreted VN remains platelet bound.7 HuMab VN18 mainly inhibited the second phase of platelet aggregation.
The involvement of VN in platelet aggregation is in agreement with a report of Asch and Podack, who already demonstrated that ADP-, epinephrin-, and thrombin-induced, but not collagen-induced, platelet aggregation could be inhibited with an antibody directed against VN.12 Additionally, Moab 13H1 against multimeric VN, which inhibits binding of VN to integrins through interaction with the somatomedin B domain, could markedly reduce ADP-induced platelet aggregation.13
In the presence of huMab VN18, trombi formed in whole plasma after ADP or TRAP (not shown) administration showed a tendency to disaggregate in time. This indicates that VN not only plays a role in the initial aggregate formation but also in stabilization of the freshly formed thrombus. Interestingly, abciximab, a Fab fragment of a chimeric mouse-human Moab directed against the β3 subunit of integrins GP IIb/IIIa and αvβ3, which binds both fibrinogen and VN, also caused destabilization of thrombi by preventing aggregate formation and dispersion of thrombi.34
When multimeric VN was incorporated into a fibrin clot, total platelet adhesion to the clot was increased 2-fold in comparison to a clot devised from fibrin alone, and the platelets showed pronounced aggregation. The VN-dependent adhesion was reduced in the presence of huMab VN18 to the level of platelet adhesion to fibrin alone (Table 1). The fibrin itself was essential for the aggregation-promoting effect of VN, because perfusion of whole blood on immobilized VN did not show any platelet adhesion. The requisite of fibrin was also shown with a Poab directed against fibrin(ogen) or an antibody against integrin βIIIa: when preincubated on a combined fibrin/VN network, platelet adhesion was almost completely blocked.
The inhibitory action of huMab VN18 was directed toward multimeric VN bound to the platelets, because huMab VN18 does not recognize fibrin-incorporated multimeric VN either in a reconstituted clot or in a plasma-derived clot (Table 1). Because huMab VN18 recognizes the carboxyl terminus of VN, our data suggest that this region is involved in the binding of multimeric VN to fibrin. This hypothesis is supported by the facts that this region was inaccessible for specific Moabs and for heparin, both binding to the carboxyl terminus, and blockage of the accessible aminoterminus of fibrin-incorporated multimeric VN with PAI-1 abrogated the VN-modulated effect on platelet aggregation.
Taken together, these results indicate that platelets become covered with VN after the initial tethering on fibrin and that VN incorporated into a fibrin clot potentiates platelet adhesion and aggregate formation through homotypic association of VN molecules present on the platelet surface and in the clot. The actual homotypic interaction of VN multimers was demonstrated using immobilized VN and biotinylated VN in solution. This interaction was inhibited by the negatively charged compound heparin, by PAI-1, and by a positively charged peptide consisting of the heparin-binding domain, suggesting that oppositely charged domains within the VN molecule are involved in this homotypic association.
Using truncated VN mutants, Schvartz et al35 showed that VN contains 2 potential binding sites for fibrin: one within the amino-terminus (residues 1 to 44) and one within the carboxyl terminus (residues 348 to 379). In our experiments multimeric VN was used, which bound to fibrin primarily through the carboxyl terminus. However, the actual relevance of the VN-potentiating platelet-clot interaction was demonstrated by the inhibitory effect of huMab VN18 in the most physiologic ex vivo setting—namely, perfusion of whole blood over a plasma-derived clot (Table 1). So, even in the presence of normal amounts of soluble fibrinogen, native VN, PAI-1, and other αIIbβIII-binding ligands, such as von Willebrand factor and fibronectin, the VN-modulating platelet adhesion/aggregation was evident. VN shares this property with von Willebrand factor, of which self-assembly of von Willebrand factor multimers was reported to provide a relevant contribution to the arrest of flowing platelets on an injured vascular surface.36 Ni et al already pointed to VN as a possible protein that still mediates thrombus formation in mice lacking both von Willebrand factor and fibrinogen.37 Our data support this contention.
The apparent thrombus-stabilizing effect of VN was recently appreciated in experiments performed with VN-deficient mice, in which the response to vessel injury implicated thrombus/fibrin formation.38 It was shown that by challenging the coagulation system of full-grown VN-deficient mice after arterial injury, the induced thrombi were unstable and embolized frequently.16 Additionally, occlusion of the vessels after injury was delayed in time.39
Interestingly, platelet-rich plasma obtained from VN null mice did not show reduced platelet aggregation,40 suggesting that other aggregation-supporting proteins may take over. Apparently, the lack of VN does not reflect blockage of VN function in platelet aggregation using an antibody. However, the VN deficiency will also manifest itself in the clot formed upon arterial injury, resulting in unstable thrombi.16 These data emphasize the contribution of a fibrin clot, in which incorporated VN supports platelet-clot interactions and stabilization of the thrombus. Our results explain how VN can act as a thrombus-consolidating factor.
Prepublished online as Blood First Edition Paper, April 6, 2004; DOI 10.1182/blood-2003-12-4293.
Supported in part by the Dutch Cancer Society (grant UU1999-2114), the Vanderes Foundation, the Dutch Thrombosis Foundation (grant 20023), and the Netherlands Heart Foundation (grant NHS 2002B157).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Martin IJsseldijk, Cor Seinen, and Anita Jorna for their excellent technical assistance.