Abstract
Transferrin receptor 2 (TfR2) is a type 2 transmembrane protein expressed in hepatocytes that binds iron-bound transferrin (Tf). Mutations in TfR2 cause one form of hereditary hemochromatosis, a disease in which excessive absorption of dietary iron can lead to liver cirrhosis, diabetes, arthritis, and heart failure. The function of TfR2 in iron homeostasis is unknown. We have studied the regulation of TfR2 in HepG2 cells. Western blot analysis shows that TfR2 increases in a time- and dose-dependent manner after diferric Tf is added to the culture medium. In cells exposed to diferric Tf, the amount of TfR2 returns to control levels within 8 hours after the removal of diferric Tf from the medium. However, TfR2 does not increase when non–Tf-bound iron (FeNTA) or apo Tf is added to the medium. The response to diferric Tf appears to be hepatocyte specific. Real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis shows that TfR2 mRNA levels do not change in cells exposed to diferric Tf. Rather, the increase in TfR2 is attributed to an increase in the half-life of TfR2 protein in cells exposed to diferric Tf. Our results support a role for TfR2 in monitoring iron levels by sensing changes in the concentration of diferric Tf.
Introduction
The human body maintains iron homeostasis by regulating the amount of iron absorbed from the diet by the intestine. Iron must be sufficient to sustain fundamental biologic processes, but it should not exceed the storage capacity of cells, because excess iron generates free radicals, resulting in oxidative damage. Serum protein transferrin (Tf) binds iron in the circulation and carries it to cells throughout the body. Erythrocytes use most of the iron in the body for heme synthesis. Macrophages in the liver and spleen phagocytose senescent erythrocytes and recycle iron to the circulation for reuse. Hepatocytes store iron that can be released for use by erythrocytes and other cells when needed. Systemic iron homeostasis, therefore, requires the coordination of iron absorption, transport, storage, and use throughout the body.1,2
Dysregulation of iron homeostasis occurs in numerous diseases.3,4 The most common is the iron overload disorder, hereditary hemochromatosis (HH). HH types 1, 2A, 2B, and 3 are autosomal recessive diseases caused by mutations in HFE,5 hemojuvelin,6 hepcidin,7 and transferrin receptor 2 (TfR2),8 respectively. An autosomal dominant form of HH, type 4, is caused by mutations in the iron exporter, ferroportin 1 (Fpn1).9,10 A resultant deficiency in iron export causes macrophages to retain iron that would normally recycle to the blood. This leads to the accretion of iron in macrophages and to a reduction in saturation of Tf.11 In HH types 1, 2, and 3, the intestine and macrophages fail to receive or interpret signals from the liver and erythrocytes communicating that body iron levels are sufficient. Consequent excess absorption of dietary iron by the intestine and release of iron from macrophages leads to an accumulation of iron in the liver and other parenchymal tissues, saturation of Tf, and elevated secretion into the serum of the cellular iron storage protein ferritin (Ft).1,12 In severe cases of HH, cirrhosis, cancer, heart abnormalities, arthritis, and diabetes ensue if the iron overload is not treated by regular phlebotomy.
The proteins implicated in HH are critical components of incompletely defined signaling pathways between the liver, erythrocytes, macrophages, and intestine that maintain iron homeostasis. An integrated understanding of the manner in which these and other proteins involved in iron transport coordinate to sense, respond to, and regulate iron remains elusive. Iron levels have been shown to modulate synthesis and secretion by hepatocytes of the peptide hormone hepcidin, which in turn affects iron absorption by the intestine and iron export from macrophages, possibly by altering the activity of Fpn1.13-16 The pathways connecting iron levels to hepcidin expression are unknown. The concentration of diferric Tf in the blood may be an indicator of the level of iron in the body that signals to hepatocytes and regulates hepcidin.17,18 A potential mediator of this process is the recently identified receptor for Tf, TfR2.
TfR2 is a type II transmembrane protein and homolog of TfR1,19,20 the ubiquitously expressed receptor for Tf that delivers iron to cells.21 The 2 proteins are 45% identical and 66% similar in their extracellular domains. Like TfR1, TfR2 binds diferric Tf at neutral pH and apo Tf at acidic pH.22 Heterologous expression of TfR2 in Chinese hamster ovary (CHO) cells lacking transferrin receptors (TRVb cells) increases the uptake of Tf-bound iron19 and promotes cell growth under low iron conditions,22 indicating that TfR2 can function as a receptor for Tf. However, differences in the activity, regulation, and expression of TfR1 and TfR2, and in the pathophysiology of disorders caused by their deficiency, indicate that they have different roles in iron homeostasis. The affinity at pH 7.5 of TfR2 for diferric Tf, Kd approximately 27 nM, is approximately 25-fold lower than that of TfR1, Kd approximately 1 nM.22-24 Unlike TfR1, TfR2 does not appear to interact with HFE.24 TfR1 mRNA expression inversely correlates with intracellular iron levels25-29 because of posttranscriptional regulation by iron-response elements (IREs) located in the 3′ untranslated regions (UTRs) of TfR1 transcripts.30-33 In contrast, TfR2 mRNA expression does not change in K562 erythroleukemia or murine erythroleukemia (MEL) cells treated with Fe2(NO3) or with the iron chelator desferrioxamine (DFO),22,34 nor does it change in iron-deficient or iron-overloaded mice.20 This is consistent with the absence of IREs in the 3′ UTR of the TfR2 transcript.19,20 Unlike TfR1, TfR2 has limited tissue distribution, with prominent expression of protein in the liver.19,20,35-37 Finally, TfR2 cannot compensate for the loss of TfR1. Knock-out of TfR1 in mice results in embryonic lethality at day 12.5.38 Mutations in TfR2, on the other hand, result in HH type 3 (HH3) in humans and in mice.8,35 In humans homozygous for the Y250X TfR2 mutation and mice transgenic for the orthologous Y245X mutation, the liver accumulates iron, despite an absence of membrane-bound TfR2 and a reduction in TfR1,35 suggesting that the uptake of Tf-bound iron for use by the hepatocytes is not the principal role of TfR2.
The fact that mutations in TfR2 cause HH indicates that TfR2 has an important but unknown role in iron homeostasis. TfR2 may sense body iron levels by sensing the level of diferric Tf in the serum. One prediction of this hypothesis is that TfR2 should respond to changes in the extracellular concentration of diferric Tf. To test this, we characterized the response of TfR2 to Tf in HepG2 cells, a human hepatocarcinoma cell line that endogenously expresses TfR2 and other iron-related proteins. We demonstrate an increase in TfR2 protein in response to elevated exogenous diferric Tf and show that this increase is a result of an increase in the half-life of TfR2.
Materials and methods
Cell lines
HepG2 and K562 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). HuH7 cells were provided by Dr Philip Aisen (Einstein University, Bronx, NY). The TRVb2 cell line was generated by transfecting TRVb cells with a pcDNA 3 vector encoding TfR2, as described previously.37
Antibodies
Generation of monoclonal antibodies 3B82A1 and 9F81C11 against the ectodomains of human TfR1 and TfR2, respectively, was described previously.37 Rabbit anti-hTfR2 serum was produced at Pocono Rabbit Farm and Laboratory (Canadensis, PA) by immunizing rabbits with purified human TfR2 ectodomain. The specificity of the rabbit anti-TfR2 serum was verified in the manner described for the 9F81C11 mouse anti-TfR2 antibody.37 Mouse anti-TfR1 42/6 antibody was obtained from Dr Ian Trowbridge (Salk Institute, La Jolla, CA). Goat anti-Tf serum was described previously.39 Sheep antiferritin antibody was purchased from The Binding Site (Birmingham, United Kingdom). Secondary antibodies against rabbit, mouse, and sheep immunoglobulin G (IgG) conjugated to horseradish peroxidase (HRP) were purchased from Chemicon (Temecula, CA). Fluorescence-labeled Alexa 680 goat anti–rabbit IgG and IRDye 800 donkey anti–mouse IgG secondary antibodies were from Molecular Probes (Eugene, OR) and Rockland Immunochemicals (Gilbertsville, PA), respectively. Rabbit anti–mouse IgG was from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). Rabbit anti–mouse IgA was from Zymed Laboratories (San Francisco, CA). Purified mouse IgA was from Bethyl Laboratories (Montgomery, TX).
Reagent preparation
Stock solutions of Tf were prepared by dissolving diferric Tf (Intergen, Purchase, NY) or apo Tf (Serologicals, Norcross, GA) in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (HBS; pH 7.4). Protein concentration was determined by measuring the A280. Saturation of diferric Tf was verified by an A465/A280 ratio of 0.045.40 For FeNTA, stock solutions of 400 mM nitrilotriacetic acid (NTA; Sigma, St Louis, MO) in phosphate-buffered saline (PBS; pH 7.4) and 400 mM FeCl3 in 0.1 N HCl were prepared. Before each experiment, FeCl3 and NTA were combined in a 1:40 molar ratio to give a final Fe concentration of 10 mM. For control, NTA was combined with the appropriate volume of 0.1 N HCl. Stock solutions of 10 mg/mL cycloheximide (Sigma) were prepared in water.
Cell culture
HepG2 and HuH7 cells were maintained in minimal essential medium (MEM; Life Technologies, Bethesda, MD) supplemented with 1.0 mM sodium pyruvate, 0.1 mM MEM nonessential amino acids (Life Technologies), and 10% fetal bovine serum (FBS). In all experiments, HepG2 cells were seeded at 2 × 104 cell/cm2 4 days before harvesting. K562 cells were cultured in RPMI 1640 (Life Technologies) supplemented with 10% FBS. TRVb2 cells were maintained in F12-nutrient mixture (Life Technologies) supplemented with 5% FBS and 800 μg/mL G418 (Calbiochem, La Jolla, CA). Diferric Tf, apo Tf, FeNTA, cycloheximide, and antibodies were added to the culture medium of cells as described in the figure legends.
Western blot
Cells were lysed on ice in NET-Triton buffer (150 mM NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), 10 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.4, 1% Triton X-100) containing 1 × Complete Mini Protease Inhibitor Cocktail (Roche Diagnostic, Indianapolis, IN) and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were cleared by centrifugation at 16 000g for 5 minutes. Protein concentration was measured using the BCA Protein Assay (Pierce, Rockford, IL). Aliquots of lysates containing 10 to 50 μg total protein were incubated in 3.6 × Laemmli buffer41 for 5 minutes at 95°C and were subjected to reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels for analysis of TfR1, TfR2, and Tf or 12% gels for analysis of Ft. Protein was transferred to nitrocellulose and was detected using rabbit anti-hTfR2 (1:10 000), mouse anti-hTfR1 (1:10 000), goat anti-hTf (1:10 000), or sheep anti-hFt (1:100) primary antibodies and then HRP-conjugated (1:10 000) or fluorescence-labeled (1:5000) secondary antibodies. Bands were visualized by chemiluminescence (SuperSignal WestPico; Pierce) or were visualized and quantified by fluorescence imaging (Odyssey Infrared Imaging System; Li-Cor, Lincoln, NB). The species specificity of the Alexa 680 goat antirabbit and the IRDye 800 donkey antimouse fluorescent secondary antibodies enabled blots to be probed simultaneously with rabbit anti-hTfR2 and mouse anti-hTfR1 antibodies. For quantification, serial dilutions of lysate were loaded onto the same gel to ensure that samples were within the linear range of detection.
Real-Time qRT-PCR
RNA was isolated from approximately 1 × 107 HepG2 cells using the RNeasy RNA Isolation Kit (Qiagen, Valencia, CA). After treating RNA with 10 units DNase (Roche Diagnostics) to remove contaminating genomic DNA, cDNA was synthesized from 2 μg RNA using Oligo dT primers and Superscript II Reverse Transcriptase (RT) (Invitrogen, Carlsbad, CA). cDNA was diluted 1:10 and was analyzed by polymerase chain reaction (PCR) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers spanning an intron/exon junction to verify yield and to confirm the absence of product from contaminating genomic DNA. Samples were analyzed by real-time qRT-PCR using the SYBR green detection system on an ABI PRISM 7900 machine (Applied Biosystems, Foster City, CA). Primer pairs were designed with Primer Express software (Applied Biosystems) and synthesized by IDT Technologies (Coralville, IA). Primers pairs used were GAPDH 868F/968R 5′-ACCCACTCCTCCACCTTTGA-3′ and 5′-CTGTTGCTGTAGCCAAATTCGT-3′); TfR1 305F/435R 5′-CAGGAACCGAGTCTCCAGTGA-3′ and 5′-CTTGATGGTGCCGGTGAA GT-3′); and TfR2 1461F/1563R 5′-GGAGTGGCTAGAAGGCTACCTCA-3′ and 5′-GGTCTTGGCATGAAACTTGTC A-3′). Primers were verified and data were analyzed using the ΔCT (difference in threshold cycles) method, as described previously.42,43
Immunoprecipitation
Pansorbin (Calbiochem, La Jolla, CA) was coated with rabbit anti–mouse IgG for immunoprecipitation with anti-TfR1 and anti-TfR2 mouse monoclonal antibodies and with rabbit anti–mouse IgA for immunoprecipitation with 42/6 anti-TfR1 mouse monoclonal antibody. Cells (approximately 2 × 106) were washed twice with ice-cold PBS and then were lysed in 100 μL NET-Triton buffer containing 1 × Complete Mini Protease Inhibitor Cocktail (Roche Diagnostic) and 1 mM PMSF. Lysates were preadsorbed by incubation for 1 hour at 4°C with 50 μL Pansorbin/IgA or Pansorbin/IgG to reduce nonspecific interactions. Preadsorbed lysates were incubated for 1 hour at 4°C with 25 μL Pansorbin/IgG and 1.5 μL mouse anti-TfR1 or mouse anti-TfR2 antibodies or with 25 μL Pansorbin/IgA and 5 μg 42/6 anti-TfR1 antibody. Pansorbin was pelleted by centrifugation at 16 000g for 2 minutes, resuspended in 100 μL NET-Triton buffer, and washed through 1 mL NET-Triton buffer with 15% sucrose. Samples were eluted into 20 μL 2 × Laemmli buffer,41 heated at 95°C for 5 minutes, and subjected to Western blot analysis. Proteins were detected with rabbit anti-hTfR2 (1:10 000) or sheep anti-hTfR1/Tf serum (1:10 000).
Results
Response of TfR2 to diferric Tf in HepG2 cells
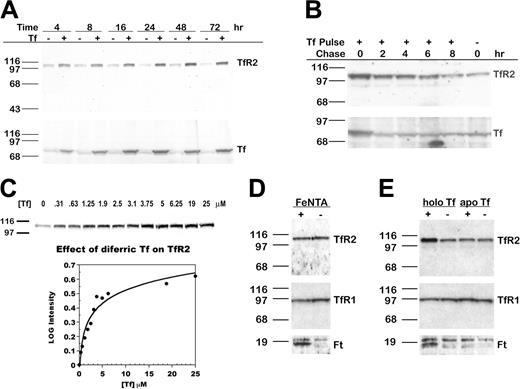
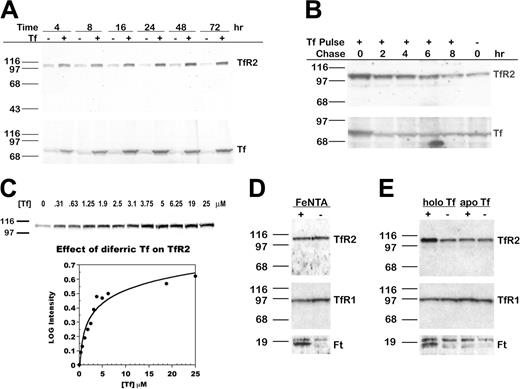
To determine whether Tf alters TfR2 protein levels, we cultured HepG2 cells in medium with 25 μM (2 mg/mL) diferric Tf for 4 to 72 hours and examined levels of TfR2 and Tf by Western blot analysis. An increase in TfR2 was evident within 4 hours, maximal at 48 hours, and sustained for at least 72 hours (Figure 1A, upper panel). The increase in TfR2 paralleled an increase in Tf associated with the cells (Figure 1A, lower panel). Treatment with Tf did not increase the levels of Tf transcript (measured by real-time qRT-PCR) or the rate of Tf protein synthesis (measured by metabolic labeling with 35S-cysteine/methionine; our unpublished results, 2004), suggesting that this Tf was exogenous rather than endogenous. When diferric Tf was withdrawn after 24 hours, TfR2 returned to basal levels within 8 hours, subsequent to the return of Tf to basal levels within 2 hours (Figure 1B).
TfR2 increases after the addition of diferric Tf to the medium of HepG2 cells. (A) TfR2 increases in a time-dependent manner. HepG2 cells were cultured for 4 to 72 hours after the addition of 25 μM diferric Tf or HBS to the medium. Lysates (20 μg total protein) were transferred to nitrocellulose, probed for TfR2 and Tf, and visualized by chemiluminescence. The increase in TfR2 was paralleled by an increase in Tf associated with the cells. (B) TfR2 returns to basal levels after withdrawal of Tf from the medium. HepG2 cells were cultured for 24 hours in the presence of 25 μM diferric Tf, then chased in medium for 0 to 8 hours. TfR2 and Tf levels were analyzed by Western blot using HRP-conjugated secondary antibodies and chemiluminescence. (C) The response of TfR2 to diferric Tf is concentration dependent. HepG2 cells were cultured for 24 hours after the addition of 0 to 25 μM diferric Tf to the medium, and lysates (20 μg total protein) were analyzed by Western blot with fluorescence-labeled secondary antibodies for quantification and HRP-conjugated secondary antibodies for chemiluminescence imaging, as described in “Materials and methods.” The intensity of each band was normalized to the intensity of the 0 μM Tf sample. The log of the normalized intensity is plotted as a function of diferric Tf concentration. The increase in TfR2 is half-maximal at approximately 2.5 μM diferric Tf. (D) TfR2 does not increase in response to nontransferrin-bound iron. TfR2, TfR1, and Ft protein levels were assessed by Western blots of lysates (20 μg total protein) from HepG2 cells cultured for 24 hours in the presence of 100 μM FeNTA (lane 1,+) or 4 mM NTA (lane 2,-). Bands were detected by chemiluminescence. Ft heavy and light chains are visible as a doublet in the lower panel. (E) TfR2 does not increase in response to apo Tf. HepG2 cells were incubated in medium containing 25 μM diferric Tf (lane 1, labeled holo Tf) or apo Tf (lane 3) for 24 hours. Lysates (20 μg total protein) were analyzed by Western blot for TfR2, TfR1, and Ft protein. Bands were detected by chemiluminescence.
TfR2 increases after the addition of diferric Tf to the medium of HepG2 cells. (A) TfR2 increases in a time-dependent manner. HepG2 cells were cultured for 4 to 72 hours after the addition of 25 μM diferric Tf or HBS to the medium. Lysates (20 μg total protein) were transferred to nitrocellulose, probed for TfR2 and Tf, and visualized by chemiluminescence. The increase in TfR2 was paralleled by an increase in Tf associated with the cells. (B) TfR2 returns to basal levels after withdrawal of Tf from the medium. HepG2 cells were cultured for 24 hours in the presence of 25 μM diferric Tf, then chased in medium for 0 to 8 hours. TfR2 and Tf levels were analyzed by Western blot using HRP-conjugated secondary antibodies and chemiluminescence. (C) The response of TfR2 to diferric Tf is concentration dependent. HepG2 cells were cultured for 24 hours after the addition of 0 to 25 μM diferric Tf to the medium, and lysates (20 μg total protein) were analyzed by Western blot with fluorescence-labeled secondary antibodies for quantification and HRP-conjugated secondary antibodies for chemiluminescence imaging, as described in “Materials and methods.” The intensity of each band was normalized to the intensity of the 0 μM Tf sample. The log of the normalized intensity is plotted as a function of diferric Tf concentration. The increase in TfR2 is half-maximal at approximately 2.5 μM diferric Tf. (D) TfR2 does not increase in response to nontransferrin-bound iron. TfR2, TfR1, and Ft protein levels were assessed by Western blots of lysates (20 μg total protein) from HepG2 cells cultured for 24 hours in the presence of 100 μM FeNTA (lane 1,+) or 4 mM NTA (lane 2,-). Bands were detected by chemiluminescence. Ft heavy and light chains are visible as a doublet in the lower panel. (E) TfR2 does not increase in response to apo Tf. HepG2 cells were incubated in medium containing 25 μM diferric Tf (lane 1, labeled holo Tf) or apo Tf (lane 3) for 24 hours. Lysates (20 μg total protein) were analyzed by Western blot for TfR2, TfR1, and Ft protein. Bands were detected by chemiluminescence.
The dose response of TfR2 to diferric Tf was examined by adding 0 to 25 μM (0-2 mg/mL) diferric Tf to the medium for 24 hours. Western blot analysis showed that TfR2 increased as the concentration of diferric Tf increased from approximately 0 to 12.5 μM (Figure 1C). In contrast, TfR1 decreased at the lowest concentration of diferric Tf assayed and remained at this level as the concentration of diferric Tf increased (results not shown). We quantified the effect of diferric Tf on TfR2 by Western blot detection with fluorescence-labeled secondary antibodies. The increase in TfR2 was half-maximal when the concentration of diferric Tf was approximately 2.5 μM (Figure 1C).
Because diferric Tf supplies cells with iron, we considered that the increase in TfR2 might be a response to elevated cellular iron levels. To address this, we added 100 μM FeNTA, a non–Tf-bound iron source, to the medium of cells for 24 hours and examined protein levels by Western blot. The levels of TfR1 and Ft, which are regulated by the IRE system in response to changes in intracellular iron, served as positive controls. As expected, Ft increased and TfR1 decreased in cells treated with FeNTA (Figure 1D). TfR2 levels, however, remained the same, indicating that TfR2 responds specifically to diferric Tf rather than to iron loading of cells. When cells were cultured in medium with 25 μM apo Tf for 24 hours, TfR2 remained the same (Figure 1E). Because the interaction of apo Tf with its receptors is weak at neutral pH, the results suggest that the response of TfR2 to diferric Tf requires interaction of Tf with TfR1 or TfR2 or the delivery of iron specifically by diferric Tf.
Response of TfR2 to Tf in other cell lines
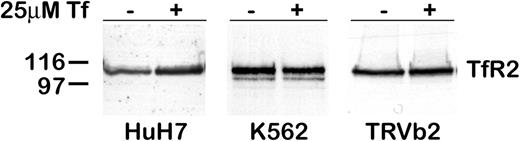
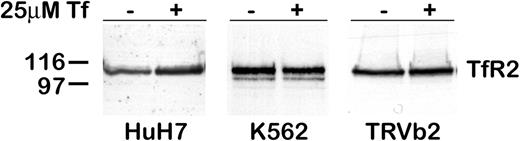
The effect of diferric Tf on TfR2 in other cells lines was investigated (Figure 2). In TRVb2 cells (CHO cells lacking detectable expression of endogenous TfR144 and stably transfected with TfR2), adding diferric Tf to the medium had no effect on TfR2 protein level. Similarly, in K562 cells, an erythroleukemia cell line endogenously expressing TfR2, diferric Tf had no effect. However, in HuH7 cells, a human hepatoma cell line endogenously expressing TfR2, adding 25 μM diferric Tf to the medium for 24 hours produced an increase in TfR2. These results suggest that the regulation of TfR2 by Tf involves a hepatocyte-specific mechanism.
TfR2 increases in HuH7 cells, but not in K562 or TRVb2 cells. Cells were cultured for 24 hours after the addition of 25 μM diferric Tf to the medium. The level of TfR2 in lysates from HuH7 (50 μg), K562 (20 μg), and TRVb2 (10 μg) cells was determined by Western blot with chemiluminescence detection. In cells endogenously expressing TfR2, treatment with diferric Tf increased TfR2 in HuH7 human hepatoma cells but not in K562 erythroleukemia cells. TRVb cells stably transfected with TfR2 (TRVb2) did not respond to diferric Tf.
TfR2 increases in HuH7 cells, but not in K562 or TRVb2 cells. Cells were cultured for 24 hours after the addition of 25 μM diferric Tf to the medium. The level of TfR2 in lysates from HuH7 (50 μg), K562 (20 μg), and TRVb2 (10 μg) cells was determined by Western blot with chemiluminescence detection. In cells endogenously expressing TfR2, treatment with diferric Tf increased TfR2 in HuH7 human hepatoma cells but not in K562 erythroleukemia cells. TRVb cells stably transfected with TfR2 (TRVb2) did not respond to diferric Tf.
Effect of diferric Tf on TfR2 mRNA and protein stability
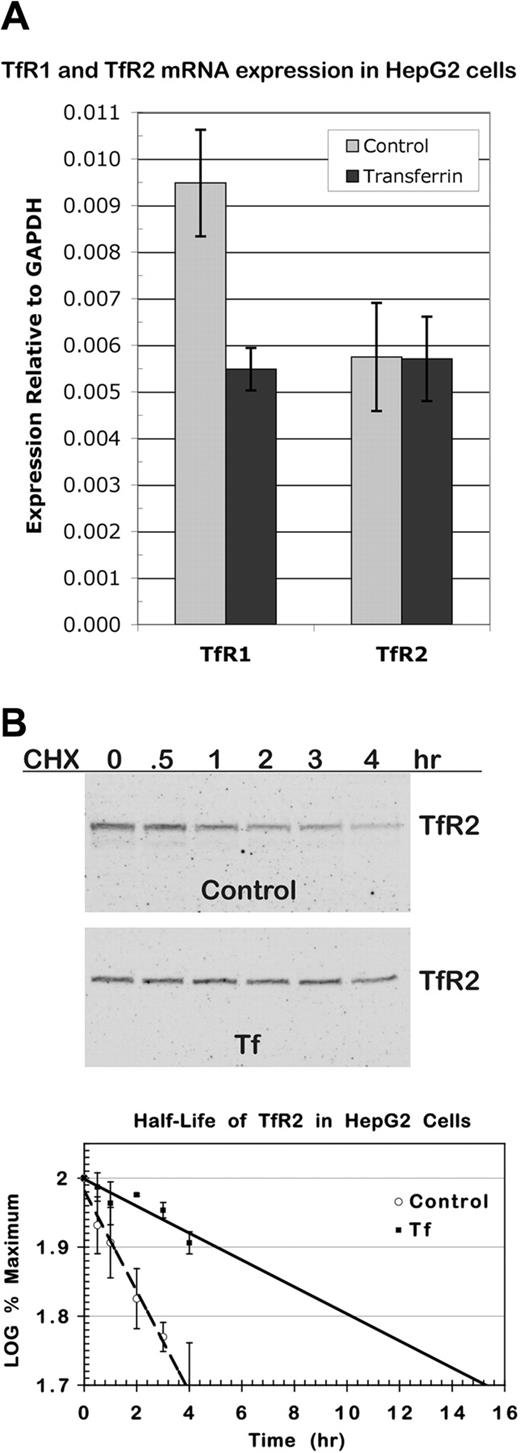
To determine whether the increase in TfR2 was a consequence of an increase in TfR2 transcript, we measured TfR2 mRNA in control cells and in cells cultured in medium with 25 μM diferric Tf for 24 hours using real-time qRT-PCR. As a positive control for the effect of diferric Tf, TfR1 mRNA was also measured. Levels of TfR1 and TfR2 transcripts were quantified relative to GAPDH levels. Three independent experiments were conducted, and for each experiment samples were analyzed twice in triplicate. As expected, TfR1 mRNA decreased when cells were cultured with diferric Tf (Figure 3A). In contrast, TfR2 mRNA did not change, indicating that the increase in protein does not derive from an increase in TfR2 transcript.
Regulation of TfR2 occurs at the protein level. (A) TfR2 transcript in HepG2 cells does not increase in response to diferric Tf. Total RNA was isolated from approximately 1 × 107 HepG2 cells 24 hours after the addition of 25 μM diferric Tf or an equal volume of HBS to the medium. Expression of TfR2, TfR1, and GAPDH transcripts was measured by real-time qRT-PCR analysis of cDNA synthesized from 2 μg total RNA. Levels of TfR2 and TfR1 transcripts are shown relative to GAPDH levels. The graph represents the mean of 3 separate experiments in which each sample was analyzed twice in triplicate. Error bars depict SD. (B) Diferric Tf stabilizes TfR2 protein. HepG2 cells seeded at 2 × 104 cells/cm2 were incubated in normal medium or medium with 25 μM diferric Tf for 24 hours before the addition of 100 μg/mL cycloheximide for 4, 3, 2, 1, 0.5, and 0 hours. Cells were solubilized, lysates from duplicate wells were pooled, and half of each sample was analyzed by Western blot. TfR2 and TfR1 were detected with fluorescence-labeled secondary antibodies for quantification and then with HRP-conjugated secondary antibodies for chemiluminescence imaging. The integrated intensity of TfR2 was normalized to that of TfR1, which did not change detectably over the time-course of the experiment. The normalized intensity was expressed as a percentage of the normalized intensity at time 0, and the log of this value was plotted. Half-life was determined by linear regression analysis. The graph shows the mean of 3 experiments. Error bars indicate average deviation from the mean.
Regulation of TfR2 occurs at the protein level. (A) TfR2 transcript in HepG2 cells does not increase in response to diferric Tf. Total RNA was isolated from approximately 1 × 107 HepG2 cells 24 hours after the addition of 25 μM diferric Tf or an equal volume of HBS to the medium. Expression of TfR2, TfR1, and GAPDH transcripts was measured by real-time qRT-PCR analysis of cDNA synthesized from 2 μg total RNA. Levels of TfR2 and TfR1 transcripts are shown relative to GAPDH levels. The graph represents the mean of 3 separate experiments in which each sample was analyzed twice in triplicate. Error bars depict SD. (B) Diferric Tf stabilizes TfR2 protein. HepG2 cells seeded at 2 × 104 cells/cm2 were incubated in normal medium or medium with 25 μM diferric Tf for 24 hours before the addition of 100 μg/mL cycloheximide for 4, 3, 2, 1, 0.5, and 0 hours. Cells were solubilized, lysates from duplicate wells were pooled, and half of each sample was analyzed by Western blot. TfR2 and TfR1 were detected with fluorescence-labeled secondary antibodies for quantification and then with HRP-conjugated secondary antibodies for chemiluminescence imaging. The integrated intensity of TfR2 was normalized to that of TfR1, which did not change detectably over the time-course of the experiment. The normalized intensity was expressed as a percentage of the normalized intensity at time 0, and the log of this value was plotted. Half-life was determined by linear regression analysis. The graph shows the mean of 3 experiments. Error bars indicate average deviation from the mean.
The elevation in TfR2 could be a consequence of an increase in protein stability. To investigate this possibility, we measured the half-life of TfR2 protein in untreated and diferric Tf–treated cells. After culturing cells for 24 hours in 25 μM diferric Tf, 100 μg/mL cycloheximide was added to the medium for 0 to 4 hours to inhibit protein synthesis. Levels of TfR2 and TfR1 protein were quantitated by Western blot (Figure 3B). TfR1 did not change detectably over the period assayed. As measured from 3 independent experiments, in untreated cells the half-life of TfR2 was 4 hours, and in Tf-treated cells the half-life of TfR2 increased to 14 hours.
Requirement for TfR1 in mediating the effect of diferric Tf on TfR2
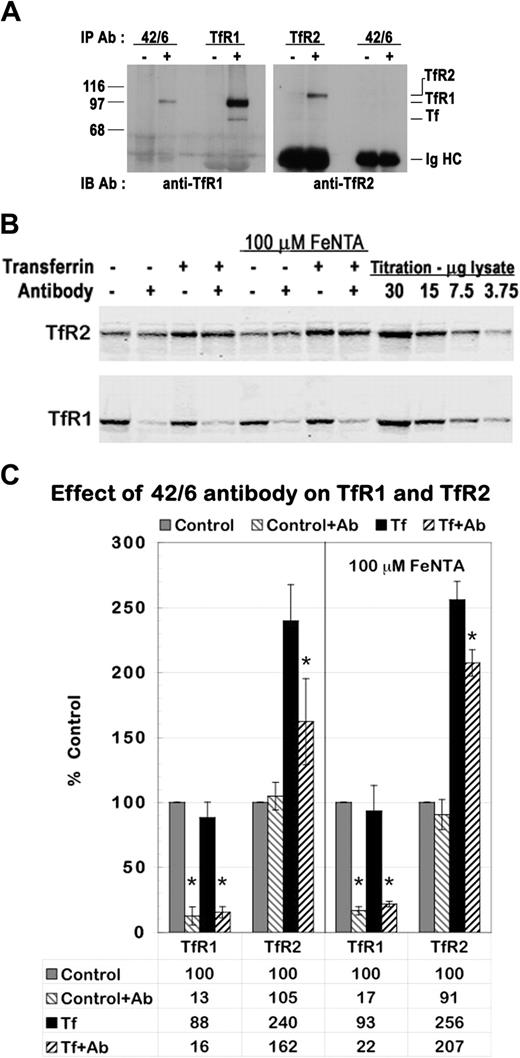
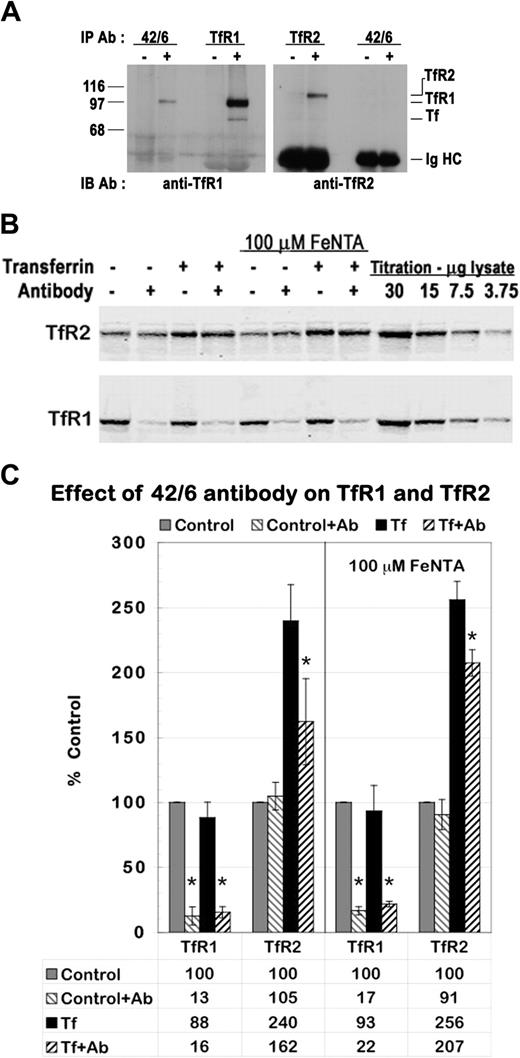
HepG2 cells express both TfR1 and TfR2; thus, regulating TfR2 stability may involve the binding of diferric Tf to either or both of its receptors. To investigate whether interaction of TfR1 is required to mediate the effect of diferric Tf on TfR2, we used the 42/6 monoclonal antibody to TfR1, which has been previously shown to block binding of diferric Tf to TfR1 and to reduce TfR1 levels in a variety of cell lines.45,46 The 42/6 antibody is isotype IgA. Dimerization between IgA molecules induces clustering of TfR1, which is consequently targeted to the lysosome for degradation. We first verified the specificity and activity of this antibody in HepG2 cells. Immunoprecipitation of lysates from HepG2 cells demonstrated interaction of 42/6 antibody with TfR1 but not with TfR2 (Figure 4A), indicating the 42/6 antibody interacts specifically with TfR1. Cells cultured in medium with 25 μg/mL 42/6 antibody for 28 hours showed a marked reduction in TfR1 protein when compared with cells cultured in normal medium (Figure 4B, bottom panel, lanes 1-2) or medium containing 25 μg/mL nonspecific IgA (data not shown). Treatment with 42/6 antibody for 4 hours was sufficient to produce this reduction in TfR1 (data not shown). Given that Tf competes with 42/6 for binding to TfR1, 25 μM diferric Tf was added to the medium 4 hours after the addition of 42/6 antibody. TfR1 remained down-regulated in the presence of Tf and 42/6 antibody (Figure 4B, bottom panel, lanes 3-4).
Down-regulation of TfR1 reduces the increase in TfR2 protein. (A) The 42/6 anti-TfR1 antibody interacts with TfR1 but not with TfR2. HepG2 cell lysates were immunoprecipitated with (+) or without (-) 42/6 anti-TfR1, 3B82A1 anti-TfR1, or 9F81C11 anti-TfR2 monoclonal antibodies and were analyzed by Western blot with sheep anti-TfR1/Tf serum and rabbit anti-TfR2 serum. Bands corresponding to TfR2, TfR1, Tf, and the immunoglobulin heavy chains (Ig HC) are indicated. (B-C) Treatment with 42/6 diminishes the effect of diferric Tf on TfR2. Anti-TfR1 antibody 42/6 was added to the medium of HepG2 cells at a concentration of 25 μg/mL 4 hours before the addition of 25 μM diferric Tf to the medium for 24 hours. To control for possible effects of iron deprivation, a second set of cells was treated identically, but 100 μM FeNTA was added concomitantly with 42/6 antibody. Lysates (20 μg total protein) were analyzed by Western blot. (B) TfR1 and TfR2 bands were visualized with fluorescence-labeled secondary antibodies. (C) Fluorescence imaging was used to quantitate TfR1 and TfR2 protein levels. The integrated intensity of each band is expressed as a percentage of control. The graph depicts the mean ± SD of 4 independent experiments. *P < .05, as determined by Student one-tailed paired t test.
Down-regulation of TfR1 reduces the increase in TfR2 protein. (A) The 42/6 anti-TfR1 antibody interacts with TfR1 but not with TfR2. HepG2 cell lysates were immunoprecipitated with (+) or without (-) 42/6 anti-TfR1, 3B82A1 anti-TfR1, or 9F81C11 anti-TfR2 monoclonal antibodies and were analyzed by Western blot with sheep anti-TfR1/Tf serum and rabbit anti-TfR2 serum. Bands corresponding to TfR2, TfR1, Tf, and the immunoglobulin heavy chains (Ig HC) are indicated. (B-C) Treatment with 42/6 diminishes the effect of diferric Tf on TfR2. Anti-TfR1 antibody 42/6 was added to the medium of HepG2 cells at a concentration of 25 μg/mL 4 hours before the addition of 25 μM diferric Tf to the medium for 24 hours. To control for possible effects of iron deprivation, a second set of cells was treated identically, but 100 μM FeNTA was added concomitantly with 42/6 antibody. Lysates (20 μg total protein) were analyzed by Western blot. (B) TfR1 and TfR2 bands were visualized with fluorescence-labeled secondary antibodies. (C) Fluorescence imaging was used to quantitate TfR1 and TfR2 protein levels. The integrated intensity of each band is expressed as a percentage of control. The graph depicts the mean ± SD of 4 independent experiments. *P < .05, as determined by Student one-tailed paired t test.
Having verified that the 42/6 antibody interacts specifically with TfR1 to down-regulate TfR1 expression in HepG2 cells, we assessed the effect of this antibody on TfR2 regulation by diferric Tf. HepG2 cells were cultured with and without 25 μg/mL 42/6 antibody for 4 hours, then with and without 25 μM diferric Tf for 24 hours. In a parallel set of experiments, 100 μM FeNTA was added at the same time as antibody to control for any possible effects of iron deprivation brought about by the down-regulation of TfR1. TfR1 and TfR2 protein levels were assessed simultaneously using two-color Western blot detection with fluorescence-labeled secondary antibodies that specifically detect rabbit (anti-TfR2) and mouse (anti-TfR1) IgG (Figure 4B). The 42/6 antibody reduced TfR1 by 80% to 90% in all conditions tested but had no effect on TfR2 in control cells. An increase in TfR2 induced by diferric Tf is evident in cells cultured with or without 42/6. However, in the absence of 42/6 antibody, diferric Tf caused an approximately 2.5-fold increase inTfR2, whereas in the presence of 42/6 antibody this increase abated to approximately 1.6-fold (Figure C). The difference was statistically significant (P = .02) when evaluated by Student one-tailed paired t test. Supplementing cells with iron did not alter the ability of the 42/6 antibody to down-regulate TfR1 or the response of the cells to diferric Tf. These results indicate that down-regulating TfR1 partially inhibited the increase in TfR2 by Tf.
Discussion
After its identification in 1999,19 TfR2 was shown to bind Tf and to mediate Fe uptake,22 functions that are consistent with its homology to the classical transferrin receptor, TfR1. Unlike TfR1, however, TfR2 expression did not respond to iron and, in a mouse model of HH1 (Hfe-/-), persisted when TfR1 expression was minimal despite iron overload.20 The presence of TfR2 in hepatocytes offered a plausible explanation for the accumulation of iron that occurs in the livers of mice and of patients with HH1. The finding that TfR2 mutation caused hemochromatosis with the same phenotype of iron overload in the liver was a surprise, therefore, and suggested that TfR2 might have a role in maintaining iron homeostasis.8,35 We hypothesized that TfR2 might sense iron levels through its interaction with Tf. To test this, we examined the response of TfR2 to diferric human Tf in HepG2 human hepatoma cells. We found that TfR2 increased when the concentration of diferric Tf in the medium increased. We detected no change in TfR2 transcript, but we measured a 3.5-fold elongation of TfR2 protein half-life when diferric Tf was added to the medium of cells. This does not preclude the possibility that diferric Tf might also affect the rate of TfR2 translation. Because the low level of TfR2 expression in HepG2 cells prohibits detection by metabolic labeling, future experiments will assay the effect of Tf on TfR2 translation using hepatoma cells stably transfected with the full-length TfR2 transcript.
TfR2 is sensitive to changes in diferric Tf concentration that would occur physiologically in response to normal fluctuations in iron. Human serum contains 30 to 60 μM Tf comprising apo (Tf), monoferric (Fe-Tf), and diferric (Fe2-Tf) forms. Saturation of Tf is approximately 30% in nonpathologic states, but it may reach 100% during severe iron overload. At 30% saturation, 10% of the total Tf is diferric.47 Thus, concentrations of diferric Tf in healthy persons range from approximately 3 to 6 μM. In our studies, TfR2 increased as diferric Tf increased from 0.3 to 13 μM, with half-maximal response at approximately 2.5 μM. By sensing changes in the concentration of diferric Tf, the body could monitor iron levels and modulate intestinal iron uptake to maintain diferric Tf within this range. This would likely involve the regulation of hepcidin expression and secretion. Given that hepcidin expression occurs in the liver,13,48,49 sensing of iron levels is likely to occur there. TfR2 binds Tf, is expressed at high levels in hepatocytes,20 and, thus, is a good candidate to signal serum Tf saturation to the hepatocyte.
Diferric Tf regulates TfR2 stability through an undefined mechanism. This mechanism could involve the interaction of Tf with TfR2, with TfR1, or with both receptors. We knocked down TfR1 expression to investigate whether the regulation of TfR2 by Tf requires TfR1. Results were inconclusive. In cells expressing reduced levels of TfR1, TfR2 still increased in response to Tf, but not to the same extent as in cells expressing normal levels of TfR1. Down-regulation of TfR1 by 80% to 90% reduced TfR2 by 36% in Tf-treated cells. Multiple mechanisms could account for this result. First, the interaction of Tf with TfR1 alone could regulate TfR2 through a signal transduction cascade. This would account for the disproportionate reductions in TfR2 and TfR1. Alternatively, total uptake of Tf may be the mediating factor. By adding diferric Tf to the medium, an increase in the uptake of Tf would still occur when TfR1 is down-regulated, leading to higher TfR2 levels that could partially offset the reduction in uptake by TfR1. Finally, Tf could regulate TfR2 directly. A small proportion of TfR2 immunoprecipitates with TfR1 in extracts from liver,50 K562 cells,50 and HepG2 cells (our unpublished results, 2004). The 36% reduction of TfR2 in cells treated with 42/6 antibody and diferric Tf may be a consequence of this interaction and may not be due to an effect on the regulation of TfR2 by Tf. Down-regulation of TfR2 by the 42/6 antibody as a side effect of heterodimerization with TfR1 is undetectable in untreated cells but may become pronounced as TfR2 levels increase in cells treated with Tf. Additional future experiments are required to distinguish between these possibilities and to define the mechanism by which Tf regulates TfR2 stability. Our finding that Tf increases TfR2 in HepG2 and HuH7 hepatoma cell lines, but not in other nonhepatoma cell lines tested, suggests that the mechanism may involve proteins or compartments specific to hepatocytes.
Our finding that diferric Tf regulates the half-life of TfR2 predicts that TfR2 levels will be altered in diseases that affect the concentration of diferric Tf in the blood. In diseases in which the saturation of serum Tf increases, such as HH and β-thalassemia, TfR2 levels would be elevated. Conversely, TfR2 would be reduced in diseases in which levels of saturated serum Tf decrease, such as hypotransferrinemia. In a companion paper in this issue, Robb and Wessling-Resnick examine TfR2 levels in mouse models of HH1 (Hfe-/-),51 β-thalassemia (Hbbth1),52 and hypotransferrinemia (hpx)53 and show that this does indeed occur. Relative to congenic controls, TfR2 levels are increased in Hfe-/- and Hbbth-1 mice but are decreased in hpx mice.
The phenotypes of mice deficient in TfR2 or Tf are consistent with a role for TfR2 in regulating iron homeostasis in the body by sensing diferric Tf. Hpx mice are deficient in serum Tf because of a mutation within the Tf gene that disrupts splicing. As a consequence, there is a lack of Tf-bound iron, causing severe iron deficiency in erythrocytes, and an elevation in non–Tf-bound iron, producing iron overload in other cell types. Hpx mice express low levels of hepcidin despite iron loading in parenchymal tissues.54,55 Consistent with the low level of hepcidin, intestinal iron absorption is high in these mice. Interestingly, infusing erythrocytes into hpx mice to remedy anemia does not reduce iron uptake, but transfusing Tf does.17 This suggests that Tf contributes to the regulation of iron absorption56 and that the absence of Tf produces a discontinuity between iron levels and intestinal absorption. It is plausible that TfR2 senses the lack of diferric Tf in the serum and, in turn, regulates hepcidin. The decreased TfR2 and hepcidin, accurately reflecting levels of diferric Tf but not of iron, may aberrantly signal that iron levels are low. This would account for the occurrence of iron overload in hpx mice and the effects of Tf transfusion. We would predict an increase in TfR2 and hepcidin after Tf transfusion in hpx mice.
As with hpx mice, the phenotype of TfR2Y245X mice is consistent with disruption of a signaling pathway that senses iron through Tf saturation and alters intestinal iron uptake. TfR2Y245X mice have a mutation in TfR2 that is orthologous to a mutation that causes HH in humans. They develop hemochromatosis, as do Hfe-/- mice, with parenchymal iron loading, increased Tf saturation, and elevated serum ferritin.35 Tf is present in TfR2Y245X mice, unlike in hpx mice, and the erythrocytes in TfR2Y245X mice acquire sufficient iron. However, according to our hypothesis, in the absence of TfR2, hepatocytes would not be able to sense Tf or to regulate hepcidin secretion appropriately.
Based on published studies of TfR2 and current understanding of the consequences of mutations in TfR2, Tf, and hepcidin, we speculate that these proteins may be part of an iron-sensing system whose disruption causes the body to respond as if iron levels are low. Under normal conditions, serum Tf saturation indicates iron levels in the body. TfR2 mediates a signal in proportion to Tf saturation that regulates hepcidin expression and, consequently, iron uptake by the intestine. Low iron levels would reduce Tf saturation, attenuate this signal, lower hepcidin secretion, and increase intestinal iron uptake. Deficiency in TfR2 or Tf would disrupt this system. In humans and mice with mutations in TfR2, the absence of functional TfR2 is akin to the attenuation of TfR2 signaling that occurs when iron levels are low.
If hepatocytes sense iron through this system, treatment with diferric Tf should result in an increase in hepcidin expression in HepG2 cells. Contrary to this expectation, no significant increase in hepcidin mRNA was observed when HepG2 cells were incubated with 63 μM diferric Tf for 24 hours.57 In HepG2 cells, intermediary proteins intermediate between TfR2 and hepcidin might not be expressed at appropriate levels. HFE, for example, is expressed at high levels in hepatocytes of the rat liver but at low levels in HepG2 cells (our unpublished results, 2003). Hemojuvelin, the newly identified gene that causes juvenile hemochromatosis, is expressed in the liver but is not detectable in HepG2 cells6,42 (and our unpublished results, 2004). Alternatively, the iron-sensing system that regulates hepcidin expression may require factors provided by other cell types in the liver or by the serum.
In vitro Kd measurements of the affinity of Tf for TfR222,24 complicate the hypothesis that TfR2 senses diferric Tf. These measurements predict that TfR2 would be saturated by the concentrations of Tf found in the blood. We have shown, though, that TfR2 protein responds to changes in diferric Tf at physiologic concentrations. Understanding how the stability of TfR2 is regulated by concentrations of diferric Tf that are 100-fold higher than the Kd of TfR2 and determining whether the response of TfR2 to diferric Tf affects other iron-related proteins will contribute significant details to our understanding of iron homeostasis.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-06-2477.
Supported by National Institutes of Health grant RO1-DK54488 (C.A.E.). M.B.J. was supported in part by National Institutes of Health grant T32-HL00781.
Presented in abstract form at Experimental Biology 2004; April 17 to 21, 2004; Washington, DC.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.