Graft-versus-host disease (GVHD) and failure of engraftment limit clinical bone marrow transplantation (BMT) to patients with closely matched donors. Engraftment failure of purified allogeneic hematopoietic stem cells (HSCs) has been decreased in various BMT models by including donor BM–derived CD8+/αβγδTCR- facilitating cells (FCs) or CD8+/αβTCR+ T cells in the BM inoculum. To aggressively investigate the GVHD potential of these donor CD8+ populations, a purified cell model of lethal GVHD was established in a murine semiallogeneic parent → F1 combination. Lethally irradiated recipients were reconstituted with purified donor HSCs alone or in combination with splenic T cells (TSP), BM-derived T cells (TBM), or the FC population. In marked contrast to the lethal GVHD present in recipients of HSCs plus TSP or CD8+ TBM, recipients of donor HSC+FC inocula did not exhibit significant clinical or histologic evidence of GVHD. Instead, HSC+FC recipients were characterized by increased splenocyte expression of transforming growth factor-β (TGF-β) and the induction of the regulatory T-cell genes CTLA4, GITR, and FoxP3. These findings suggest that the FCs, which express a unique FCp33-TCRβ heterodimer in place of αβTCR, permits HSC alloengraftment and prevents GVHD through the novel approach of regulatory T-cell induction in vivo.
Introduction
Allogeneic bone marrow transplantation (BMT) plays an important role in the treatment of various hematologic maladies such as lymphoma, leukemia, aplastic anemia, and severe immunodeficiencies. However, even in the 30% of patients with matched donors who undergo BMT, clinical success has been limited by graft-versus-host disease (GVHD).1-3 The initial enthusiasm that followed the decreased incidence of GVHD noted with the transplantation of T cell–depleted (TCD) marrow or purified hematopoietic stem cells (HSCs) was tempered by an increase in graft failure and a recurrence of malignant disease.1,3-7 Attempts to add-back titrated doses of mature T lymphocytes to restore engraftment and the graft-versus-leukemia effect in unmanipulated BM have been hampered in multiple donor/recipient antigen disparities by the induction of lethal GVHD, even with limited numbers of mature T cells.8,9 However, several animal studies have recently shown that BM-derived CD8+ populations, T and non–T cell, are an important means to facilitate the engraftment of purified HSCs.6,10,11 We have previously identified such a non–T cell population, known as the donor facilitating cell (FC), which dramatically enhances allogeneic engraftment of a mixed syngeneic and allogeneic TCD BM inoculum in lethally irradiated major histocompatibility complex (MHC)–disparate murine recipients.10 Characterized as CD8+/αβγδTCR- (CD8+/TCR-), adding only 30 000 to 50 000 of these donor BM-derived FCs to the mixed BM inoculum reliably facilitated engraftment of the allogeneic HSCs across complete MHC barriers.10 The incidence of alloengraftment increased from 43% to 100%, and the average level of donor chimerism rose from 13% to more than 90%. In contrast, supplementation of the inocula with similar numbers of CD8+/αβγδTCR+ (CD8+/TCR+) BM-derived T cells (TBM) resulted in the failure of allogeneic engraftment, with most recipients exhibiting syngeneic reconstitution. Even among TBM recipients with low levels of donor chimerism, 75% exhibited histologic evidence of GVHD. Despite recent demonstrations of tumor-specific CD8+ T cells in BM from patients with hematologic malignancies and the evidence that BM-derived CD8+ T cells can elicit a tumor response in vivo, these findings suggest that supplementation with CD8+/TCR+ TBM may not improve alloengraftment and may, in fact, prove to be clinically disastrous, regardless of other potential antitumor benefits.12-15 Therefore, understanding differences in the GVHD potential of CD8+/TCR+ TBM and CD8+/TCR- FC donor cell populations in a clinically relevant model of purified HSC transplantation is critical before attempts at clinical application can be considered.
To date, the induction of GVHD in an irradiated allogeneic host has characteristically required supplementation of the TCD donor BM inocula with large numbers of mature T lymphocytes obtained from donor spleen, thymus, or lymph nodes.16,17 The exact composition of each inoculum depends on the thoroughness of TCD and the source and composition of peripheral T-cell supplementation. Although extremely helpful in our understanding of GVHD, clinical relevance of these prior models is diminished because GVHD potential is assessed for mature peripheral T cells, whereas the T-cell subsets within the donor BM are removed. Furthermore, the TCD donor inoculum contains a variety of cell types that differ in cell-cell interactions, maturation, activation, and cytokine profiles, all of which may alter engraftment and GVHD potential. Given that the current state of the art in clinical BMT uses purified donor HSC inocula, where other donor populations are less available to modulate the graft-versus-host response, the question of GVHD effector activity for specific BM-derived populations in patients who undergo purified HSC transplantation has become clinically relevant.
To better understand the contribution of individual donor cell subsets, and of BM-derived CD8+ populations in particular, to the induction of acute GVHD after purified HSC transplantation, we have established a purified cell-based model of lethal GVHD using a (parent) P → F1 (semiallogeneic) murine combination. Transplantation of purified HSCs alone, as the source of donor BM, excludes all other donor hematopoietic cell populations from potentially affecting engraftment and modulating the risk for GVHD. This approach permits one to assess the GVHD potential of various individual donor cell populations by adding back only purified GVHD effectors to the HSC inocula. Using this model, 3 separate populations of potential effectors, all of which contain CD8+ cells, were assessed for the ability to induce GVHD in lethally irradiated semiallogeneic recipients. The subsets evaluated were (1) αβTCR+ splenic T cells (TSP), (2) CD8+/TCR+ BM-derived T cells (TBM), and (3) the BM-derived CD8+/TCR- FC population. The results of the current study demonstrate that the recipients of HSCs + αβTCR+ TSP or CD8+/TCR+ TBM acquire lethal GVHD, whereas recipients of HSCs + CD8+/TCR- FCs fail to exhibit clinical or histologic evidence of significant GVHD. Subsequent gene analysis of recipients after HSC+FC transplantation has shown that an immunoregulatory profile characterized by the expression of regulatory T-cell genes and transforming growth factor-β (TGF-β) is induced. These findings suggest, for the first time, that a regulatory immune state by which GVHD is controlled and HSC engraftment and immune reconstitution are favored can be induced in vivo through the use of FC transplantation.
Materials and methods
Mice
B10.BR SgSnJ (BR, H-2k), C57BL/6 (B6, H-2b), and B6D2 F1 (F1, H-2b/d) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in sterile microisolator cages and received autoclaved food and acidified water for 2 weeks after BMT. Care was in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Dana-Farber Cancer Institute.
Multiparameter live sterile cell sorting
HSCs, FCs, and TBM were isolated from donor BM as previously described.10 Briefly, BM was isolated from the long bones of mice and washed in Hanks balanced salt solution (HBSS; Gibco, Grand Island, NY). Monoclonal antibodies (mAbs) chosen to isolate murine HSCs were Ly6A/E (Sca-1) phycoerythrin (PE), c-kit biotin, and a mixture of fluorescein isothiocyanate (FITC)–conjugated antilineage (Lin-) mAbs: B220, CD8α, GR-1, MAC-1, αβTCR, and γδTCR. After 45 minutes of incubation at 4°C, cells were washed, resuspended at 200 × 106 cells/mL, and incubated with streptavidin Cychrome for 30 minutes. Cells were washed and resuspended in sterile sort media consisting of HBSS, 2% fetal calf serum (Gibco), and 2 μl/mL HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Gibco) before multiparameter sterile live cell sorting for HSCs as Sca+/c-kitdim/intermediate/Lin- cells within the conventional lymphoid gate on a MoFlo flow cytometric cell sorter (Cytomation, Denver, CO). BM-derived TBM and FCs were similarly isolated as CD8+/TCR+ and CD8+/TCR- populations, respectively, using CD8α-PE, αβTCR-FITC, and γδTCR-FITC. TSP was isolated from single-cell suspensions of donor murine splenocytes after incubation with αβTCR-FITC mAbs. All mAbs were from BD Biosciences PharMingen (San Diego, CA). Purity after sorting was reanalyzed with respect to forward and side scatter parameters and the designated cell surface markers. Purity for all experimental samples was 85% to 98%. Cells were portioned into aliquots in 1 mL HBSS and were injected into lethally irradiated mice.
Bone marrow transplantation
For allogeneic BMT, 10 000 HSCs plus 50 000 FCs or TBM from B6 donors were transplanted into lethally irradiated (950 cGy) B10.BR recipients through tail vein injection. Semiallogeneic (haploidentical) P → F1 BMT entailed the intravenous transplantation of 2000 HSCs alone or together with 50 000 to 400 000 FCs, CD8+ TBM, or αβTCR+ splenocytes (TSP), all sorted from B6 donors, into B6D2F1 recipients conditioned with 1100 cGy in a split dose separated by 3 hours to limit gastrointestinal toxicity. To maintain as much homogeneity within a given experiment as possible, recipients were of the same age, were from same shipment of mice, and underwent transplantation with donor populations isolated at the same sorting session, and all control animals were prepared on the same day. Survival was monitored daily.
GVHD clinical assessment
After BMT, mice were scored for clinical evidence of GVHD in a blinded fashion once a week for the first 2 weeks and then daily until the animals were killed.9 A more sensitive indicator of GVHD severity than weight alone, the clinical GVHD score was generated by the cumulative sum of grade 0 (no GVHD) to grade 2 (severe GVHD) for each of 5 clinical parameters: weight loss, posture (hunching), activity, fur texture and skin integrity. Total scores of 2 or lower were indicative of no GVHD, scores higher than 4 suggested moderate to severe GVHD, and scores higher than 7 signified moribund lethal disease.
Flow cytometric analysis of allogeneic donor engraftment
Peripheral blood lymphocytes (PBLs) were collected into heparinized vials and analyzed for H-2 antigen expression to determine the extent of donor (parental or fully allogeneic) chimerism. Each PBL sample included a negative control, and separate aliquots were stained with H-2b FITC (donor) or H-2k FITC (recipient) for B6 → BR chimeras or H-2b FITC and H-2d PE for confirmation of B6 parental engraftment (H-2b+/H-2d-) in B6D2F1 recipients. Data were collected on a FACScan flow cytometer and were analyzed in the lymphoid gate with Cell Quest (Becton Dickinson, San Jose, CA) or WinList (Verity, Topsham, ME) software.
Tissue procurement and histopathology
Recipients were killed at the end of the designated GVHD assessment period of 28 days (unless otherwise stated) or before they became moribund, and small intestines were harvested and placed in 10% neutral buffered formalin. Tissue samples were embedded in paraffin, and 6-μm–thick samples were sectioned, stained with hematoxylin and eosin (H&E), mounted with standard mounting medium, and assessed for GVHD in a blinded fashion. Images were obtained using an Olympus BX45 microscope (Olympus, Melville, NY) with UPlan-F1 × 20 (numerical aperture 0.5) and × 40 (numerical aperture 0.75) objective lenses and an Olympus QColor 5 camera. Acquisition and processing software used was Adobe Photoshop 7.0 (Adobe, San Jose, CA). GVHD was recognized histologically by the presence of crypt epithelial cell degeneration and apoptosis (grade 1), apoptotic crypt abscesses (grade 2), crypt dropout (grade 3), and mucosal erosion or ulceration (grade 4) in accordance with previously described criteria.18
Statistics
Statistically significant differences in survival among various treatment groups were determined using Kaplan-Meier analysis. Comparison of GVHD scores was performed with the Mann-Whitney U nonparametric test. Means and variances for real-time polymerase chain reaction (PCR) data were calculated with JMP statistical software (SAS Institute, Cary, NC). Student t test analysis was used to determine the statistical significance of differential gene expression between HSC+FC and HSC+TBM recipients, and geometric fold change analysis was used to determine the extent of differential expression.
Real time-quantitative PCR
Twenty-eight days after BMT, RNA from purified FCs, TBM, TSP, or whole spleens of HSC+FC or HSC+TBM recipients was isolated using TriReagent (Sigma, St Louis, MO). RNA was treated with DNase I and was reverse transcribed to cDNA using SuperScript II (Invitrogen, Carlsbad, CA). cDNA was mixed with diethylpyrocarbonate (DEPC)–treated water, SYBR Green PCR Master Mix, and the primer pair of interest. The specific primer pairs used were designed with Primer Express software (Applied Biosystems, Foster City, CA). Gene-specific real-time PCR products were continuously measured by the Gene Amp 5700 Sequence Detection System (Applied Biosystems) for 40 cycles. All experiments were run in duplicate. The cycle threshold (CT) was determined at the same fluorescence signal intensity during the most exponential phase, was inversely proportional to the copy number of the target template, and was related to the CT of the housekeeping gene GAPDH. The percentage of GAPDH calculations for each gene was (100/2 CTGene - CTGAPDH). Log 2-fold change calculations for each gene were log 2 - (2 -(CTGene - CTGAPDH) - (CTGene - CTGAPDH)). Nontemplate controls and dissociation curves were used to detect primer-dimer formation and nonspecific amplification.
Results
Fully allogeneic HSC transplantation—FCs required for long-term HSC engraftment without acute GVHD
We have previously demonstrated that donor alloengraftment is markedly improved when the mixed syngeneic and allogeneic TCD inocula are supplemented with donor BM–derived CD8+/TCR- FCs.10 We hypothesized that the absence of GVHD in this model may be secondary to suppression of FC GVHD effector activity by other donor or recipient cell populations present in the mixed BM inoculum. Therefore, an allogeneic BMT model incorporating only purified donor HSCs and FCs was used to assess the GVHD effector activity of the FC in the absence of all other non-HSC populations.10 B10.BR recipients were lethally irradiated and reconstituted with 10 000 purified Sca+/c-kit+/Lin- HSCs and 50 000 CD8+/TCR- FCs, both isolated by rare event cell sorting from B6 donor BM (HSC+FC → B10.BR).10 All HSC+FC recipients were PBL typed for evidence of allogeneic engraftment and, for the first 8 weeks, assessed weekly for survival and evidence of acute GVHD. Previous studies have shown that transplantation with HSCs and donor CD8+ T cells of BM or splenic origin does not facilitate allogeneic HSC engraftment; thus, recipients succumb to radiation-induced aplasia in this purified HSC model.10 Therefore, allogeneic HSC+TBM and HSC+TSP recipients were not evaluated in this model because too few recipients survive to be reliably assessed for evidence of GVHD.
As shown in Table 1, allogeneic HSC+FC recipients exhibited excellent survival (88.9% at 4 weeks) and high levels of fully allogeneic donor engraftment at 3 months (94.1% ± 1.8% donor). Clinical scores for mild to severe GVHD (0 to 10, respectively) were compared with those in syngeneic HSC recipients as a treatment control for radiation conditioning. Scores were not significantly different between animals undergoing allogeneic HSC+FC (n = 8) or syngeneic HSC reconstitution (n = 6). The respective peak clinical scores of 1.25 ± 0.3 and 1.0 ± 0.3 are consistent with the absence of clinical GVHD.
Semiallogeneic HSC transplantation—establishment of a purified cell model of lethal GVHD
The failure of TBM or TSP to reliably rescue recipients of allogeneic HSCs across complete MHC barriers prevented comparison of GVHD potential between FCs and T cells in the previous fully allogeneic model of HSC engraftment. Furthermore, radioresistant recipient cells can generate an antidonor response that suppresses GVHD effector activity of the FCs. However, the semiallogeneic parent into F1 recipient combination of B6 → B6D2F1 permits unopposed GVHD by alloreactive donor cell populations and HSC engraftment at significantly lower HSC numbers, with or without the FCs. Individual donor populations may be added to the HSC inoculum and may be directly assessed for lethal GVHD potential. Therefore, to assess the potential GVHD effector activity of the FCs in a rigorous GVHD model, in the absence of other donor non-HSC populations, a purified cell model of lethal GVHD was developed using semiallogeneic HSCs.
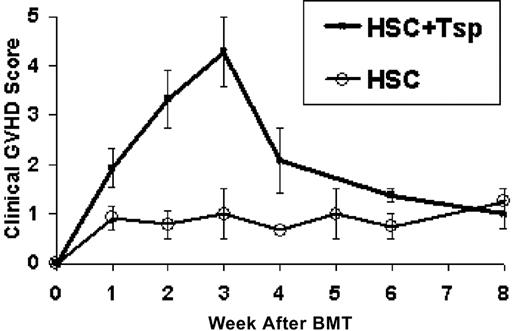
B6D2F1 recipients were lethally irradiated and reconstituted with 2000 purified HSCs of B6 origin. The HSC inoculum was supplemented with αβTCR+ splenocytes sorted from B6 donors as positive GVHD effector controls. Recipients of donor HSCs alone or HSCs plus 100 000 or 200 000 αβTCR+ TSP were assessed weekly for survival and severity of GVHD, as described. As expected, recipients of HSCs alone (n = 8) exhibited 100% survival without evidence of GVHD, as reflected in the peak morbidity score of 1.5 ± 0.7 (Figure 1). In contrast, recipients of HSCs plus TSP exhibited a dose-dependent effect on GVHD severity. Recipients of HSCs plus 100 000 TSP survived with clinical evidence of moderate GVHD between 2 and 3 weeks after BMT. The peak GVHD morbidity score was 3.7 ± 0.3. Adding 200 000 TSP (n = 14) resulted in lethal GVHD marked by decreased survival at 1 month (35.7%; Figure 2) and increased morbidity. As shown in Figure 1, the peak morbidity scores for this group occurred 3 weeks after BMT, and the peak score of surviving animals was 4.5 ± 0.5. Therefore, morbidity and mortality rates were significantly higher than those in mice treated with HSCs alone (P = .0005).
Establishment of a purified cell model of GVHD. Lethally irradiated B6D2F1 recipients reconstituted with B6-derived HSCs (n = 8) or with HSCs plus 200 000 αβTCR+ splenocytes from B6 donors (n = 14) were assessed for evidence of acute GVHD. Mean ± SE clinical GVHD scores are displayed for each week after BMT. The recipients of HSCs alone showed no clinical GVHD. In contrast, recipients of 2000 HSC + 200 000 αβTCR+ donor TSP exhibited severe and often lethal GVHD, with the peak severity and incidence occurring at week 3 (P = .0005 vs HSCs alone).
Establishment of a purified cell model of GVHD. Lethally irradiated B6D2F1 recipients reconstituted with B6-derived HSCs (n = 8) or with HSCs plus 200 000 αβTCR+ splenocytes from B6 donors (n = 14) were assessed for evidence of acute GVHD. Mean ± SE clinical GVHD scores are displayed for each week after BMT. The recipients of HSCs alone showed no clinical GVHD. In contrast, recipients of 2000 HSC + 200 000 αβTCR+ donor TSP exhibited severe and often lethal GVHD, with the peak severity and incidence occurring at week 3 (P = .0005 vs HSCs alone).
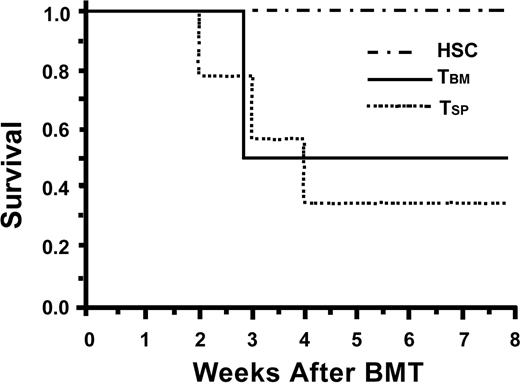
TBM induce severe lethal GVHD. Lethally irradiated B6D2F1 recipients were reconstituted with B6-derived HSCs (n = 8), HSCs plus 200 000 CD8+ TBM (n = 9), or HSCs plus 200 000 TSP (n = 14) and were assessed for survival. The inoculum containing CD8+TBM resulted in death from lethal GVHD, similar to that seen with αβTCR+ TSP used as a positive GVHD control (P = NS). Survival was markedly decreased for TBM and TSP compared with HSCs alone (P < .05 for both groups at 30 days).
TBM induce severe lethal GVHD. Lethally irradiated B6D2F1 recipients were reconstituted with B6-derived HSCs (n = 8), HSCs plus 200 000 CD8+ TBM (n = 9), or HSCs plus 200 000 TSP (n = 14) and were assessed for survival. The inoculum containing CD8+TBM resulted in death from lethal GVHD, similar to that seen with αβTCR+ TSP used as a positive GVHD control (P = NS). Survival was markedly decreased for TBM and TSP compared with HSCs alone (P < .05 for both groups at 30 days).
HSC supplementation with CD8+/TCR+ TBM
Given the mild GVHD seen when mixed TCD inocula were supplemented with TBM in the allogeneic model and the recent evidence of antitumor and proengraftment properties evident in CD8+ TBM in other models, we hypothesized that TBM supplementation of the purified semiallogeneic HSC inocula may be possible without a significant increase in the risk for GVHD. Irradiated B6D2F1 recipients were reconstituted with a donor B6 inoculum of 2000 HSCs plus 200 000 CD8+/TCR+ TBM and were assessed for survival and GVHD severity. Similar to recipients of HSC+TSP, recipients of HSC+TBM rapidly exhibited lethal GVHD with decreased survival (30-day mortality rate, 50%; Figure 2) and significant morbidity. The peak GVHD score of surviving HSC+TBM recipients at 3 weeks was 4.3 ± 0.7 (n = 9). This GVHD score and 30-day survival rate are not statistically different from those of HSC+TSP recipients.
FCs do not exhibit acute or chronic GVHD effector activity
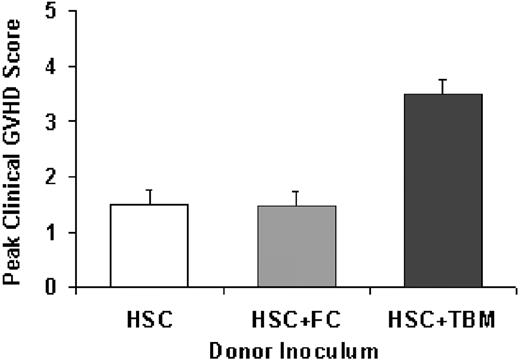
Given the marked increase in GVHD seen with CD8+/TCR+ TBM in this purified HSC semiallogeneic model, it was critical to determine the GVHD potential of the CD8+/TCR- BM–derived FC population. Therefore, lethally irradiated B6D2F1 recipients underwent transplantation with 2000 HSCs, alone or together with 200 000 FCs or 200 000 TBM, all from B6 donors. As demonstrated in Figure 3, recipients of HSC+FC (n = 9) failed to exhibit significant morbidity or mortality secondary to GVHD compared with recipients of HSC+TBM (n = 7). The 6-week survival rate for HSC+FC recipients was 100%, and the peak score was 1.5 ± 0.2. This score is similar to that for HSC controls (1.55 ± 0.3; NS) but was significantly less than the 3.5 ± 0.8 score for surviving TBM recipients (n = 7; P < .05). Adding as many as 400 000 FCs did not result in adverse clinical scores.
Absence of clinical GVHD after FC transplantation. Lethally irradiated B6D2F1 recipients reconstituted with 2000 B6-derived HSCs alone (n = 8) or HSCs plus 200 000 FCs (n = 9), also from a B6 donor, failed to exhibit significant evidence of GVHD even at 3 weeks after BMT. This is in contrast to HSC+200 000 TBM recipients (n = 7) in which clinical scores consistent with moderate to severe GVHD were evident (P ≤ .05). Mean ± SE peak clinical scores for each group are shown.
Absence of clinical GVHD after FC transplantation. Lethally irradiated B6D2F1 recipients reconstituted with 2000 B6-derived HSCs alone (n = 8) or HSCs plus 200 000 FCs (n = 9), also from a B6 donor, failed to exhibit significant evidence of GVHD even at 3 weeks after BMT. This is in contrast to HSC+200 000 TBM recipients (n = 7) in which clinical scores consistent with moderate to severe GVHD were evident (P ≤ .05). Mean ± SE peak clinical scores for each group are shown.
Depletion of donor T-cell subsets from the inoculum has been previously demonstrated to result in chronic GVHD in B6 → B6D2F1 models by 12 weeks.19,20 To ensure that the appearance of GVHD was not merely delayed or converted to chronic GVHD, HSC+FC recipients were followed up for 14 weeks without any clinical evidence of chronic GVHD. The average peak clinical score for HSC+FC recipients between 9 and 14 weeks after BMT (n = 5) was 0.75 ± 0.16. These results demonstrate that the FC population does not induce acute or chronic GVHD in this model.
Morbidity and mortality after HSC plus T-cell transplantation are not caused by engraftment failure
A limited number of HSCs are present in the donor inoculum; therefore, to confirm that the increased mortality and morbidity rates of TBM or TSP recipients were secondary to GVHD and not to engraftment failure, F1 recipients were PBL typed 28 days after BMT to document donor engraftment. This also provided confirmatory evidence that sufficient donor cells existed for the induction of GVHD in HSC+FC recipients. The level of B6 donor chimerism (H-2b+/H-2d-) was high in all treatment groups, with no significant differences evident between groups (Table 2). These results demonstrate that although FCs are required for HSC engraftment across complete MHC barriers, facilitation is not required for HSC engraftment in semiallogeneic recipients.
Histologic absence of significant GVHD in FC recipients
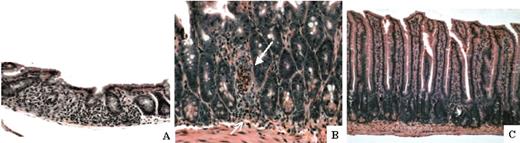
F1 recipients reconstituted with HSC+TBM or HSC+FCs were electively killed 28 days after BMT, and the small intestine was assessed for early histologic evidence of acute GVHD (n = 7 each). Histopathologic evidence of severe GVHD was present in HSC+TBM, but not in HSC+FC, recipients. Consistent with the fact that 50% of HSC+TBM recipients succumb to lethal GVHD in this model after 4 weeks, 57% of these recipients exhibited histologic evidence of acute GVHD within the gut, as manifested by villous shortening, lymphocytic infiltration, and crypt apoptosis (Figure 4A-B). Gut GVHD in HSC+TBM recipients was moderate to severe in intensity, with an average histologic grade of 2.4 ± 0.4 on a scale of 0 to 4. In contrast, villous architecture was maintained without evidence of lymphocytic infiltrate or crypt abscess in HSC+FC recipients (Figure 4C). Histologic analysis was independently scored as mild GVHD (occasional crypt apoptosis) or no GVHD in 86% of HSC+FC recipients. Only a single HSC+FC recipient showed evidence of crypt dropout, which was nonspecific and was not associated with any clinical evidence of GVHD.
Histopathologic evidence of acute GVHD after donor TBM, but not FC, transplantation. Small intestines were harvested from HSC+TBM and HSC+FC recipients on day 28. Paraffin-embedded sections were stained with H&E and were histologically analyzed for evidence of GVHD. (A) Small intestine of a representative HSC+TBM recipient (n = 7) exhibits villous shortening, crypt dropout, and extensive lymphocytic infiltration of the lamina propria (original magnification, × 200). (B) In these same mice, lymphocytic infiltration of crypts with apoptosis (open arrow) and the formation of a crypt abscess (closed arrow) is observed at higher magnification (original magnification, × 400). These findings are indicative of moderate to severe GVHD. (C) Small intestine of a representative HSC+FC recipient (n = 7). There is no evidence of GVHD (original magnification, × 200).
Histopathologic evidence of acute GVHD after donor TBM, but not FC, transplantation. Small intestines were harvested from HSC+TBM and HSC+FC recipients on day 28. Paraffin-embedded sections were stained with H&E and were histologically analyzed for evidence of GVHD. (A) Small intestine of a representative HSC+TBM recipient (n = 7) exhibits villous shortening, crypt dropout, and extensive lymphocytic infiltration of the lamina propria (original magnification, × 200). (B) In these same mice, lymphocytic infiltration of crypts with apoptosis (open arrow) and the formation of a crypt abscess (closed arrow) is observed at higher magnification (original magnification, × 400). These findings are indicative of moderate to severe GVHD. (C) Small intestine of a representative HSC+FC recipient (n = 7). There is no evidence of GVHD (original magnification, × 200).
FC recipients characterized by increased gene expression of TGF-β
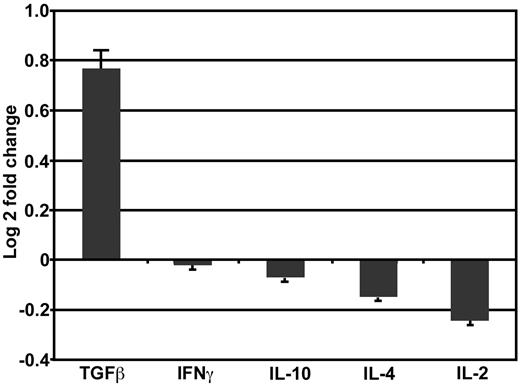
Several recent studies have demonstrated that acute GVHD can be suppressed by the addition of immunoregulatory T cells.21-25 To determine the mechanism by which FC recipients avoid GVHD even though TBM induce lethal GVHD, splenocyte mRNA isolated from B6D2F1 recipients 28 days after HSC+FC (n = 4) or HSC+TBM (n = 3) transplantation was analyzed for differences in gene expression of cytokines commonly associated with the immunosuppressive function of regulatory T cells (TGF-β, IL-4, or IL-10) or down-regulated in the presence of immune tolerance networks (IL-2, IFN-γ). HSC+FC recipients, in comparison with HSC+TBM recipients, exhibited a nearly 2-fold increase in splenocyte TGF-β expression. In contrast, there was little difference in IFN-γ, IL-10, IL-4, or IL-2 gene expression. In Figure 5, log-2 transformation was used to enhance visualization of up- and down-regulation patterns of these cytokines, with the log-2 transformation of the 1.72-fold change in TGF-β expression equal to 0.76 ± 0.09. This demonstrates a marked trend toward increased gene expression of the immunosuppressive cytokine TGF-β after FC transplantation (P = .2; Student t test.). Given the importance of TGF-β in inducing immunoregulatory tolerance,26,27 the trend toward increased TGF-β gene expression in HSC+FC recipients suggested that the FC is a regulatory T cell or that regulatory T cells are induced after FC transplantation.
Increased TGF-β gene expression in HSC+FC recipients. Splenocytes harvested 28 days after BMT from HSC+FC (n = 4) and HSC+TBM (n = 3) recipients were analyzed using real-time PCR for cytokine gene expression. Relative differences in expression levels among HSC+FC recipients compared with HSC+TBM recipients are shown as log 2-fold change mean ± SD.
Increased TGF-β gene expression in HSC+FC recipients. Splenocytes harvested 28 days after BMT from HSC+FC (n = 4) and HSC+TBM (n = 3) recipients were analyzed using real-time PCR for cytokine gene expression. Relative differences in expression levels among HSC+FC recipients compared with HSC+TBM recipients are shown as log 2-fold change mean ± SD.
FCs do not express regulatory T-cell genes
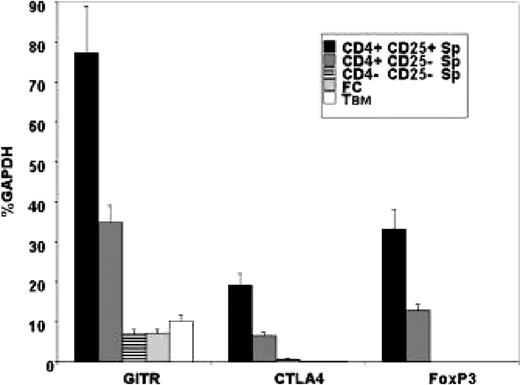
We evaluated the possibility that FCs were regulatory T cells by analyzing the FCs before BMT for the expression of genes commonly present at increased levels in regulatory T cells, namely glucocorticoid-induced TNF receptor (GITR), cytotoxic T lymphocyte–associated antigen 4 (CTLA4), and FoxP3. Although GITR and CTLA4 can also be expressed by activated T-cell subsets, FoxP3 is considered essential for regulatory T-cell development and function.28-30 Therefore, purified FC (n = 4) and TBM (n = 4) populations were isolated from donor B6 bone marrow and analyzed by real-time PCR for GITR, CTLA4, and FoxP3 gene expression. A representative experiment is shown in Figure 6. GITR, CTLA4, and FoxP3 expression in FCs and TBM is statistically significantly lower than expression in the characteristic CD4+CD25+ regulatory T cells identified in the spleens of B6 donors (GITR, P = .0005; CTLA4, P = .0004; FoxP3, P = .0003; Student t test analysis). Compared with the nontolerogenic CD4-CD25- splenocyte population, there was no statistically significant difference in GITR expression by FCs (P = .8734), and FoxP3 and CTLA4 expression were significantly lower (P < .05), though the absolute change in expression is small (less than 0.5%, GAPDH). These results demonstrate that donor BM–derived FCs do not express the genes classically associated with the regulatory T-cell phenotype; thus, the FC is not a regulatory T cell.
Regulatory T-cell gene expression is not increased in the FC or the TBM populations. Real-time PCR analysis of CD8+/TCR- FCs (n = 4) or CD8+/TCR+ TBM (n = 4) isolated from donor BM compared with unstimulated control B6 splenocytes sorted for the CD4+CD25+ regulatory T-cell phenotype or the CD4+CD25- or CD4-CD25- nontolerogenic phenotype. Individual samples were analyzed for the presence of mRNA encoding GITR, CTLA4, and FoxP3 and were semiquantitated as a mean ± SD percentage of baseline GAPDH expression.
Regulatory T-cell gene expression is not increased in the FC or the TBM populations. Real-time PCR analysis of CD8+/TCR- FCs (n = 4) or CD8+/TCR+ TBM (n = 4) isolated from donor BM compared with unstimulated control B6 splenocytes sorted for the CD4+CD25+ regulatory T-cell phenotype or the CD4+CD25- or CD4-CD25- nontolerogenic phenotype. Individual samples were analyzed for the presence of mRNA encoding GITR, CTLA4, and FoxP3 and were semiquantitated as a mean ± SD percentage of baseline GAPDH expression.
FC recipients are characterized by the in vivo induction of regulatory T-cell genes
We subsequently investigated whether the absence of GVHD in HSC+FC recipients was associated with the in vivo induction of regulatory T-cell genes. Lethally irradiated B6D2F1 recipients were reconstituted with HSC+FCs (n = 4) or HSC+TBM (n = 3) and were electively killed 28 days after BMT. RNA from individual spleens was analyzed using real-time PCR for regulatory T-cell marker gene expression. Log 2-fold change was used to determine the extent of differential gene expression, and, as evident in Figure 7, GITR, CTLA4, and FoxP3 gene expression were all significantly increased within the spleens of HSC+FC recipients compared with HSC+TBM recipients. FC recipients expressed a 2.06 ± 0.29–, 2.45 ± 0.30–, and 12.33 ± 2.61–fold increase in respective GITR, CTLA4, and FoxP3 expression over HSC+TBM controls. Student t test analysis of these gene expression patterns in HSC+FC and HSC+TBM recipients 28 days after BMT shows a highly statistically significant difference for FoxP3 (P = .02), GITR (P = .02), and CTLA-4 (P = .01) gene expression. These findings indicate that though the FC is not a regulatory T cell, FC transplantation results in the absence of GVHD in the setting of an in vivo induction of regulatory T cells within the recipient.
Induction of regulatory T-cell genes after FC transplantation. Splenocytes isolated from HSC+FC (n = 4) and HSC+TBM (n = 3) recipients 28 days after BMT were individually analyzed using real-time PCR for mRNA encoding GITR, CTLA-4, and FoxP3. Mean ± SD log 2-fold changes of gene expression for HSC+FC recipients compared with HSC+TBM recipients from 2 independent experiments are shown.
Induction of regulatory T-cell genes after FC transplantation. Splenocytes isolated from HSC+FC (n = 4) and HSC+TBM (n = 3) recipients 28 days after BMT were individually analyzed using real-time PCR for mRNA encoding GITR, CTLA-4, and FoxP3. Mean ± SD log 2-fold changes of gene expression for HSC+FC recipients compared with HSC+TBM recipients from 2 independent experiments are shown.
Discussion
We have previously established that failure of allogeneic engraftment across MHC class I and II disparities can be decreased with the assistance of a unique CD8+/TCR- population derived from donor BM, known as the facilitating cell.10 The initial description of the FC has been substantially expanded in the current study by demonstrating the absence of GVHD effector function within the FC and the in vivo induction of regulatory T cells after FC transplantation. We have now demonstrated that despite the absence of other non-HSC populations in the BM inoculum, which could potentially down-regulate GVHD, HSC+FC recipients exhibit substantial donor chimerism without clinical evidence of GVHD across fully allogeneic MHC barriers. However, it is well established that radioresistant recipient cells may mitigate potential GVHD activity, and GVHD effector activity can be markedly enhanced when recipient alloreactivity is lowered, as in the lethally irradiated P → F1 models. Earlier GVHD models using the B6 → B6D2F1 combination reconstituted irradiated recipients with a donor inoculum of 5 × 106 T cell–depleted BM cells and induced lethal GVHD through the addition of 1 to 2 × 106 peripheral T cells.31 Given a 0.5% incidence of HSCs within murine marrow, a significant number of non-HSC donor cells are included in this TCD inoculum. There has been much discussion of the contribution and need for other cell populations in the initiation and development of acute GVHD, and our goal was to specifically assess the GVHD potential of the FCs. Therefore, a semiallogeneic P → F1 model of lethal GVHD was established using only purified donor cells, specifically HSCs, T cells, and FCs. Using only 2000 HSCs as the source of donor BM, cells conventionally included in the TCD inoculum are absent, thereby permitting the assessment of potential GVHD effector activity of specific purified cell subsets added in isolation. The current model uses only 200 000 purified αβTCR+ splenocytes added to the donor HSC inoculum to induce lethal GVHD in a dose-dependent manner, with a mortality rate exceeding 50%. This represents an approximately 5-fold increase in sensitivity for GVHD effector activity compared with the TCD model, for which 1 to 2 × 106 splenocytes are used.
The current studies have elucidated 3 fundamental observations with potential clinical significance in relation to GVHD. First, to the best of our knowledge, this is the most clinically relevant model of GVHD, using limited numbers of purified HSCs and bone marrow cells to induce lethal GVHD. This model is particularly important because several recent studies have suggested that donor CD8+ T cells decrease the risk for engraftment failure and leukemic relapse.6,11-14 However, our data demonstrate that surprisingly severe GVHD is elicited by purified TBM in a semiallogeneic model, suggesting that BMT supplementation with donor BM–derived CD8+ T cells could be clinically devastating, at least if purified HSCs are transplanted into a recipient after the recipient has undergone myeloablation.
Second, we have determined that FCs do not exhibit GVHD effector activity. Although the ability of FCs to facilitate engraftment of purified HSCs has been well documented in a fully allogeneic model, GVHD effector potential of FCs has not previously been assessed in a GVHD-sensitive model. Therefore, FCs were evaluated in a semiallogeneic BMT model in which effector activity was not opposed by radioresistant alloreactive recipient cell populations. Furthermore, the ability of HSCs to engraft in this semiallogeneic model without FCs permitted a direct comparison of GVHD potential for TBM and FCs in vivo. Despite the induction of severe GVHD by limited numbers of αβTCR+ TSP or CD8+/TCR+ TBM, escalation of the number of transplanted FCs from the usual 50 000 required for allogeneic engraftment to as many as 400 000 FCs failed to show clinical or histologic evidence of significant GVHD effector activity. Furthermore, HSC+FC recipients did not develop the clinical appearance, nor did they manifest low levels of donor engraftment or decreased cell numbers, classically present in chronic GVHD.
Third, we have focused on understanding the mechanism by which severe GVHD is readily induced by CD8+/TCR+ TBM but is absent after the transplantation of CD8+/TCR- FCs. The current findings demonstrate that in marked contrast to recipients of HSC+TBM in whom lethal GVHD develops, FC transplantation is characterized by an increased expression of factors linked to T cell–mediated immunosuppression. HSC+FC recipients demonstrate significant increases in TGF-β transcription, a key factor necessary for the induction and development of regulatory T cells, and a several-fold increase in CTLA-4, GITR, and FoxP3 gene expression, which are characteristic of regulatory T-cell and suppressor T-cell function.27,28,32-34 These factors are not induced in the splenic T cells of HSC+TBM recipients, indicating that FC transplantation avoids GVHD through the in vivo establishment of regulatory T cells in the reconstituted recipient. Others have shown that adding ex vivo–activated regulatory T cells to the donor inoculum can inhibit GVHD and prevent the expansion of alloreactive donor T cells.21-23,25 However, the FC is not a CD4+CD25+ T cell, nor does it express the established regulatory T-cell genes CTLA4, GITR, or FoxP3, indicating that the FC is not a regulatory T cell. Instead, FC transplantation induces the development of immunoregulatory T cells in vivo, a previously unknown characteristic of the FC and of FC-mediated tolerance. This ability to induce regulatory T cells is particularly intriguing because the FC expresses a TCR-β heterodimer containing a novel 33-kDa protein, FCp33, in place of the conventional αβTCR and γδTCR heterodimers on the GVHD effectors TSP and TBM.35 The role of the FCp33 receptor and the mechanism by which the FC induces immunoregulatory T cells in the reconstituted host after BMT are important areas of future study.
In summary, the data presented here establishes that the purified semiallogeneic HSC model of GVHD is sensitive and specific and that GVHD effector activity of individual purified donor cell populations can be accurately assessed in a clinically relevant model of HSC transplantation. Significant mortality and morbidity resulting from GVHD were demonstrated after the cotransplantation of HSCs and αβTCR+ TSP or CD8+/TCR+ TBM. These findings suggest that using CD8+ T cells for adoptive therapy potentially carries a significant risk for GVHD. This may be particularly true in the clinically relevant setting of purified donor HSC transplantation between parent and child, from which additional cell populations with the potential to mitigate the GVHD response have been removed. Of greater potential importance, however, is the absence of significant GVHD after the transplantation of the CD8+/TCR- FC population. Interestingly, the lack of GVHD after HSC+FC transplantation is associated with the in vivo induction of regulatory T cells during immune reconstitution. These findings demonstrate that inducing immunoregulatory T cells as a means to prevent GVHD can be achieved in vivo through a novel FC-mediated mechanism, without previous antigen exposure or ex vivo T-cell expansion. The ability to decrease engraftment failure and to avoid GVHD after allogeneic BMT through the in vivo induction of regulatory T cells suggests that donor FCs may potentially offer a safer, more efficient, and clinically relevant approach to HSC supplementation and donor organ tolerance.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-01-0393.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Sincere thanks to Rahilya Napoli for her hard work and technical support and to the staff at the Redstone Animal Facility at the Dana-Farber Cancer Institute for outstanding animal care.