Based on the hypothesis that long-term fetomaternal microchimerism is associated with acquired immunologic hyporesponsiveness to noninherited maternal antigens (NIMAs) or inherited paternal antigens (IPAs), several groups have recently reported successful cases of non-T-cell–depleted hematopoietic stem cell transplantation (SCT) from HLA-haploidentical family members mismatched for NIMAs. In this study, we examined the outcomes of 35 patients with advanced hematologic malignancies who underwent HLA-2-antigen– or HLA-3-antigen–incompatible SCT from a microchimeric NIMA-mismatched donor. After standard-intensity or reduced-intensity preparative regimens, all patients had sustained hematopoietic recovery with tacrolimus-based graft-versus-host disease (GVHD) prophylaxis. Grade II/IV acute GVHD occurred in 19 (56%) of 34 evaluable patients, while extensive chronic GVHD developed in 13 (57%) of 23 patients who could be evaluated. Multivariate analysis demonstrated that NIMA mismatch in the GVH direction was associated with a lower risk of severe grade III-IV acute GVHD when compared with IPA mismatch (P = .03). Fifteen patients were alive and 14 of them were disease-free with a median follow-up of 20 (range, 8 to 37) months. These results indicate that T cell–replete SCT from an HLA-haploidentical NIMA-mismatched donor can offer durable remission with an acceptable risk of GVHD in selected patients with advanced hematologic malignancies who lack immediate access to a conventional stem cell source.
Introduction
The lack of donor availability has been a major limitation to the widespread use of allogeneic hematopoietic stem cell transplantation (SCT), which is a curative treatment for various hematologic malignancies, bone marrow failure syndromes, and genetic disorders. Despite the presence of an increasing pool of unrelated volunteer donor registries, many patients who need allogeneic SCT are not able to find a histocompatible donor because of the inheritance of a rare or private HLA haplotype. Currently, unrelated umbilical cord blood is validated as an alternative stem cell source allowing less HLA restriction for children with malignant diseases.1 However, its application to adult recipients is still associated with a high probability of early nonrelapse mortality mostly due to delayed or unsuccessful engraftment,2 although a few centers have reported encouraging results.3-6
Genetically HLA-haploidentical family members are more readily accessible to most of the patients who fail to find an HLA-compatible related or unrelated donor. However, transplants from such donors are limited by a number of historical barriers such as intractable graft-versus-host disease (GVHD) or graft failure.7,8 In the past decade, extensive efforts have been made to overcome these problems, especially in transplantations from HLA-2-antigen– or HLA-3-antigen–mismatched donors; those include partial T-cell depletion combined with intensive immunosuppression,9 megadose CD34+ cell transplantation,10 and ex vivo anergy induction.11 Although these studies clearly became the leads to safer and more effective ways to undergo highly HLA-disparate transplantations, their introduction into routine clinical practice still awaits further validation.
An alternative way to overcome histocompatible barriers in transplantation medicine would be to identify “permissible” or “acceptable” HLA mismatches.12 The concept that some mismatches are not harmful in terms of allograft acceptance was originally proposed from earlier observations that different HLA mismatches had different influences on survival rates of kidney allografts.13,14 In this scenario, more attention should be focused on the relatively low immunogenicity of the noninherited maternal HLA antigens (NIMAs) to which one had been previously exposed in utero. In the late 1980s, Claas and colleagues in Leiden reported that about half of the patients who received multiple blood transfusions as adults exhibited reduced alloreactivity against NIMAs, while they had much higher reactivity against noninherited paternal antigens (NIPAs).15 The clinical significance of this observation was later revived through 2 retrospective analyses showing the superior long-term survival rates in NIMA-mismatched kidney allografts, although the introduction of new immunosuppressive drugs appeared to obscure such effects.16,17 Furthermore, another study performed by the International Bone Marrow Transplant Registry (IBMTR) also revealed that bone marrow transplants from NIMA-mismatched siblings were significantly associated with a lower incidence of grade II to IV acute GVHD (aGVHD) when compared with those from the other HLA-mismatched familial donors.18 However, the true existence of such tolerogenic “NIMA effect” has still been the subject of debate due to lack of the knowledge of the underlying immunologic mechanisms.19,20
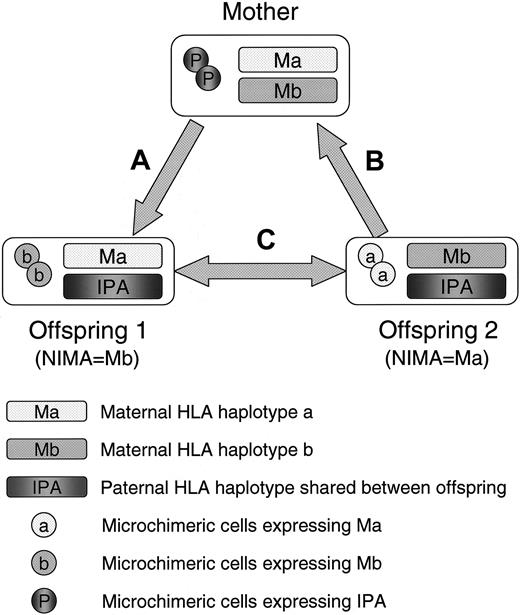
Recently, Andrassy et al have shown in a murine experimental model that more than half of the H-2b/b offspring that had experienced both oral and in utero exposure to noninherited H-2d antigens of semiallogenic H-2b/d mothers could accept fully allogeneic H-2d/d vascularized heart allografts for a long time.21 Importantly, they also demonstrated that the tolerant mice had higher levels of maternal cell microchimerism after their birth as compared with the nontolerant mice, suggesting the role of long-term maternal microchimerism for the induction and maintenance of NIMA-specific allotolerance. In this context, we previously found that more than two thirds of healthy adults have hematopoietic cell microchimerism presumed to be of maternal or fetal origin and proposed that the presence of such microchimerism might have beneficial effects on the transplantation outcome by reducing host-versus-graft (HVG) or graft-versus-host (GVH) alloreactivity not only against NIMAs but also against inherited paternal HLA antigens (IPAs) in the setting of maternal donation.22,23 On the basis of this hypothesis, several transplantation centers in Japan have started clinical trials to test the feasibility of HLA-haploidentical allo-SCT from microchimeric NIMA-mismatched relatives without using either T-cell depletion or intensive posttransplantation immunosuppression. In these transplantations, the HLA haplotypes that are not shared between the donor and recipient always include at least one of the noninherited maternal haplotypes of their own, mimicking the condition that NIMA is complementarily selected as an acceptable mismatch (NIMA-“complementary” transplantation) (Figure 1). To evaluate the safety and efficacy of this novel principle of alternative donor selection, we performed a nationwide collective analysis of the clinical outcomes of T cell–replete allogeneic SCT for advanced hematologic malignancies from HLA-2-antigen– or HLA-3-antigen–incompatible (in the GVH direction) familial donors that were mismatched for NIMAs.
A scheme of 3 different types of NIMA-complementary HLA-haploidentical stem cell transplantation. (A) Stem cell transplantation (SCT) from mother to offspring: GVH reaction is directed against the inherited paternal HLA antigens (IPA), while HVG reaction is directed against the NIMAs of offspring 1 (Mb). (B) SCT from offspring to mother: GVH reaction is directed against the NIMAs of offspring 2 (Ma), and HVG reaction is directed against IPAs. (C) SCT between NIMA-mismatched siblings who shared the inherited paternal HLA haplotype: These siblings are bidirectionally mismatched for NIMAs in both the GVH and HVG directions.
A scheme of 3 different types of NIMA-complementary HLA-haploidentical stem cell transplantation. (A) Stem cell transplantation (SCT) from mother to offspring: GVH reaction is directed against the inherited paternal HLA antigens (IPA), while HVG reaction is directed against the NIMAs of offspring 1 (Mb). (B) SCT from offspring to mother: GVH reaction is directed against the NIMAs of offspring 2 (Ma), and HVG reaction is directed against IPAs. (C) SCT between NIMA-mismatched siblings who shared the inherited paternal HLA haplotype: These siblings are bidirectionally mismatched for NIMAs in both the GVH and HVG directions.
Patients and methods
Study patients and data collection
This registry study included a total of 35 patients with advanced hematologic malignancies who underwent non-T-cell–depleted SCT from an HLA-haploidentical NIMA-mismatched family member at 16 transplantation centers affiliated with the Japan Society for Hematopoietic Cell Transplantation (JSHCT)24 between May 2000 and June 2003. All the patients, the donors, or their guardians provided written informed consents on the protocols for the NIMA-complementary haploidentical transplantation, which were approved by the institutional review boards at participating centers. The detailed clinical course of 8 of these patients was previously reported in several papers.25-29 The eligibility criteria for the study were as follows: (1) having advanced leukemia or lymphoma; (2) receiving T cell–replete bone marrow and/or peripheral blood stem cell (PBSC) grafts mismatched for 2 or 3 HLA-A, -B, or -DR antigens in the GVH direction harvested from mother, offspring (to mother), or an NIMA-mismatched sibling who was shown to have recipient-specific microchimerism presumed to be of fetal or maternal origin; (3) receiving tacrolimus-based GVHD prophylaxis. Using a standardized questionnaire form, participating centers were requested to consecutively report data with respect to the patient/donor characteristics and clinical outcomes in terms of neutrophil recovery, platelet recovery, aGVHD, chronic GVHD (cGVHD), graft failure, relapse, and survival after transplantation to the office of the Japanese Collaborative Study Group for NIMA-Complementary Haploidentical Stem Cell Transplantation (Department of Hematology/Oncology, Kyoto University Hospital, Japan). The reported data sets including the clinical severity of aGVHD and cGVHD were thoroughly reviewed by 2 independent physicians. All the patients were longitudinally followed until February 29, 2004.
Histocompatibility testing and detection of long-term fetomaternal microchimerism
HLA-A, -B, and -DR antigens of the donor and recipient were typed by the standard serologic technique or low- to middle-resolution DNA typing before transplantation and were designated according to the World Health Organization nomenclatures for factors of the HLA system.30 HLA-A, -B, and -DR mismatches in the GVH direction were defined as the presence of recipient antigens determined by serologic or low-resolution DNA typing not shared by the donor and vice versa; mismatches in the HVG direction were defined as the presence of the donor antigens not shared by the recipient. NIMA and IPA haplotypes were deduced from the HLA typing results obtained from family members of 2 or 3 generations. The presence of the recipient-specific long-term microchimerism presumed to be of maternal or fetal origin was confirmed in all the donors with IPA- or NIMA-specific nested polymerase chain reactions (PCRs) using sequence-specific primers as previously described.26,31 No patients were reported to be positive for antidonor lymphocyte antibodies before transplantation.
Preparative regimens, transplantation procedures, and GVHD prophylaxis
Recipients were prepared for transplantation with various conditioning regimens used by each center. Regimens that included more than 4 Gy total-body irradiation (TBI) or more than 8 mg/kg busulfan were considered to be myeloablative (standard-intensity regimens). According to this definition, 21 patients (60%) received standard-intensity regimens based on 6 to 12 Gy TBI (median dose, 10 Gy) combined with cyclophosphamide in 5, melphalan in 3, cyclophosphamide plus cytarabine in 9, cyclophosphamide plus busulfan in 3, and fludarabine plus melphalan plus thiotepa in 1. Three patients (9%) received non-TBI standard-intensity conditioning based on busulfan plus cyclophosphamide. The remaining 11 patients (31%), who were at least 50 years of age or who had a history of extensive prior therapy, received fludarabine-based reduced-intensity regimens that included busulfan in 6 and melphalan in 5. The types of stem cell source were granulocyte colony-stimulating factor (G-CSF)–mobilized PBSCs in 30 patients (86%) (the median number of CD34+ cells infused [× 10-6/kg of recipient's body weight], 5.0; range, 1.3 to 9.5), bone marrow in 4 (11%) (the number of nucleated cell doses infused [× 10-8/kg of recipient's body weight], 0.5, 4.7, 5.5, 6.1), and 1.2 × 108/kg of bone marrow–nucleated cells plus PBSCs containing 1.0 × 106/kg of CD34+ cells in 1 (3%). Posttransplantation G-CSF was administered to 28 patients. Twenty-three patients (63%) received GVHD prophylaxis consisting of tacrolimus and methotrexate (MTX). The other regimens used for GVHD prophylaxis included tacrolimus alone in 4 (11%), tacrolimus plus corticosteroids or mycophenolate mofetil in 2 (6%), and tacrolimus plus MTX plus corticosteroids in 6 (16%). All the patients received standard antibiotics, antifungal agents, and blood products according to the protocols of each institution.
Assessment of engraftment, GVHD, and survival
The date of neutrophil recovery was defined as the first of 3 consecutive days in which the absolute neutrophil count (ANC) exceeded 0.5 × 109/L. The date of platelet recovery was defined as the first of 7 consecutive days during which the nontransfused platelet count was at least 20 × 109/L. Donor cell engraftment was determined on marrow samples using sex chromosome–specific fluorescence in situ hybridization (FISH) or quantitative PCR of informative short tandem repeat regions in the recipient and donor. Complete donor chimerism was defined as the detection of more than 95% donor cells in the marrow-nucleated cell populations. Late graft failure was defined among the patients who attained neutrophil engraftment as a decline of ANC to less than 0.5 × 109/L for at least 7 consecutive days with evidence of severe hypocellularity of bone marrow confirmed by histopathological examination. Acute and chronic GVHD were diagnosed and graded according to standard criteria.32,33 All the patients who had evidence of donor cell engraftment were considered to be evaluable for aGVHD, excluding 1 patient who developed life-threatening multiple organ failure before engraftment. Chronic GVHD was evaluated in patients who survived without relapse or disease progression for at least 100 days after transplantation. Overall survival was applied to all the patients and measured from the date of transplantation to the date of death from any cause. Event-free survival was applied to the patients who survived in remission for at least 30 days after transplantation and measured from the date of transplantation to the date of relapse or death. Patients were censored at the time of the last follow-up.
Statistical analysis
Probabilities and their 95% confidence intervals (CIs) by binomial distribution for obtaining neutrophil recovery were calculated using the number of subjects obtaining target values as numerator and total number of subjects as denominator. Cumulative incidence curves were used to obtain probability of aGVHD that has survival event as a competing risk.34 Kaplan-Meier estimates were applied to obtain the probability of survival at specific time points, and difference in survival was tested by the log-rank test. The extended Fisher exact test was employed to evaluate the independence between categoric variables between groups separated by the donor type, while the Mann-Whitney test was used for age. Unconditional logistic regression analysis was applied to obtain odds ratios (ORs) for the effect of possible risk factors on the development of grade III/IV aGVHD. Univariate Cox proportional hazard analysis was applied to estimate hazard ratios for possible risk factors for survival.35 A multivariate logistic regression model was constructed by stepwise method with forward selection. P values for inclusion and exclusion were .15 and .25, respectively. The factors evaluated in the model were as follows: the number of mismatched HLA antigens in the GVH direction and in the HVG direction, type of GVH target (IPA versus NIMA), recipient age at transplantation (20 years or more versus less than 20 years), donor-recipient sex pairs (female-to-male versus other combination), disease status at transplantation, type of preparative regimens (standard intensity versus reduced intensity; TBI-containing versus non-TBI), and type of GVHD prophylaxis. Recipient age and the number of GVH mismatches were forced to be in the model. All tests were 2-sided, and a P value less than .05 was considered to be suggestive evidence of an association. All statistical analyses were conducted by STATA version 8 software (STATA, College Station, TX) based on data available on February 29, 2004.
Results
Characteristics of the study patients
The characteristics of the patients, the donors, and the transplantation procedures are summarized in Table 1. Diagnoses included acute myelogenous leukemia (AML) in 12, acute lymphoblastic leukemia (ALL) in 11, lymphoblastic lymphoma in 1, chronic myeloid leukemia (CML) in 7, diffuse large B-cell lymphoma in 3, and adult T-cell leukemia in 1. Five patients received NIMA-complementary SCT after failures of the prior bone marrow or cord blood allografts (primary rejection in 3 and relapse in 2). The remaining 30 recipients, including 2 patients with a history of previous autograft, lacked an immediate access to a conventional donor until the time of the transplantation and underwent NIMA-complementary SCT as a first allograft. Disease status at transplantation was as follows: first complete remission with poor-risk clinical features in 2, advanced remission or late chronic phase in 11, and chemorefractory in 22. With respect to the types of HLA-A, -B, and -DR antigens serologically mismatched in the GVH direction at the unshared HLA haplotype, 6 donor-recipient pairs were mismatched for HLA-A and -B, 18 pairs were mismatched for HLA-DR plus either HLA-A or -B, and the remaining 11 had a mismatch at HLA-A, -B, and -DR. In particular, these transplantations can be separated into the following 2 categories as to whether the type of HLA mismatch in the GVH direction is IPA or NIMA: (1) SCT performed from mother to offspring (GVH target: IPA; n = 15); (2) SCT from offspring to mother (n = 9) or SCT between NIMA-mismatched siblings (n = 11) (GVHD target: NIMA). Recipients of maternal donor transplants (IPA-targeted group) were younger, had more female-into-male transplantations, and had less HVG incompatibility than the NIMA-targeted group. However, they had similar characteristics in the other background factors such as diagnosis, disease status at SCT, the number of mismatched HLA antigens in the GVH direction, type of conditioning, source of stem cell, use of posttransplantation G-CSF, and type of GVHD prophylaxis (Table 1).
Engraftment
All the patients attained successful neutrophil recovery at a median of 14 days (range, 9 to 21 days). Analyses of donor cell chimerism using marrow aspirates were available in 33 patients, excluding 2 patients who could not be assessed due to early toxic death. All the 22 evaluable patients receiving standard-intensity regimens and 10 of 11 patients conditioned with reduced-intensity regimens were shown to achieve complete donor chimerism between days 14 and 94 after transplantation. One patient with chemorefractory AML who received reduced-intensity conditioning had mixed chimerism due to the persistence of leukemic cells in the peripheral blood. Recovery of platelet counts of more than 20 × 109/L was observed in 31 patients (89%) at a median of 14 days (range, 0 to 104 days). The principal causes of failure of platelet recovery in the remaining 4 patients were early toxic death in 2, early relapse in 1, and thrombotic microangiopathy in 1. No late graft failure was reported.
Graft-versus-host disease
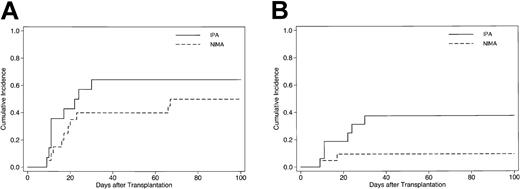
Acute GVHD was evaluable in 34 patients. The respective number of patients who developed grade 0, I, II, III, or IV aGVHD was 6 (18%), 9 (26%), 11 (32%), 5 (15%), and 3 (9%). One patient assigned to grade II developed treatment-resistant aGVHD after the early cessation of tacrolimus on day 45 and the subsequent donor lymphocyte infusions for relapse. The other 10 patients who developed grade II and 2 of 5 patients who developed grade III aGVHD completely responded to the primary treatment with corticosteroids. The cumulative incidence estimate of grades II to IV and III/IV aGVHD among all evaluable patients by 100 days after transplantation was 56% (95% confidence interval [CI], 38%-71%) and 22% (95% CI, 10%-37%), respectively. In univariate analysis, the incidence of grade III/IV aGVHD was not significantly associated with the number of mismatched HLA antigens in either the GVH or HVG direction, age of patient at SCT, donor-patient sex combination, disease status at SCT, type of conditioning, and regimen of GVHD prophylaxis (Table 2). However, it was notable that transplantations from offspring or sibling donors (GVH target: NIMA; “NIMA-targeted”group) had a lower risk of developing grade III/IV severe aGVHD when compared with those from maternal donors (GVH target: IPA; “IPA-targeted”group) (P = .04). It seems unlikely that the difference in the risk of developing severe aGVHD between the IPA-targeted and NIMA-targeted transplantations originates from other factors in each patient group, because 2 groups had similar background characteristics except a few variables (Table 1), and NIMA mismatch in the GVH direction was still associated with a lower risk for grade III/IV aGVHD after adjustment by a multivariate regression model (P = .03) (Table 2). A similar result was obtained by another multivariate model including donor multiparity (multiparous females versus other females or males) instead of simple donor-recipient sex mismatch as a potential risk factor for severe aGVHD. The respective cumulative incidence of grade II to IV aGVHD in the NIMA-targeted group and IPA-targeted group was 50% (95% CI, 27%-69%) and 64% (95% CI, 34%-83%) (Figure 2A), whereas that of grade III/IV was 10% (95% CI, 2%-26%) in the NIMA-targeted group and 38% (95% CI, 15%-60%) in the IPA-targeted group (Figure 2B).
Cumulative incidence of acute GVHD in patients who underwent T cell–replete NIMA-complementary stem cell transplantation. Cumulative incidence curves of grades II-IV (A) and III-IV (B) acute GVHD according to the type of GVH target among 34 evaluable patients who received NIMA-complementary SCT. Solid lines represent SCT from mother to offspring (GVH target: IPA), while dashed lines indicate SCT from offspring or NIMA-mismatched siblings (GVH target: NIMA).
Cumulative incidence of acute GVHD in patients who underwent T cell–replete NIMA-complementary stem cell transplantation. Cumulative incidence curves of grades II-IV (A) and III-IV (B) acute GVHD according to the type of GVH target among 34 evaluable patients who received NIMA-complementary SCT. Solid lines represent SCT from mother to offspring (GVH target: IPA), while dashed lines indicate SCT from offspring or NIMA-mismatched siblings (GVH target: NIMA).
Chronic GVHD was observed in 19 (83%) of 23 evaluable patients who survived without evidence of relapse for at least 100 days after transplantation; 6 patients had limited-stage disease, and the remaining 13 patients had extensive disease. Extensive cGVHD occurred in 4 (44%) of 9 evaluable patients in the IPA-targeted group and 9 (64%) of 14 evaluable patients in the NIMA-targeted group.
Relapse and causes of death
Twenty deaths were reported until the time of last follow-up. Primary causes of death were disease-related in 9 and transplantation-related in 11 (Table 3). Two patients died from multiorgan failure due to regimen-related toxicity by day 30. Four chemorefractory patients never attained remission after transplantation and died of infection (n = 1), treatment-resistant GVHD after donor lymphocyte infusions (n = 1), and disease progression (n = 2) between days 65 and 121. Among the remaining 29 patients who survived for more than 30 days with evidence of achieving remission after transplantation, 8 patients experienced clinical or cytogenetic relapse between days 77 and 377. Seven of the relapsed patients eventually succumbed to disease progression between 117 and 756 days after transplantation. Seven other patients died without evidence of recurrent disease from treatment-resistant GVHD (n = 4), pulmonary infection (n = 2), and myocarditis (n = 1) between 37 and 230 days after transplantation.
Survival
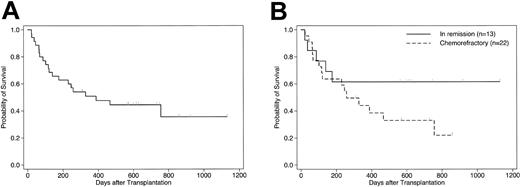
As of February 29, 2004, 15 (43%) of the 35 patients were alive and 14 (40%) of these were disease-free, with a median follow-up of 20 months (range, 8 to 37 months). The estimated probability of survival at 3 years after transplantation was 38% (95% CI, 17%-60%) for the whole cohort (Figure 3A). The survival rate for patients who underwent transplantation in remission or chronic phase was 62% at 3 years, compared with 22% at 2.3 years for those who had a chemorefractory disease at transplantation (P = .17) (Figure 3B). When the analysis was confined to the 29 patients who survived in remission for at least 30 days after transplantation, the estimated event-free survival at 3 years was 47% (95% CI, 29%-66%). Notably, 5 (23%) of the 22 chemoresistant patients were alive in continuous remission for more than 18 months after transplantation (2 with relapsed or primary refractory AML, 1 with relapsed ALL, 1 with CML in blastic phase, and 1 with primary refractory diffuse large B-cell lymphoma). All surviving patients in remission had a Karnofsky score of 80 or more. In univariate analysis, no factors including the degree of HLA incompatibility and the type of GVH target (IPA versus NIMA) showed significant association with survival.
Probability of survival in patients who underwent T cell–replete NIMA-complementary stem cell transplantation. Kaplan-Meier estimates of overall survival in 35 patients who underwent HLA-haploidentical SCT from a microchimeric NIMA-mismatched donor for advanced hematologic malignancies. (A) All patients; (B) comparison according to disease status at transplantation (P = .17 by log-rank test).
Probability of survival in patients who underwent T cell–replete NIMA-complementary stem cell transplantation. Kaplan-Meier estimates of overall survival in 35 patients who underwent HLA-haploidentical SCT from a microchimeric NIMA-mismatched donor for advanced hematologic malignancies. (A) All patients; (B) comparison according to disease status at transplantation (P = .17 by log-rank test).
Discussion
In this study, we evaluated the feasibility of T cell–replete HLA-haploidentical SCT from a microchimeric NIMA-mismatched family member (NIMA-complementary SCT) in terms of engraftment, incidence and severity of GVHD, and the events that affected survival among a selected cohort of patients with advanced hematologic malignancies. Our analyses revealed previously unknown benefits of non-T-cell–depleted SCT based on such novel criteria for HLA-haploidentical donor selection, although the heterogeneity of these transplantations currently demands careful interpretation of each result. First, in contrast to the previous experiences of “nonmegadose” CD34+-selected SCT or umbilical cord blood transplantations in adults,2,36 our series of patients had a high probability of prompt hematopoietic engraftment following both standard-intensity and reduced-intensity conditioning regimens. Second, regardless of the great degree of HLA disparity between donor and recipient, 27 (79%) of the 34 evaluable patients did not develop treatment-resistant aGVHD using standard GVHD prophylaxis based on tacrolimus. Moreover, although most of the patients had an advanced or chemorefractory disease at the time of transplantation, most of these patients still remained alive and disease-free with a relatively good performance status, yielding a product-limit survival estimate of 38% at 3 years. Additionally, it is less likely that these favorable results might be attributable to the relative homogeneity of the histocompatibility antigens among the Japanese populations, because the Japanese nationwide analyses of T cell–replete HLA-2-loci– or HLA-3-loci–mismatched SCT without considering NIMA complementarity also revealed a higher incidence of severe aGVHD and inferior survival rates as have been previously reported from the non-Japanese centers.37,38
To date, several population studies have provided evidence in favor of the presence of the tolerogenic “NIMA effect,” including the suppression of humoral immune responses,15 superior graft survival of kidney transplants from an HLA-haploidentical sibling or cadaveric donor,16,17 and low rates of aGVHD in umbilical cord or bone marrow transplantation from an NIMA-mismatched sibling.18,39 In addition, although pregnancy can induce long-persisting primed cytotoxic T cells specific for fetal alloantigens of paternal origin,40,41 peripheral blood lymphocytes (PBLs) obtained from mothers were reported to exhibit a markedly depressed proliferative response to PBLs of their infants but not to those of their husbands at 6 months after delivery,42 suggesting that the postpartum maternal immune system can preserve IPA-specific protective mechanisms at least for a certain period of time. In accordance with this observation, Polchi et al reported an unexpectedly low rate of severe GVHD in children who received unmanipulated bone marrow from fully HLA-haploidentical mothers using standard preparative regimens combined with peritransplantation administration of antithymocyte globulin (ATG).43 Furthermore, a retrospective study performed by the JSHCT showed that even adult patients who received SCT from maternal donors had a better survival rate when compared with those receiving paternal blood and marrow grafts. Although there have been conflicting reports on the true presence of such tolerogenic or protective effects among the “mother-child union,” recent studies using murine models have demonstrated the involvement of multiple mechanisms44 and highlighted the role of regulatory T-cell subsets and the longlasting fetomaternal microchimerism in the establishment of IPA- or NIMA-specific hyporesponsiveness.21,45
The phenomenon of long-term fetomaternal microchimerism, which is naturally acquired as a result of 2-way cell traffic between mother and fetus during pregnancy,22 was first described in the late 1990s through the development of PCR-based techniques that are capable of detecting less than 0.01% of microchimeric cells among the background cells.46,47 Earlier studies extensively investigated the role of such microchimerism in the pathogenesis of various autoimmune diseases; however, it has been difficult to demonstrate a true causal link, because a number of healthy individuals were also shown to be naturally microchimeric.48,49 Given the common incidence of fetomaternal microchimerism among healthy subjects without any autoimmune attacks, we subsequently raised a hypothesis that long-term acceptance of maternal cells in offspring and, vice versa, that of offspring's cell in the maternal circulation might be an indicator of acquired immunologic hyporesponsiveness to NIMAs and IPAs, respectively (Figure 1).22,49 In support of this concept, nearly half of our series of patients did not develop aGVHD of grade II or more, irrespective of the number of mismatched HLA antigens between donor and recipient. However, this striking finding does not seem to be a definitive proof of principle, because 8 (24%) of the 34 evaluable patients still experienced severe grade III/IV aGVHD, suggesting that the presence of the recipient-specific microchimerism in the donor is a relative indicator, rather than a hallmark, of appropriate NIMA-mismatched donor selection. Therefore, for the “NIMA-complementary” transplantations to be safer and more feasible, it is essential to establish additional methods for identifying the NIMA-mismatched donor-recipient pairs who are at high risk for developing severe GVHD.
Importantly, 6 (43%) of the 14 evaluable patients receiving SCT from a microchimeric maternal donor developed grade III or IV aGVHD, 3 of which became fatal. Although the result should be interpreted with caution due to small size of this study, multivariate analysis revealed that the only factor significantly associated with the incidence of severe aGVHD among recipients of NIMA-complementary SCT was IPA mismatch in the GVH direction. If the maternal donation is more likely to result in severe aGVHD even when long-term fetal cell microchimerism can be detected in the mother, what are the underlying mechanisms for that? One possibility is that maternal GVH reactions against offspring may be modulated by presensitization against minor histocompatibility antigens that are presented on the inherited maternal HLA antigens of the offspring.41 Recently, G-CSF–mobilized PBSC grafts obtained from some female donors were reported to contain a significant amount of male DNA presumed to be of fetal origin.50 Although we could not find any statistically significant association between donor multiparity and the incidence of severe aGVHD in our small cohort of patients, it is important to further investigate the correlation between the presence of H-Y microchimerism and the severity of aGVHD in SCT from multiparous women with a history of male pregnancy into a male recipient. Alternatively, fetal cell microchimerism in the mother and maternal cell microchimerism in the offspring might have different immunologic implications in the context of modifying alloreactivity against NIMAs or IPAs. Anderson and Matzinger demonstrated in a murine skin allograft model that donor T-cell microchimerism can result in either immunity or tolerance depending on the immunologic maturity of the host and the antigenic disparity.51 On the other hand, Burlingham and colleagues described in an intriguing report a patient who was tolerant of a maternal kidney allograft without any immunosuppressive therapy.52 PBLs and skin of this patient were found to contain microchimeric maternal cells, and depletion of these maternal cells could specifically inhibit the in vitro activity of cytotoxic T cells against NIMAs in a dose-dependent manner. More recently, Cai et al showed the coexistence of effector and regulatory CD8+ T cells specific for the HA-1 minor histocompatibility antigen in renal allograft recipients who had long-term HA-1 microchimerism that was possibly first induced from mother and later from the donor kidney allograft.53
The risk of aGVHD might be also influenced by other genetic factors different from HLA or minor histocompatibility antigens, such as killer immunoglobulin-like receptor (KIR) haplotypes and cytokine gene polymorphisms.54,55 Recently, the type of interleukin-10 (IL-10) promotor haplotypes in the recipient was shown to significantly influence the risk of grade III/IV aGVHD and the nonrelapse mortality after HLA-identical sibling hematopoietic cell transplantation.55 The interaction between donor T cells and host antigen-presenting cells (APCs) is considered an essential step in the development of aGVHD, and an elegant murine study by Teshima et al showed that the expression of the major histocompatibility complex antigens in the target tissue is not essential for the development of aGVHD.56 If this also holds true in human beings, the magnitude of GVH reactions might be determined by the profile of preferential cytokine production from recipient APCs, which regulate functions of the donor effector T-cell repertoire. Because the effect of cytokine gene polymorphisms on the severity of aGVHD might be greater in the setting of HLA-incompatible SCT, it would be beneficial to investigate the relationship between the cytokine polymorphisms of NIMA-mismatched donor-recipient pairs and their correlations with clinical outcomes.
Finally, the type of stem cell source might also have an influence on the development of aGVHD and cGVHD after NIMA-complementary SCT. Transplantation of unmanipulated PBSCs has been reported to be associated with a higher incidence of cGVHD, particularly of extensive type, as compared with bone marrow among SCT from HLA-identical siblings.57,58 Apparently, the high incidence of cGVHD in our series of patients might be partly explained by the fact that most of them had received PBSC allografts, although whether the difference in quantity or characteristics of microchimeric cells contained in the marrow or PBSC graft may affect the development of cGVHD has yet to be investigated.
In conclusion, our results showed that T cell–replete HLA-haploidentical SCT from a microchimeric NIMA-mismatched family member can reconstitute long-term hematopoiesis with acceptable rates of treatment-resistant GVHD and also can induce sustained remission in selected patients with chemorefractory hematologic malignancies. Because this approach may greatly increase the donor availability and open a way to more appropriate donor selection in HLA-haploidentical SCT, future research is required to more precisely evaluate NIMA- or IPA-specific allotolerance and to identify genetic factors that are associated with GVHD and nonrelapse mortality in a given NIMA-mismatched donor-recipient pair.
Appendix
In addition to the authors, the following JSHCT members and colleagues participated in the nationwide study of NIMA-mismatched hematopoietic stem cell transplantation: Tetsuya Nishida, Yasuhiro Mochiduki, Yoshihisa Nagatoshi, Hiroyasu Ogawa, Seitaro Terakura, Yasunori Ueda, Naoya Ochiai, Shin-ichi Fuchida, Mikiya Endoh, Hiromasa Yabe, Katsuyoshi Ko, Kazuhiro Masuoka, Kentaro Aritaki, Ken Tabuchi, Mika Kuroiwa, Kumiko Goi, Norifumi Katayama, Akihiko Yokohama, Kozo Masuda, Masahiro Sako, Hirofumi Teshima, Satoru Hamada, Takahisa Yamane, Takashi Shiga, Tatsuyuki Kai, Nobuo Masauji, Naoto Fujita, Hiroshi Matsubara, and Koji Nagafuji.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-03-1212.
A complete list of the members of the Japanese Collaborative Study Group for NIMA (noninherited maternal antigen)-Complementary Haploidentical Stem Cell Transplantation appears in the “Appendix.”
Supported in part by grants from the Ministry of Health, Labor, and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Yasuo Morishima (Aichi Cancer Center), Dr Takayuki Ishikawa (Graduate School of Medicine, Kyoto University), and Dr Takanori Teshima (Okayama University Graduate School of Medicine and Dentistry) for their critical reading of the manuscript and to Dr Shinji Nakao (Kanazawa University Graduate School of Medical Sciences) and Dr Yoshinobu Kanda (University of Tokyo Hospital) for helpful discussions. We also thank Hiroko Muramatsu and Mika Kobayashi for their expert data management and secretarial assistance and all the staff members of the participating centers for their dedicated care to the patients and the donors in this study.