Oncogenic mutations of the receptor tyrosine kinase KIT occur in gastrointestinal stromal tumors (GISTs), some cases of acute myelogenous leukemia (AML), and systemic mastocytosis (SM). GISTs commonly contain mutations of the KIT juxtamembrane region while SM and AML harbor active site KIT mutations. Imatinib, which potently inhibits juxtamembrane mutants, is effective for the treatment of GISTs but has no activity against active site mutants. We analyzed the inhibitory potential of 2 small molecule inhibitors, MLN518 and PD180970, against different classes of KIT mutants. Both compounds inhibit the growth of cell lines expressing juxtamembrane mutant KIT. MLN518 additionally targets active site mutant cell lines, inhibiting cell proliferation, KIT, and signal transducer and activator of transcription-3 (Stat3) phosphorylation and inducing apoptosis at concentrations that may be clinically achievable. As phase 1 clinical trials of MLN518 in AML have shown little toxicity, our data suggest MLN518 is a promising candidate for the treatment of SM or AML with KIT mutations.
Introduction
KIT is a receptor tyrosine kinase (RTK) of the type III subgroup, characterized by 5 extracellular immunoglobulin-like domains and a split tyrosine kinase domain.1 Stem cell factor (SCF) binding induces dimerization and transphosphorylation, activating multiple signaling pathways, including mitogen-activated protein (MAP) kinases, Src kinases, Janus kinases/signal transducers and activators of transcription (Jaks/Stats), and phosphatidylinositol-3 (PI3) kinase.1
Oncogenic mutations of KIT occur in diverse malignancies including gastrointestinal stromal tumors (GISTs), sinonasal natural killer (NK)/T-cell lymphoma, seminomas/dysgerminomas, acute myelogenous leukemia (AML), and systemic mastocytosis (SM).2-4 The site of mutation tends to be cell-type specific. For example, 57% to 70% of GISTs have activating point mutations or small deletions in the juxtamembrane region of KIT.3 In contrast, nearly all patients with SM exhibit a mutation in codon 816, typically an aspartate to valine substitution (D816V).2,3,5 The D816V mutation, within the activation loop of the kinase, produces ligand-independent activation, increases the catalytic activity of KIT, and alters substrate specificity leading to aberrant signaling.6-8 Codon 816 mutations were also reported in AML, as were small deletions in exon 8 in the extracellular region of KIT, typically concurrent with core binding factor mutations.9-11
Treatment of SM is primarily symptomatic, although cladribine and interferon-α have some effect.12,13 AML with core binding factor mutations generally carries a good prognosis, but KIT mutations in this group confer a higher risk of relapse.9 Therefore, specifically targeting D816 mutant KIT may improve treatment of these malignancies.
Various small molecule inhibitors of KIT have been developed, including FLT3 tyrosine kinase inhibitors14-16 and imatinib mesylate, initially designed to target Bcr-Abl.17 The sensitivity of KIT mutants to small molecule inhibitors depends on the nature of the mutation. Imatinib, for example, potently inhibits KIT juxtamembrane mutants and induced partial responses in 54% of GIST patients in phase 2 clinical trials.3,18 In contrast, imatinib does not target D816 mutant KIT and is therefore ineffective in mastocytosis patients harboring these mutations.19-22
Study design
Cell lines
The Ba/F3 mouse pro-B-cell line and P815, a mouse mastocytoma cell line expressing murine D814Y KIT, were purchased from American Type Culture Collection (ATCC; Manassas, VA). The HMC-1 human mast cell line containing an activating juxtamembrane domain point mutation (V560G) 25 and Bcr-Abl-expressing Ba/F3 26 were described previously. The previously described retroviral construct, M5 hKIT Neo,27 was used as a template for site-directed mutagenesis to generate M5 hKIT D816V Neo. To generate cells expressing human D816V KIT, we transduced Ba/F3 cells with supernatants from retroviral packaging cells transiently transfected with M5 hKIT D816V Neo. Stably transduced Ba/F3 cells were first selected for functional resistance to G418 and subsequently for interleukin-3 (IL-3)-independent growth.
Cell proliferation assays
Tetrazolium-based proliferation assays were performed as described.26 Briefly, cell viability following 72 hours of treatment with MLN518 (Millennium Pharmaceuticals, Cambridge, MA) or PD180970 (Pfizer Global Research and Development, Ann Arbor, MI) at 0 to 1000 nM was determined by spectrophotometric measure of methylthiazoletetrazolium (MTT) reactivity. P815 was assayed at 48 hours to avoid assay saturation due to rapid growth. Assays were repeated 3 times, each plated in triplicate, and averaged.
KIT phosphorylation assays
Cells were serum starved for 3 hours and then treated for 1 hour with MLN518 or PD180970 (0 to 1000 nM). KIT was immunoprecipitated from Nonidet P-40 (NP40) cell lysates (107 cells per immunoprecipitation) with 2 μg anti-KIT Ab-1 (Oncogene, San Diego, CA). KIT phosphorylation was determined by 4G10 (Upstate, Charlottesville, VA) immunoblots. Anti-KIT (Ab-1) was a loading control. Bands were detected and quantitated by chemiluminescence on a Lumi-Imager (Roche, Indianapolis, IN). Phosphorylation levels were normalized for KIT loading. Fifty percent inhibitory concentration (IC50) values are reported as the average of 3 independent assays.
Apoptosis assays
Induction of apoptosis was determined using the Annexin-V-Fluos Staining Kit (Roche) and a FACSAria (BD Biosciences, San Diego, CA) flow cytometer following 72 hours (48 hours for P815) of MLN518 or imatinib mesylate (Novartis, Basel, Switzerland) treatment (0 to 2 μM). Three independent assays were averaged to determine the percent apoptotic cells.
Stat3 phosphorylation
Stat3 phosphorylation levels were determined by phosphotyrosine-705 Stat3 (Santa Cruz Biotechnology, Santa Cruz, CA) and total Stat3 (Santa Cruz Biotechnology) immunoblots of cell lysates treated with 1 μM MLN518 or imatinib as described in “Apoptosis assays.” Three independent assays were performed.
Results and discussion
The quinazoline-based type III RTK inhibitor MLN518 (Figure 1 and Table 1), initially developed as an FLT3 inhibitor, has been shown to inhibit wild-type KIT kinase with an IC50 of 170 nM in cell-based assays.14 The efficacy of MLN518 against various KIT mutants was, however, unknown. We examined the effect of MLN518 on a KIT juxtamembrane mutant cell line, HMC-1, and 2 active site mutant cell lines, D816V Ba/F3 and P815 m-D814Y, to predict whether this inhibitor might have clinical activity in patients with different types of KIT mutations.
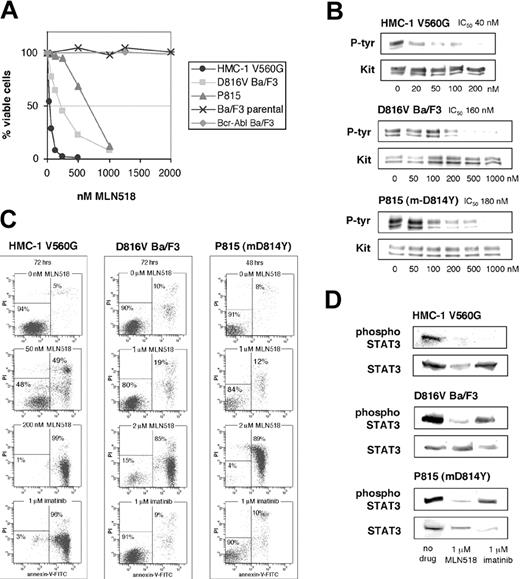
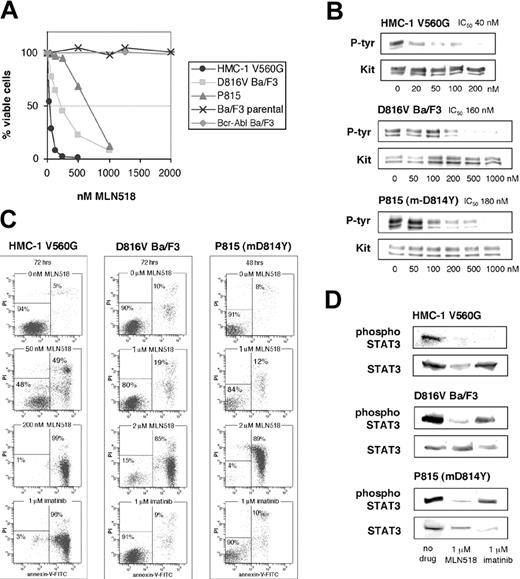
MLN518 potently inhibited cell proliferation (Figure 2A) and KIT phosphorylation (Figure 2B) of juxtamembrane mutant HMC-1 with IC50 values of 40 nM, similar to its effect on FLT3 internal tandem duplication (ITD)-positive cell lines.14 MLN518 also inhibited cell proliferation of D816V Ba/F3 and P815 with respective IC50 values of 250 nM and 600 nM (Figure 2A). Five micromolar MLN518 did not affect the Ba/F3 parental cell line, suggesting that inhibitory doses do not induce nonspecific toxicity. Consistent with the inhibition of proliferation, MLN518 inhibited KIT tyrosine phosphorylation in D816V- and m-D814Y-expressing cell lines with respective IC50 values of 160 nM and 180 nM (Figure 2B), implying down-regulation of KIT kinase activity by MLN518.
Inhibitory profile of MLN518 against KIT mutant cell lines. (A) KIT juxtamembrane and activation loop mutant-expressing cell lines were assessed for cell viability by MTT assay at 72 hours in the presence of escalating doses of MLN518. Bcr-Abl Ba/F3 and Ba/F3 parental cell lines were used as controls. Graphs represent the average of 3 independent experiments, each plated in triplicate. (B) KIT mutant cell lines incubated in escalating doses of MLN518 were immunoblotted for tyrosine phosphorylation of the KIT receptor. Simultaneous blots for total KIT receptor were used as loading controls. Phosphotyrosine content was normalized for total KIT, and IC50 values were calculated as the average of 3 independent experiments. Representative gels are shown. (C) KIT mutant cell lines were assessed for an increase in the number of apoptotic cells in response to escalating doses of MLN518. Apoptotic cell numbers were determined by flow cytometric measurement of annexin V binding. Representative experiments are shown. (D) KIT mutant cell lines were assessed for inhibition of phosphorylation of the downstream target Stat3. Phosphospecific immunoblots for tyrosine 705 of Stat3 were performed on cells incubated in the presence of either 1 μM MLN518 or 1 μM imatinib. Simultaneous blots of total Stat3 were used as a loading control. Representative gels are shown.
Inhibitory profile of MLN518 against KIT mutant cell lines. (A) KIT juxtamembrane and activation loop mutant-expressing cell lines were assessed for cell viability by MTT assay at 72 hours in the presence of escalating doses of MLN518. Bcr-Abl Ba/F3 and Ba/F3 parental cell lines were used as controls. Graphs represent the average of 3 independent experiments, each plated in triplicate. (B) KIT mutant cell lines incubated in escalating doses of MLN518 were immunoblotted for tyrosine phosphorylation of the KIT receptor. Simultaneous blots for total KIT receptor were used as loading controls. Phosphotyrosine content was normalized for total KIT, and IC50 values were calculated as the average of 3 independent experiments. Representative gels are shown. (C) KIT mutant cell lines were assessed for an increase in the number of apoptotic cells in response to escalating doses of MLN518. Apoptotic cell numbers were determined by flow cytometric measurement of annexin V binding. Representative experiments are shown. (D) KIT mutant cell lines were assessed for inhibition of phosphorylation of the downstream target Stat3. Phosphospecific immunoblots for tyrosine 705 of Stat3 were performed on cells incubated in the presence of either 1 μM MLN518 or 1 μM imatinib. Simultaneous blots of total Stat3 were used as a loading control. Representative gels are shown.
We next analyzed the capacity of MLN518 to induce apoptosis in KIT mutant cell lines. HMC-1 cells displayed more than 90% apoptotic cells at 200 nM MLN518 (Figure 2C), consistent with cell proliferation assays. Induction of apoptosis was also observed, albeit at higher inhibitor concentrations, in D816V Ba/F3 cells and P815 cells with an average of 74% and 95% annexin V-positive cells at 2 μM MLN518, respectively (Figure 2C). No induction of apoptosis was seen up to 5 μM MLN518 in the parental cell line. Although lower concentrations did not result in a dramatic increase in apoptotic cells, treatment of the active site mutant cell lines with 2 μM MLN518 showed comparable results to 1 μM treatment of the FLT3 ITD-expressing Molm-14 cell line.14
The occurrence of either a juxtamembrane deletion or the D816V mutation of KIT results in constitutive phosphorylation of Stat3, an event required for full transforming potential.28,29 We reasoned that inhibition of KIT kinase activity should decrease the level of Stat3 phosphorylation. Indeed, we observed a 60% reduction of Stat3 phosphorylation in all 3 cell lines following incubation with 1 μM MLN518 (Figure 2D). These data suggest that MLN518 can reduce abnormal signaling essential to KIT mutant transformation.
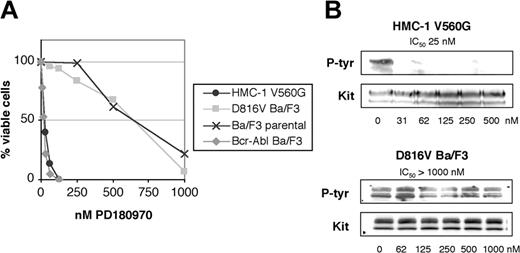
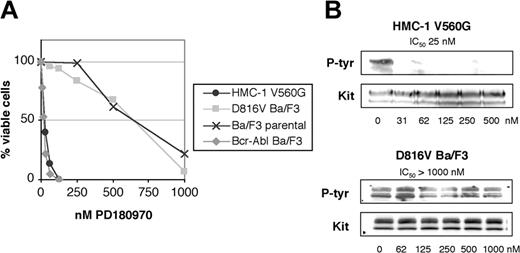
PD180970 is a pyrido[2,3-d]pyrimidine inhibitor of Abl, Src, and KIT23,24 (Figure 1). Given the activity of PD180970 against many imatinib-resistant active site mutants of Bcr-Abl (IC50 values for cell proliferation from 5 to 200 nM),26 we reasoned that this compound may inhibit active site mutant KIT. We tested PD180970 against the HMC-1 and D816V Ba/F3 cell lines. PD180970 inhibited cell proliferation and KIT phosphorylation of HMC-1 with IC50 values of 40 nM and 25 nM, respectively, similar to wild-type KIT24 (Figure 3). In contrast, the KIT D816V Ba/F3 cell line was insensitive to PD180970 in cell proliferation assays with an inhibition profile matching the parental cell line (Figure 3A). Additionally, 1 μM PD180970 did not reduce the level of D816V KIT phosphorylation (Figure 3B). These data suggest that compounds of the pyrido[2,3-d]pyrimidine class would not be effective against malignancies expressing KIT active site mutations.
Inhibition of cell proliferation and KIT phosphorylation by PD180970. (A) KIT juxtamembrane and activation loop mutant-expressing cell lines were assessed for cell proliferation by MTT assay at 72 hours in the presence of escalating doses of PD180970 and graphed as the average of 3 independent experiments plated in triplicate. (B) KIT immunoprecipitates from the same cell lines were blotted for phosphotyrosine content in the presence of escalating doses of PD180970. Total KIT immunoblots were performed as a loading control. IC50 values were calculated as the average of 3 independent experiments. Representative gels are shown.
Inhibition of cell proliferation and KIT phosphorylation by PD180970. (A) KIT juxtamembrane and activation loop mutant-expressing cell lines were assessed for cell proliferation by MTT assay at 72 hours in the presence of escalating doses of PD180970 and graphed as the average of 3 independent experiments plated in triplicate. (B) KIT immunoprecipitates from the same cell lines were blotted for phosphotyrosine content in the presence of escalating doses of PD180970. Total KIT immunoblots were performed as a loading control. IC50 values were calculated as the average of 3 independent experiments. Representative gels are shown.
Structural analysis of KIT predicts that the fidelity of the D816 residue is critical to maintaining an inactive conformation of the activation loop.30 Mutation of 816 would therefore shift the equilibrium of the kinase to an open, adenosine triphosphate (ATP)-binding conformation. In fact, mutation of D816 to practically any other amino acid increases the intrinsic kinase activity of the receptor.8 While pyrido[2,3-d]pyrimidine inhibitors bind to the active conformation of Bcr-Abl and, thus, effectively target its activation loop mutants,26 it may be that specific features of the KIT D816V mutant do not allow PD180970 to bind.
The mode of binding of MLN518 to its targets has not been elucidated, and modeling studies to determine specific points of contact of the inhibitor are inconclusive (personal communication with Neill Giese, Millennium Pharmaceuticals, October, 2003). Further investigation of the mechanism of inhibition by MLN518 will be required to explain the effectiveness of the compound against the activation loop mutants of KIT and the differential sensitivity of different classes of KIT mutants to the compound.
Several indolinone derivates have recently been described to inhibit KIT mutants with activities comparable to MLN518 15 ; however, no pharmacokinetic or toxicity data have been reported, and it is not clear if these compounds have potential for drug development. MLN518 is currently in clinical trials of relapsed and refractory AML. The mean steady-state plasma trough level at a 525 mg oral, twice-daily dose was 453 nM, with up to 1464 nM observed in individual patients.31 Dose-limiting toxicity was observed only at more than 2 μM, a value well above the IC90 for growth inhibition of D816V and m-D814Y cell lines in our study. Thus, MLN518 may have clinical activity in patients with KIT D816 mutant malignancies, such as SM.
Our data demonstrate that MLN518 can effectively target both juxtamembrane mutant and D816V mutant KIT at clinically achievable doses, and the data provide evidence that this compound could be beneficial in the treatment of SM, D816V-positive AML, and other D816V KIT-driven malignancies as well as GIST patients who are intolerant to imatinib. These results warrant further investigation of MLN518 as a viable therapeutic option for patients with these diseases.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-06-2189.
Supported by Howard Hughes Medical Institute (B.J.D.), The Leukemia and Lymphoma Society (B.J.D.), The Doris Duke Charitable Foundation (B.J.D., M.C.H., K.W.H.Y.), and a Veterans Administration Merit Review Grant (M.C.H.). M.W.N.D. is a recipient of a junior faculty award of the American Society of Hematology.
One of the authors (C.L.R.) has declared a financial interest in a company (Millennium Pharmaceuticals) whose product was studied in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Chris Koontz and Sarah Anderson for administrative assistance and Tom O'Hare for advice and careful review of this paper.