Although both of the small Rho guanosine triphosphatases (GTPases) Rac1 and Rac2 have been demonstrated to play a role in chemotaxis, the precise and possible unique roles performed by each of these 2 Rac isoforms in neutrophil chemotaxis have not been defined. To elucidate the specific roles of Rac1 and Rac2 in neutrophils during the process of chemotaxis, we generated mice deficient in Rac1, Rac2, or in both Rac1 and Rac2 in cells of myeloid lineage including neutrophils by mating Rac2 null mice with mice carrying a conditional allele for Rac1 and expressing the Cre recombinase downstream of a specific myeloid promoter, lysozyme M. We demonstrate here that although Rac1 null neutrophils display normal chemokinesis, they are unable to migrate toward the source of the chemoattractant. By contrast, Rac2 null neutrophils can orient toward the chemoattractant source but are unable to migrate efficiently. We show that Rac1 is essential for gradient detection and orientation toward the chemoattractant source through spatially constrained regulation of phosphoinositol-3,4,5-trisphosphate (PIP3) and Akt in the leading edge and confirm that Rac2 is the primary regulator of actin assembly providing the molecular motor for neutrophil translocation during chemotaxis.
Introduction
Directed migration or cell chemotaxis involves initial recognition of a chemoattractant gradient followed by the development of a dominant leading lamella directed toward the source of the chemoattractant.1 The critical chemoattractant gradient detection step has been demonstrated to depend on an internally generated gradient of phosphoinositol-3,4,5-trisphosphate (PIP3) that accumulates at the leading edge membrane facing the source of the chemoattractant.2 This accumulation of PIP3 at the leading edge acts as an internal compass to direct cell migration toward the source of the chemical signal.3 This compass has been demonstrated to rely on an unidentified member of the Rho family of small guanosine triphosphatases (GTPases),2 phosphoinositide 3 kinase γ (PI3Kγ), and its lipid product PIP3.3
Recent studies have demonstrated that Akt, which is recruited to the leading edge membrane of chemotaxing cells by PIP3, is a critical component of neutrophil chemotaxis.4,5 Furthermore, recent studies document that Akt, PI3Kγ, and Vav are involved in the regulation of Rac1 activity in T cells.6 However, the possible roles of Akt and Rac1 in regulation of the chemotactic compass have not been demonstrated.
Cell migration up a chemoattractant concentration gradient depends on a series of actin-dependent polymerization-depolymerization cycles that result in extension of the leading lamella and retraction of the cell tail. It has been previously demonstrated that the actin-dependent processes driving neutrophil chemotaxis are downstream of the Rac members of the Rho family of small GTPases.7-11 Moreover, regulation of the actin cytoskeleton that is required for normal cell polarization and chemotaxis is tightly regulated by Rac GTPases in discrete cytoplasmic domains both within the leading edge lamella and within the tail of chemotaxing neutrophils.12,13
Recent work has focused on the specific roles played by Rac1 and Rac2 in hematopoietic cell functions including neutrophil chemotaxis.7-11,14,15 Within the Rac subfamily, Rac1 and Rac2 play key roles in cell motility and chemotaxis.10,11 These 2 proteins are 92% homologous16 and recent interest has focused on identifying the distinct roles performed by these proteins.10,11,15 Whereas Rac1 is ubiquitously expressed and is the isoform most often studied, recent studies on Rac2, which is restricted to cells of the hematopoietic lineage, demonstrate that there are important differences in the functions of these 2 proteins.10,11,15,17 Although Rac1 and Rac2 may play unique roles in neutrophil chemotaxis,11,15 their specific roles during this complex event have yet to be determined.
To investigate the individual roles played by Rac1 and Rac2 in neutrophil chemotaxis in primary neutrophils, we used mice with neutrophils deficient in either Rac1 (Rac1 null), Rac2 (Rac2 null), or Rac1 and Rac2 (Rac1/2 null) or expressing both Rac1 and Rac2 (wild-type). Analysis of neutrophil chemotaxis in vitro to several chemoattractants demonstrated that whereas Rac1 null neutrophils were able to move at normal speeds compared with wild-type cells when activated, they displayed an inability to migrate up the chemoattractant gradient. Conversely, Rac2 null neutrophils demonstrated a severe impairment in actin assembly, which prevented cell translocation. We provide evidence that Rac1 plays a role in the cell's chemotaxis compass via regulation of accumulation of Akt at the leading edge facing the chemoattractant gradient. These data provide strong evidence that Rac1 is required for the detection of a chemoattractant gradient and generation of a single dominant lamella and confirm that Rac2 is the isoform responsible for regulating the actin cytoskeletal machinery required for efficient neutrophil translocation.
Materials and methods
Antibodies and reagents
Rabbit polyclonal antibody against phosphorylated Akt (Ser473) was purchased from Cell Signaling Technology (Beverly, MA). A mouse monoclonal antibody against Rac1 was purchased from Upstate Cell Signaling Solutions (Charlottesville, VA). A mouse monoclonal antibody against Cdc42 (B-8) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mouse monoclonal antibody against PIP3 was purchased from Echelon Biosciences (Salt Lake City, UT). Biotinylated goat antimouse IgM was from Jackson ImmunoResearch Laboratories (West Grove, PA). Streptavidin–Alexa 488, goat antimouse Alexa 488, and goat antirabbit Alexa 488 were purchased from Molecular Probes (Eugene, OR). N-formyl-methyonyl-leucyl-phenylalanine (fMLP) and phalloidin-tetramethylrhodamine isothiocyanate (TRITC) were from Sigma (St Louis, MO). KC (the murine orthologue of human growth–related protein α-GRO and interleukin 8 [IL-8]) was purchased from R&D Systems (Minneapolis, MN). Goat serum was from Cedarlane Laboratories (Hornby, ON, Canada). Anti-Rac2 was a kind gift from Dr Gary Bokoch (The Scripps Research Institute, La Jolla, CA).
Mouse breeding
All procedures were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals and were approved by the University of Toronto Animal Care Committee.
Previously generated and characterized mice in which Rac1 was selectively deleted in neutrophils and monocytes,11 Rac1c/-LysMcre were bred with Rac2-/- mice10 and the resulting offspring were bred over at least 6 generations to generate optimal breeding pairs (Rac1c/-LysMcreRac2+/- × Rac1c/-LysM-Rac2+/-), which would enable the generation of Rac1 null (Rac1c/-LysMcreRac2+/+), Rac2 null (Rac1+/+LysMcreRac2-/-), Rac1/2 null (Rac1c/-LysMcreRac2-/-), and wild-type mice (Rac1+/+LysMcre Rac2+/+) from the same litters. Rac1 is disrupted in a granulocyte/neutrophil-specific fashion by breeding of mice carrying a loxP-flanked Rac1 allele with LysMcre mice in which the Cre-recombinase is expressed under control of the murine lysozyme M gene regulatory region, which is active during early embryogenesis.18 Using this approach Rac1 is deleted in the neutrophils from birth.11 All experiments were performed with mice 6 to 13 weeks old. This breeding strategy allows for the controlling of background variations. Genotyping for Rac1, Rac2, and LysM alleles was carried out as described previously.10,11
Neutrophil preparations
Mice were killed by CO2 inhalation. Femurs and tibias were removed and bone marrow was isolated. Bone marrow cells were layered onto discontinuous Percoll (Sigma, Oakville, ON, Canada) gradients of 82%/65%/55%.19 Mature neutrophils were recovered at the 82%/65% interface and were positive for Gr-1 and Mac-1 as shown by flow cytometry. More than 85% of cells isolated were neutrophils as assessed by Wright-Giemsa staining. Viability as determined by trypan blue exclusion was more than 90%.
F-actin assembly assay
Bone marrow neutrophils were incubated at 37°C for 30 minutes following isolation in Hanks balanced salt solution (HBSS). One micromolar fMLP was added for 30 seconds. Cells were immediately fixed with 3.7% paraformaldehyde, permeabilized (0.1% Triton X-100), and stained with 1 U/500 μL Alexa-phalloidin (Molecular Probes) to detect F-actin content. Cellular fluorescence intensity was measured with a Becton Dickinson FACScan flow cytometer10 (Becton Dickinson, Franklin Lakes, NJ). fMLP-mediated F-actin assembly was completely inhibited by cytochalasin D in all samples, verifying that we were observing actin assembly. Preliminary data revealed that peak actin assembly is reached at 30 seconds for all 4 genotypes.
Sodium periodate peritonitis
To induce an experimental peritonitis, 1 mL of 5 mM sodium periodate (Sigma, Oakville, ON, Canada) in phosphate-buffered saline (PBS) was injected intraperitoneally. The mice were killed 3 hours later and the peritoneal exudate was collected by lavage with chilled PBS (5 mL/mouse). Neutrophils were counted by a hemocytometer and an electronic cell counter (Becton Dickinson).
Immunoblotting assay
Peritoneal neutrophil lysates were prepared, boiled for 10 minutes at 95°C, and subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting as previously described.11 Each membrane was incubated with the indicated primary antibody (1:2000 for Rac1 [Upstate Biotechnology], 1:1000 for Rac2 [courtesy of Dr Gary Bokoch], and 1:8000 for β-actin [Sigma]) in Tris-buffered saline–Tween (TBS-T) containing 5% fat-free milk at 4°C overnight. Membranes were washed 3 times for 10 minutes with TBS-T followed by incubation with donkey antirabbit or sheep antimouse IgG peroxidase conjugates (Amersham Pharmacia Biotech, Buckinghamshire, England). Antigen-antibody complexes were visualized on x-ray film by enhanced chemiluminescence (ECL; Perkin Elmer Life Sciences, Boston, MA).
Double-headed neutrophils
Chemotaxing neutrophils on coverslips were fixed and stained for F-actin using a solution containing rhodamine phalloidin (Sigma, St Louis, MO), 0.1% Nonident, and PBS (Sigma, St Louis, MO) for 20 minutes at room temperature. Fluorescent microscope images from the slides were captured using a fluorescent microscope (Leitz Orthoplan Microscope, Wetzlar, Germany) with a × 100 oil immersion lens (NA, 1.25) where the percentage of double-headed neutrophils was calculated by counting the double-headed and total number of cells. Single cells with 2 distinct lamellae containing F-actin (rhodamine-phalloidin staining) were considered double-headed. Images were captured using Nikon (Mississauga, ON, Canada) Coolpix 995 camera.
Chemotaxis assays
Bone marrow neutrophils were suspended in HBSS and 1% gelatin. A neutrophil suspension (1 × 106/mL) was allowed to attach to bovine serum albumin (BSA)–coated glass coverslips (22 × 40 mm) at 37°C for 20 minutes. The coverslip was inverted onto a Zigmond chamber, and 100 μL HBSS media was added to the left chamber with 100 μL HBSS media containing fMLP[10-6] added to the right chamber. Time-lapse video microscopy was used to examine neutrophil movements in Zigmond chambers. The microscope (Nikon Eclipse E400) was equipped with differential interference contrast optics and a × 40 objective. Images were captured at 60-second intervals with a Nikon Coolpix 995 camera. Cell-tracking software (Retrac version 2.1.01 Freeware) was used to characterize cellular chemotaxis from the captured images.
Immunofluorescence microscopy cell preparation
The neutrophils that had undergone chemotaxis were fixed in 4% paraformaldehyde for 10 minutes at room temperature. The cells were washed twice with 1 × TBS (1:10 10 × TBS, 1000 mM Tris [tris(hydroxymethyl) aminomethane] base, 1.5 M sodium chloride, pH 7.6) and permeabilized by 0.5% Triton X-100 at room temperature for 15 minutes, incubated in blocking buffer, 10% goat serum in 1 × TBS for 30 minutes at 37°C, and immunostained for PIP3, phosphorylated Akt, Rac1, or Rac2. All the primary antibodies were used at a dilution of 1:50. For PIP3 immunostaining, the cells were incubated in PIP3 antibody solution for 30 minutes at 37°C, followed by biotinylated goat antimouse IgM (1:500) incubation for 30 minutes at 37°C and then, streptavidin–Alexa 488 (1:250) incubation for 30 minutes at 37°C. For phosphorylated Akt immunostaining, the cells were incubated in phosphorylated Akt antibody solution for 30 minutes at 37°C, followed by goat antirabbit Alexa 488 (1:250) incubation for 40 minutes at room temperature.
Confocal immunofluorescence microscopy
A Zeiss Axioplan 2 imaging microscope with LSM510 confocal attachment was used for acquisition and analysis of neutrophils immunostained with PIP3 and phospho-AKT. A Plan-Apochromat 100 ×/1.4 oil differential interference contrast (DIC) objective was used for all microscopy. The middle section image (2-μm slice) was used for all figures. Zeiss LSM 510 image acquisition software Axiovision 3.0 was used for the analysis of all images.
Measurement and comparison of phospho-Akt levels in different regions of the cell
ImageJ software version 1.31v (National Institutes of Health, Bethesda, MD) was used to measure the fluorescent intensity of phospho-Akt staining. A line was drawn from the leading edge to the end of the tail slicing the polymorphonuclear neutrophil (PMN) into 2 equal parts longitudinally and a plot profile of the fluorescent intensity of the underlined region was analyzed. As a result, a histogram and a text record of these fluorescent intensities was generated. The peak value at the leading edge, mid-body, and tail for neutrophils from each genotype was recorded and the average was calculated.
The p21-binding domain assay
The Pak-binding domain (PBD) assay was carried out as described previously.20 Briefly, isolated bone marrow neutrophils were exposed to fMLP at 37°C for the indicated period of time. They were immediately lysed and glutathione-S-transferase (GST)–PBD was added to the lysates. The GST-PBD was recovered with Sepharose beads and immunoblotting was completed using a Cdc42 antibody (B-8; Santa Cruz Biotechnology). ImageJ software version 1.31v (National Institutes of Health) was used for blot densitometry. Data were normalized to resting wild-type neutrophils and are expressed as the mean value from 4 separate experiments.
Statistics
Analysis of variance and Student t tests were performed. The Bonferroni test was used for post hoc testing. All experiments were repeated on at least 3 separate occasions and error bars in figures represent SEM.
Results
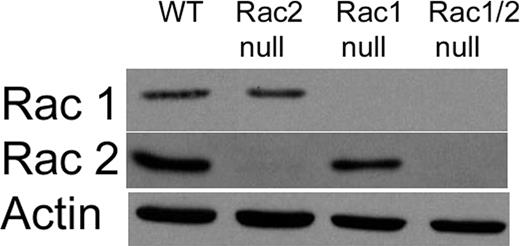
To study directly the specific roles of the Rac1 and Rac2 isoforms during chemotaxis of primary neutrophils, we generated a mouse colony using previously described knock-out mice10,11 in which single litters produced mice with neutrophils deficient in Rac1 (Rac1 null), Rac2 (Rac2 null), or both Rac1 and Rac2 (Rac1/2 null), or sufficient in both Rac1 and Rac2 (wild type). The offspring from these litters were grossly normal, able to breed, and demonstrated no signs of pathology up to 6 months of age. Rac1 and Rac2 protein expression levels in neutrophils from these mice were assessed by immunoblotting protein extracts from peritoneal neutrophils recruited by sodium periodate–induced acute peritonitis (Figure 1). As expected from the efficient genomic deletion and from previous studies describing the Rac1 null and Rac2 null mice,10,11 there was complete deletion of Rac1 and Rac2 protein from the neutrophils of the corresponding genotypes. By contrast, there was no difference in the expression of Cdc42 among the 4 genotypes (data not shown).
Immunoblot analysis of Rac1 and Rac2 levels in the mouse peritoneal neutrophils. Representative immunoblot of Rac1 and Rac2 expression in peritoneal neutrophils. Neutrophils were recruited using an intraperitoneal injection of sodium periodate. After 4 hours a peritoneal lavage was performed to collect neutrophils. As expected from genotyping, we were able to generate mice with neutrophils lacking Rac1 expression (Rac1 null), Rac2 expression (Rac2 null), and both Rac1 and Rac2 expression (Rac1/2 null). Antibody against β-actin was used as a loading control.
Immunoblot analysis of Rac1 and Rac2 levels in the mouse peritoneal neutrophils. Representative immunoblot of Rac1 and Rac2 expression in peritoneal neutrophils. Neutrophils were recruited using an intraperitoneal injection of sodium periodate. After 4 hours a peritoneal lavage was performed to collect neutrophils. As expected from genotyping, we were able to generate mice with neutrophils lacking Rac1 expression (Rac1 null), Rac2 expression (Rac2 null), and both Rac1 and Rac2 expression (Rac1/2 null). Antibody against β-actin was used as a loading control.
In vitro single-cell chemokinesis and chemotaxis analysis
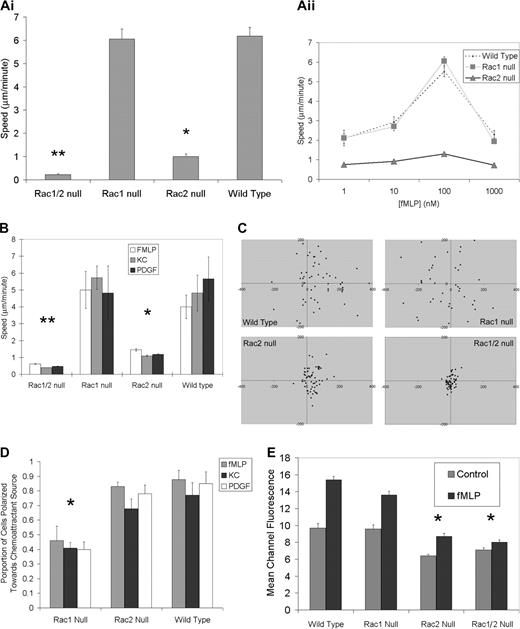
Both Rac1- and Rac2-deficient neutrophils display a chemotactic defect as assessed using Boyden chamber–type assays.10,11,15 However, unless a checkerboard analysis approach is used, the Boyden chamber assay simultaneously assesses both chemokinesis and chemotaxis. To measure these 2 processes separately we directly investigated at the single-cell level the specific nature of these previously reported chemotactic defects in the Rac1 null, Rac2 null, and Rac1/2 null neutrophils using Zigmond chambers.21 Chemokinesis of fMLP-stimulated bone marrow neutrophils was studied at the optimal concentration of 100 nM placed into both lateral compartments of the Zigmond chamber (ie, maximal chemokinetic response for all genotypes; Figure 1Aii). These experiments revealed that Rac1 null neutrophils demonstrate normal chemokinesis with cell speeds matching wild-type controls. By contrast, Rac-2–deficient neutrophils demonstrated significantly diminished chemokinesis compared with either Rac1 null or wild-type neutrophils (Figure 2A) as previously reported.10 Neutrophil chemotaxis assays were carried out using several distinct chemoattractants including fMLP and KC that function through G protein–coupled receptors22 and platelet-derived growth factor (PDGF) that signals through a receptor tyrosine kinase.23,24 In a chemoattractant gradient, Rac1 null neutrophils were able to match the speed of wild-type control neutrophils (Figure 2B), but analysis of migration direction revealed that Rac1 null neutrophils were unable to carry out directed migration toward the source of the chemoattractant; rather, they displayed random directional migration (Figure 2C). Although Rac2 null neutrophils displayed significantly reduced speed compared with wild-type and Rac1 null neutrophils in the chemotactic gradient, the small amount of migration that did occur resulted in translocation toward the chemoattractant source (Figure 2C). Assessment of expression levels of the adhesion molecule CD11b required for chemotaxis and the receptor for fMLP (fMLP-R) revealed no differences among any of the genotypes (data not shown).
In vitro single-cell chemokinesis and chemotaxis analysis. (Ai) Normal chemokinesis in Rac1 null neutrophils but not in Rac2 null neutrophils. Chemokinesis mediated by fMLP (10-6 M) was assessed in a Zigmond chamber as described (see “Materials and methods”). There was no significant difference between Rac1 and wild-type neutrophil chemokinesis; however, Rac2 null neutrophils were significantly slower than either wild-type or Rac1 null cells (*P > .001), whereas Rac1/2 null cells were significantly slower than Rac2 null cells (**P < .01). (Aii) Chemokinesis concentration kinetics. (B) Abnormal chemotaxis speeds in both Rac2 and Rac1/2 null neutrophils. The absolute speed of neutrophils undergoing chemotaxis was calculated using the indicated chemoattractant in a Zigmond chamber. Rac2 null and Rac1/2 null cells were significantly slower than both Rac1 null and wild-type cells. (*P > .01;**P < .001). (C) Rac1 null neutrophils display a direction defect in chemotaxis plots. Plots of neutrophil chemotaxis migration in the Zigmond chamber assay are shown. Neutrophils undergoing chemotaxis in response to fMLP (10-6 M) were recorded using time-lapse imaging. Tracings were used to plot final positioning of cells after 17 minutes. Positive x values represent movement up the gradient. Note the random migration in the Rac1 null cells compared to both wild-type and Rac2 null cells. (D) Rac1 required for orientation toward chemoattractant source. Time-lapse images of chemotaxing neutrophils were used to quantify the proportion of neutrophils moving toward the chemoattractant source. Rac1 null neutrophils are unable to orient toward the source of a chemoattractant. The proportions of neutrophils exposed to a gradient of chemoattractant that are polarized toward the source (leading edge within 180° of the source) were counted for the 3 genotypes shown. A complete inability to polarize preferentially toward the source (random polarization) would be reflected with a 0.5 proportion (*P < .001, Rac1 null compared to wild-type). (E) F-actin assembly following fMLP stimulation requires Rac2. Impaired fMLP-mediated (10-6 M) actin polymerization (30 seconds after stimulation) is noted in neutrophils lacking Rac2 or both Rac1 and Rac2. Note the pronounced actin polymerization defect in Rac2 and Rac1/2 neutrophils along with the lower initial resting levels of F-actin (representative of 3 experiments; significantly different at 30 seconds between wild-type and Rac2 null, wild-type and Rac1/2 null, Rac1 null and Rac2 null, and Rac1 null and Rac1/2 null; *P < .01). Error bars represent standard error of the means.
In vitro single-cell chemokinesis and chemotaxis analysis. (Ai) Normal chemokinesis in Rac1 null neutrophils but not in Rac2 null neutrophils. Chemokinesis mediated by fMLP (10-6 M) was assessed in a Zigmond chamber as described (see “Materials and methods”). There was no significant difference between Rac1 and wild-type neutrophil chemokinesis; however, Rac2 null neutrophils were significantly slower than either wild-type or Rac1 null cells (*P > .001), whereas Rac1/2 null cells were significantly slower than Rac2 null cells (**P < .01). (Aii) Chemokinesis concentration kinetics. (B) Abnormal chemotaxis speeds in both Rac2 and Rac1/2 null neutrophils. The absolute speed of neutrophils undergoing chemotaxis was calculated using the indicated chemoattractant in a Zigmond chamber. Rac2 null and Rac1/2 null cells were significantly slower than both Rac1 null and wild-type cells. (*P > .01;**P < .001). (C) Rac1 null neutrophils display a direction defect in chemotaxis plots. Plots of neutrophil chemotaxis migration in the Zigmond chamber assay are shown. Neutrophils undergoing chemotaxis in response to fMLP (10-6 M) were recorded using time-lapse imaging. Tracings were used to plot final positioning of cells after 17 minutes. Positive x values represent movement up the gradient. Note the random migration in the Rac1 null cells compared to both wild-type and Rac2 null cells. (D) Rac1 required for orientation toward chemoattractant source. Time-lapse images of chemotaxing neutrophils were used to quantify the proportion of neutrophils moving toward the chemoattractant source. Rac1 null neutrophils are unable to orient toward the source of a chemoattractant. The proportions of neutrophils exposed to a gradient of chemoattractant that are polarized toward the source (leading edge within 180° of the source) were counted for the 3 genotypes shown. A complete inability to polarize preferentially toward the source (random polarization) would be reflected with a 0.5 proportion (*P < .001, Rac1 null compared to wild-type). (E) F-actin assembly following fMLP stimulation requires Rac2. Impaired fMLP-mediated (10-6 M) actin polymerization (30 seconds after stimulation) is noted in neutrophils lacking Rac2 or both Rac1 and Rac2. Note the pronounced actin polymerization defect in Rac2 and Rac1/2 neutrophils along with the lower initial resting levels of F-actin (representative of 3 experiments; significantly different at 30 seconds between wild-type and Rac2 null, wild-type and Rac1/2 null, Rac1 null and Rac2 null, and Rac1 null and Rac1/2 null; *P < .01). Error bars represent standard error of the means.
To verify the gradient-sensing ability of the neutrophils, we quantified the number of neutrophils oriented toward the chemoattractant source (leading edge within 180° of source). These data confirmed that Rac1 null neutrophils assume a random orientation (approximately 50% within 180° of the source), whereas the wild-type and Rac2 null cells orient toward the chemoattractant source (Figure 2D).
Polarization
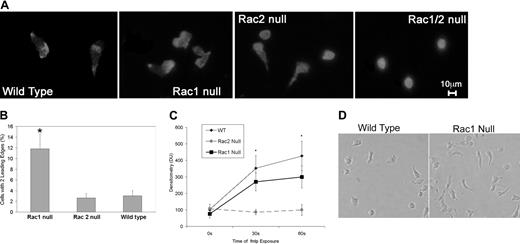
We noted that Rac1 neutrophils demonstrated abnormal polarization with a tendency to form multiple leading edges during our microscopic analysis of neutrophil chemotaxis. To investigate this phenomenon further, we assessed F-actin assembly, Cdc42 activation levels, and neutrophil morphology during polarization following fMLP stimulation. Flow cytometric analysis of F-actin as measured by Alexa 488–phalloidin fluorescence prior to and following fMLP stimulation of neutrophils from all 4 genotypes revealed that Rac2 is the primary Rac isoform responsible for regulating actin assembly in neutrophils, as described previously.10 Analysis of images of rhodamine-phalloidin–stained cells in the process of chemotaxis revealed that the Rac2 null neutrophils displayed a homogenous F-actin distribution as opposed to wildtype and Rac1 neutrophils, which displayed a “polar” F-actin distribution with F-actin levels increased in the leading and tail regions (Figure 3A). Furthermore, although Rac1 null and Rac2 null neutrophils were both able to extend lamellipodia in response to the chemoattractant fMLP, the Rac1 null neutrophils displayed a unique morphology with a significant increase in double-headed neutrophils (Figure 3A), suggesting a role for Rac1 in the development of a single dominant lamella. To quantify this phenomenon, fluorescent images of rhodamine-phalloidin–stained neutrophils exposed to an fMLP gradient were analyzed by observers blinded to the genotypes of the samples to determine the frequency of double-headed neutrophils. This analysis revealed a 3-fold increase in double-headed Rac1 null neutrophils compared with wild-type and Rac2 null neutrophils (Figure 3B). Rac1/2 null neutrophils were also unable to polarize or migrate toward a chemoattractant source (Figure 3A-B).
F-actin assembly, Cdc42 activation levels, and neutrophil morphology during polarization. (A) Rac1 null neutrophils display double leading lamellas. F-actin staining is shown in chemotaxing neutrophils. Wild-type and Rac2 null cells present with a characteristic single prominent leading edge of polymerization, whereas the Rac1 null cells showed a significant proportion of neutrophils with 2 distinct leading edges. Double null cells showed no polarization. (B) Increased proportion of Rac1 null neutrophils display more than one leading edge compared with Rac2 null and wild-type neutrophils. The proportion of neutrophils for each genotype with 2 prominent leading edges was calculated. Rac1 null neutrophils demonstrated a significantly higher likelihood of having 2 leading edges (*P < .0001). (C) fMLP-mediated Cdc42 activation neutrophils required Rac2 and not Rac1. Using the p21 PBD assay,20 we demonstrate that fMLP-mediated Cdc42 activation increases in a similar pattern in wild-type and Rac1 null neutrophils but is severely perturbed in Rac2 null neutrophils. Rac2 is significantly different from wild-type and Rac1 (*P < .04). (D) Perturbed tail retraction in Rac1 null neutrophils is shown in this representative photomicrograph of neutrophils in an fMLP gradient. It is clearly noted that Rac1 null neutrophils displayed poor tail retraction compared with wild-type neutrophils. Error bars represent standard error of the means.
F-actin assembly, Cdc42 activation levels, and neutrophil morphology during polarization. (A) Rac1 null neutrophils display double leading lamellas. F-actin staining is shown in chemotaxing neutrophils. Wild-type and Rac2 null cells present with a characteristic single prominent leading edge of polymerization, whereas the Rac1 null cells showed a significant proportion of neutrophils with 2 distinct leading edges. Double null cells showed no polarization. (B) Increased proportion of Rac1 null neutrophils display more than one leading edge compared with Rac2 null and wild-type neutrophils. The proportion of neutrophils for each genotype with 2 prominent leading edges was calculated. Rac1 null neutrophils demonstrated a significantly higher likelihood of having 2 leading edges (*P < .0001). (C) fMLP-mediated Cdc42 activation neutrophils required Rac2 and not Rac1. Using the p21 PBD assay,20 we demonstrate that fMLP-mediated Cdc42 activation increases in a similar pattern in wild-type and Rac1 null neutrophils but is severely perturbed in Rac2 null neutrophils. Rac2 is significantly different from wild-type and Rac1 (*P < .04). (D) Perturbed tail retraction in Rac1 null neutrophils is shown in this representative photomicrograph of neutrophils in an fMLP gradient. It is clearly noted that Rac1 null neutrophils displayed poor tail retraction compared with wild-type neutrophils. Error bars represent standard error of the means.
Because Cdc42 has been implicated previously in regulating fMLP-mediated actin assembly in neutrophils,7,9 we determined if either Rac isoform played a preferential role in regulating Cdc42 activation using the p21 PBD.20 Whereas fMLP-mediated Cdc42 activation was normal in Rac1 null neutrophils, it was markedly diminished in Rac2 null neutrophils (Figure 3C). In concert, these data suggest that Rac1 plays a role in the detection of the chemoattractant gradient, whereas Rac2 and CDC42 are required for the actin assembly required to drive cell translocation.
We also noted during chemotaxis that Rac1-deficient neutrophils displayed a consistent inability to retract their tails, which resulted in an elongated cell morphology during chemotaxis (Figure 3D).
Role of Racs in PIP3 localization and phosphorylation of AKT
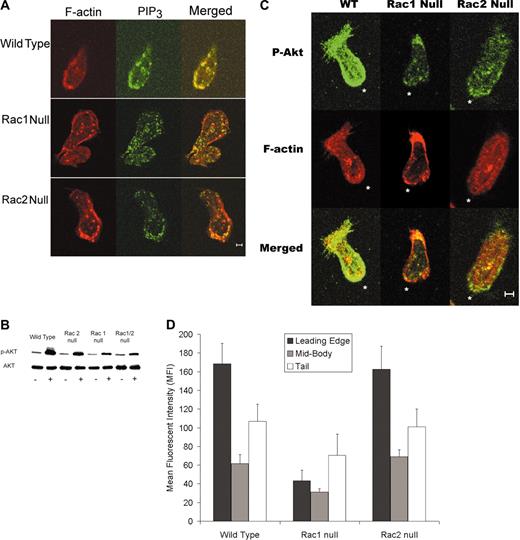
Because PIP3 has been implicated as the “chemotaxis compass” in neutrophils, we assessed the PIP3 distribution in chemotaxing neutrophils using an anti-PIP3 antibody. Immunofluorescence micrographs demonstrated that PIP3 localizes to the leading edge in wild-type and Rac2 null neutrophils. By contrast, this localization was much less frequent in Rac1 cells (Figure 4A). Previous studies have used the green fluorescent protein (GFP)–tagged PH domain of Akt to assess PIP3 localization in neutrophil-like cell lines.2 Because Akt is recruited and phosphorylated at the membrane during neutrophil chemotaxis,4-6 we assessed Akt phosphorylation levels in our neutrophils. Immunoblots for phospho-Akt (Ser473) demonstrated that fMLP-activated neutrophils lacking Rac1 exhibited significantly decreased levels of phospho-Akt compared with wild-type and Rac2 null neutrophils (Figure 4B). To assess the significance of this result, immunofluorescence localization of phospho-Akt was performed on neutrophils from the wild-type, Rac1 null, and Rac2 null genotypes. Micrographs revealed that Rac1 null neutrophils display an absence of phospho-Akt at the leading edge and lower overall phospho-Akt fluorescence compared with wild-type and Rac2 null neutrophils (Figure 4C). The relative amounts of phospho-Akt in the leading edge, midsection, and tail of neutrophils was measured using the phospho-Akt–stained micrographs and image analysis (see “Materials and methods”). This analysis revealed that there was a significant decrease in phospho-Akt in the leading edge of Rac1 null neutrophils compared with Rac2 null and wild-type neutrophils (Figure 4D).
PIP3 distribution in chemotaxing neutrophils. (A) PIP3 immunolocalization to leading edge is disrupted in Rac1 null neutrophils. Immunolocalization of PIP3 was carried out and analyzed as described in “Materials and methods.” Representative images demonstrate the PIP3 localization toward the leading edge in wild-type and Rac2 null neutrophils but not in Rac1 null cells. (B) Immunoblot analysis of phospho-Akt levels in neutrophils. Representative immunoblot of Akt and phospho-Akt levels in peritoneal neutrophils. Neutrophils were isolated from bone marrow as described. Blot is representative of 4 separate experiments that all demonstrate decreased phospho-Akt levels in Rac1 and Rac1/2 null neutrophils compared to wild-type and Rac2 null neutrophils. (C) Immunolocalization of phospho-Akt to the leading edge requires Rac1. Immunolocalization of phospho-Akt, was carried out and analyzed as described in “Materials and methods.” Representative images demonstrate phospho-Akt localization toward the leading edge in wild-type and Rac2 null neutrophils but not in Rac1 null cells. *Leading edge. (D) Quantification of mean phospho-Akt–related fluorescence intensity at the leading edge, in the cytoplasm, and in the tail was carried out as described in “Materials and methods.” These data clearly confirm that p-AKT levels are high at the leading edge in both wild-type and Rac2 null neutrophils but decreased in Rac1 null cells. (The leading edge of Rac1 was different from both wild-type and Rac2 null at the P < .05 level.) Scale bar equals 5 μm. Error bars represent standard error of the means.
PIP3 distribution in chemotaxing neutrophils. (A) PIP3 immunolocalization to leading edge is disrupted in Rac1 null neutrophils. Immunolocalization of PIP3 was carried out and analyzed as described in “Materials and methods.” Representative images demonstrate the PIP3 localization toward the leading edge in wild-type and Rac2 null neutrophils but not in Rac1 null cells. (B) Immunoblot analysis of phospho-Akt levels in neutrophils. Representative immunoblot of Akt and phospho-Akt levels in peritoneal neutrophils. Neutrophils were isolated from bone marrow as described. Blot is representative of 4 separate experiments that all demonstrate decreased phospho-Akt levels in Rac1 and Rac1/2 null neutrophils compared to wild-type and Rac2 null neutrophils. (C) Immunolocalization of phospho-Akt to the leading edge requires Rac1. Immunolocalization of phospho-Akt, was carried out and analyzed as described in “Materials and methods.” Representative images demonstrate phospho-Akt localization toward the leading edge in wild-type and Rac2 null neutrophils but not in Rac1 null cells. *Leading edge. (D) Quantification of mean phospho-Akt–related fluorescence intensity at the leading edge, in the cytoplasm, and in the tail was carried out as described in “Materials and methods.” These data clearly confirm that p-AKT levels are high at the leading edge in both wild-type and Rac2 null neutrophils but decreased in Rac1 null cells. (The leading edge of Rac1 was different from both wild-type and Rac2 null at the P < .05 level.) Scale bar equals 5 μm. Error bars represent standard error of the means.
Discussion
The neutrophil is a highly motile cell that is rapidly recruited to sites of infection and tissue trauma. Accordingly, the neutrophil is an often used model for the study of the basic mechanisms of directed cell migration (chemotaxis). The directed movement of neutrophils in a chemoattractant gradient involves 2 key steps. The first involves directional sensing though an internal chemical compass that enables the cell to translate an asymmetric extracellular chemical gradient into an internal amplified response.25,26 The second step involves activation of the cytoskeletal machinery that generates the force required for cell polarization and translocation. Although much is known about the cytoskeletal events involved in cell migration, it is only recently that the elements involved in the chemotactic compass have begun to be identified.12,26 The chemotactic compass determines where the cell establishes a leading edge and is known to require a lipid product of the phosphoinositide 3 kinases (PI3Ks) and a small Rho GTPase family member.2 Most of this work has been carried out using either Dictyostelium discoideum or neutrophil-like cell lines. In this report, we describe a murine model system that enables us to examine the unique roles played by Rac1 and Rac2 in chemotaxis by primary neutrophils. Previous studies of Rac1- and Rac2-deficient neutrophils have demonstrated chemotactic defects for both genotypes,10,11,15 but the nature of the defect (eg, whether involving propulsion or directionality) is not known. To assess the specific roles played by Rac1 and Rac2 in neutrophil chemotaxis, we have generated a mouse colony using previously described knock-out mice10,11 in which single litters produced mice with neutrophils deficient in Rac1 (Rac1 null), Rac2 (Rac2 null), or both Rac1 and Rac2 (Rac1/2 null), or sufficient in both Rac1 and Rac2 (wild type). This approach enables the use of littermate controls to overcome possible background variations and also enables the study of the specific roles of these Rac isoforms in primary neutrophils using in vitro and in vivo assays. Due to the 92% homology between these 2 GTPases and the fact that the guanine nucleotide exchange factors (GEFs) that regulate these proteins are the same, standard approaches such as expression of mutant proteins in neutrophil-like cell lines have not been effective in delineating the specific roles played by these 2 proteins in chemotaxis. Furthermore, neutrophils are relatively small, terminally differentiated end cells that are not amenable to standard biochemical techniques used to study a protein's role in chemotaxis, limiting the ability to manipulate them experimentally. Using a conditional knock-out strategy to generate mice with myeloid cells deficient in Rac1 combined with the existing Rac2 null mouse brought into the same background has enabled us to focus on the roles played by these 2 proteins in fully functional primary neutrophils.
In vitro single-cell chemotaxis analysis
Unlike the Boyden chamber chemotaxis assays, the Zigmond chambers allow for the determination of the nature of chemotactic defects including the ability to assess defects in migration speed and directionality.4 Using this approach we have demonstrated that Rac1 null neutrophils display multiple leading pseudopods along with an inability to undergo directed migration. A similar phenotype has been observed in Dictyostelium, with genetic ablation of 2 PI3Ks or treatment with PI3K inhibitors,27 neutrophils treated with intermediate concentrations of PI3K inhibitors,25 and neutrophils in which PI3Kγ was deleted.27 These observations strongly suggest that Rac1 plays a significant role in the signaling pathway that regulates the chemotactic compass, which is regulated by PI3K.26 The striking similarity between the PI3Kγ null neutrophils and the Rac1 null neutrophils is further highlighted by the observation that both neutrophil genotypes display normal chemokinesis but abnormal chemotaxis.4 It is important to note that a previous study looking at the role of Rac1 in hematopoietic cells used in vitro transduction of hematopoietic stem cells using a Cre-expressing retrovirus vector and cytokines15 to generate Rac1 null neutrophils, whereas we have used neutrophils that have developed in an intact bone marrow. Although we observed a similar defect in tail retraction (Figure 3C) in our in vivo–generated Rac1 null neutrophils, we noted a significant chemotactic defect that was not observed by Gu et al.15 Possible reasons for this difference in phenotype may be due to the methods used to obtain neutrophils (genetic manipulation and breeding versus use of a retroviral vector and in vitro differentiation of stem cells) and the chemotaxis experiments (Boyden versus Zigmond). The in vitro Zigmond chamber allows for the assessment of chemotaxis and chemokinesis separately and the results we describe here are consistent with the in vivo studies described previously, which demonstrate a chemotactic recruitment defect in neutrophils lacking Rac1.11
Role of Racs in phosphorylation of AKT and PIP3 localization
To confirm the role Rac1 plays in the chemotactic compass, we used an antibody to assess PIP3 spatial localization during chemotaxis. PIP3 localization to the leading edge has been demonstrated to be the key initial element of the chemotactic compass.2 Immunofluorescent images revealed that PIP3 that localized to the leading lamella in wild-type and Rac2 null neutrophils failed to do so in the absence of Rac1. Because the spatial localization of PIP3 has been previously assessed through the use of the pleckstrin homology (PH) domain of Akt protein kinase and given that Akt has also been recently demonstrated to play a role in neutrophil polarization and chemotaxis,4,5 we assessed the localization of Akt to the membrane by measuring phospho-Akt levels and immunolocalization of phospho-Akt in cells within a chemoattractant gradient. Because Akt is phosphorylated (Ser473) at the membrane following binding through its PH domain to PI3K lipid products on the plasma membrane,6,28 this phosphorylation event serves as a useful marker of Akt recruitment to the membrane.29 Our data demonstrate a role for Rac1 in normal Akt phosphorylation and Akt localization to the leading edge of chemotaxing neutrophils and suggest that Rac1 is part of the PI3K, PIP3, Rho small GTPase regulatory mechanism that controls the cell's chemotactic compass.
Cdc42, the Racs, and polarization
Because Rac1 null neutrophils have a directional polarization defect, we hypothesized that activation of Cdc42, the small GTPase implicated in cell polarization and the development of the leading edge,30 would be suppressed in Rac1 null neutrophils. To the contrary, however, our observations indicate that Cdc42 is in fact downstream of Rac2 and not Rac1. This concept is consistent with recent work implicating Cdc42 in the regulation of the chemotactic compass.31 In this regard, Li et al have provided evidence for 2 parallel pathways that are both required for the regulation of the chemotactic compass.31 The first involves a PI3Kγ-dependent pathway that uses Rac and PIP3 to identify the chemotactic gradient. The second pathway, which uses Pixα and Cdc42, is essential for localizing actin polymerization to the leading edge of the cell that faces the chemotactic gradient. Li et al demonstrate that the Pixα-Cdc42 pathway requires the PI3Kγ pathway to help localize the Cdc42-mediated actin assembly to the leading edge. Our current results demonstrate that Rac1, as part of the PI3Kγ pathway, plays an important role in identifying the chemoattractant gradient and localizing the actin polymerization regulating Pixα-Cdc42-Rac2 signaling pathway to the leading edge. It is important to note when considering these results that the murine system differs from human neutrophils. Specifically, in human cells, Rac1 levels are approximately 10% of Rac2 levels, whereas in murine cells, Rac1 and Rac2 levels are approximately equal.17
Summary and conclusions
Rac1 and Rac2 share 92% amino acid identity with the major divergence occurring in the C-terminus. This C-terminal region determines the subcellular localization and interactions of Rac with some downstream effecter proteins.16,30 With Rac being active in both the leading lamella and in the tail during chemotaxis,13 with the localization differences observed between GFP-Rac1 (plasma membrane) and GFP-Rac2 (cytoplasmic domains) in COS cells16 and with the key role of Rac2 in regulating actin assembly10 (Figure 2E), we suggest that Rac1 is critical to determining the location of the initial dominant lamella, whereas Rac2 is required for regulating the actin assembly dynamics required in both the leading edge and the tail. The role of Rac2 as the driver of actin assembly in neutrophils is further supported by our observation that Cdc42 activation appears to be partly regulated by Rac2. This observation is consistent with previous work by Zigmond et al7 and our group,9 which demonstrated the significant role of Cdc42 in chemoattractant-mediated actin assembly in neutrophils.
Our findings significantly advance our understanding of the chemotactic compass. We demonstrate that Rac1 plays a central role in the chemotactic compass through its upstream regulation of PI3K and Akt recruitment to the leading edge of neutrophils in a chemoattractant concentration gradient. Although we do not have evidence that Rac1 directly regulates Akt, the data presented here demonstrate that Rac1 plays a role in the signal transduction cascade that regulates Akt phosphorylation. We also provide evidence to support the notion that Rac2 is the key regulator of the chemoattractant-mediated actin assembly at the leading edge required for cell translocation during chemotaxis. Finally, our data further implicate Akt as a key effecter of PI3K lipid products responsible for the chemotaxis compass. Future work will focus on identifying the interactions and signaling intermediates that support the Rac1-PI3K-Akt chemotactic compass-signaling mechanism.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-03-0781.
Supported by Canadian Institutes of Health Research (CIHR) grants (M.G. and G.P.D.). M.G. is a CIHR Clinician Scientist and is also supported by the Bertha Rosenstadt Fund. G.P.D. currently holds the R. Fraser Elliott Chair in transplantation research from the Toronto General Hospital and is the recipient of a Tier 1 Canada Research Chair.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Drs O. Weiner and A. Kapus for their helpful comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal