We tested the in vitro and in vivo antitumor activity of the maytansinoid DM1 (N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine), a potent antimicrotubule agent, covalently linked to the murine monoclonal antibody (mAb) B-B4 targeting syndecan-1 (CD138). We evaluated the in vitro activity of B-B4–DM1 against a panel of CD138+ and CD138- cell lines, as well as CD138+ patient multiple myeloma (MM) cells. Treatment with B-B4–DM1 selectively decreased growth and survival of MM cell lines, patient MM cells, and MM cells adherent to bone marrow stromal cells. We further examined the activity of B-B4–DM1 in 3 human MM models in mice: (1) severe combined immunodeficient (SCID) mice bearing subcutaneous xenografts; (2) SCID mice bearing green fluorescent protein–positive (GFP+) xenografts; and (3) SCID mice implanted with human fetal bone (SCID-hu) and subsequently injected with patient MM cells. Tumor regression and inhibition of tumor growth, improvement in overall survival, and reduction in levels of circulating human paraprotein were observed in mice treated with B-B4–DM1. Although immunohistochemical analysis demonstrates restricted CD138 expression in human tissues, the lack of B-B4 reactivity with mouse tissues precludes evaluation of its toxicity in these models. In conclusion, B-B4–DM1 is a potent anti-MM agent that kills cells in an antigen-dependent manner in vitro and mediates in vivo antitumor activity at doses that are well tolerated, providing the rationale for clinical trials of this immunoconjugate in MM.
Introduction
Following the discovery of monoclonal antibody (mAb) technology,1 numerous attempts have been made to use mAbs targeted to cancer-associated antigens for the treatment of human cancer. Of many mAbs tested, several were found to be therapeutically active, either as naked antibodies or as immunoconjugates of radionuclides.2-4 Conjugates of antibodies with cytotoxic agents have also been explored as anticancer agents2,3 as a means of improving the selectivity of cytotoxic drugs by targeting them to antigens preferentially expressed on the surface of malignant cells. Gemtuzumab ozogamicin (Mylotarg), a conjugate of anti-CD33 humanized monoclonal antibody with a highly cytotoxic DNA-damaging agent calicheamicin, has recently been approved by the FDA as the first drug of this type for clinical treatment of myeloid leukemia.5-7
Immunoconjugates of the highly potent cytotoxic drug maytansine derivative DM1 (N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine) are also being evaluated as antigen-targeted anticancer agents.8 Maytansine9 is a natural product originally derived from the Ethiopian shrub Maytenus serrata. This drug inhibits tubulin polymerization,9-11 resulting in mitotic block and cell death. The cytotoxicity of maytansine is 200- to 1000-fold higher than that of anticancer drugs in clinical use that affect tubulin polymerization, such as Vinca alkaloids or taxol. However, clinical trials of maytansine indicated that it lacked a therapeutic window, due to high systemic toxicity. DM1 is 3- to 10-fold more cytotoxic than maytansine, and can be converted into a prodrug by linking it via disulfide bond to a mAb directed against a tumor-associated antigen. Such a conjugate (tumor-activated prodrug or TAP) is not cytotoxic in the blood compartment, since it is activated only upon binding to target cells, with subsequent release of the drug.12 Several mAb-DM1 conjugates have been developed,13 and, to date, huC242-DM1 and huN901-DM1 have been evaluated in clinical trials. These conjugates were well tolerated, did not induce any detectable immune response, and had a long circulation time.14-16
The murine IgG1 mAb B-B4 binds to a linear epitope between residues 90 to 95 of the core protein on human syndecan-1 (CD138), a member of the family of transmembrane heparan sulfate proteoglycans.17,18 This antigen was originally described as a membrane proteoglycan present on cells of epithelial origin, and was subsequently found on hematopoietic cells.19 In the normal human hematopoietic compartment, CD138 expression is restricted to plasma cells17,20 ; in particular, CD34+ stem and progenitor cells do not express CD138.17 In malignant hematopoiesis, CD138 is highly expressed on the majority of multiple myeloma (MM) cells, many Hodgkin lymphomas with classic Reed-Sternberg cells,17,20 as well as a fraction of chronic lymphocytic leukemia (B-CLL),21 acute lymphoblastic leukemia (ALL), and acute myeloblastic leukemia (AML)22,23 cells. At present, CD138 is a promising antigen for targeting MM cells. However, the lack of human CD138 on mouse tissues limits preclinical evaluation of the safety of mAbs targeting this antigen.
Here we report our preclinical study of B-B4–DM1 as a novel treatment for MM. We evaluated the in vitro activity of B-B4–DM1 against a panel of CD138+ and CD138- cell lines, as well as patient MM cells adherent to bone marrow stroma cells (BMSCs). We further examined the antitumor potency of B-B4–DM1 using 3 in vivo models of human MM in SCID mice.
Materials and methods
Preparation of mAb-DM1 conjugate
The thiol-containing maytansinoid DM1 was synthesized from the microbial fermentation product ansamitocin P-3, as previously described.8 Characterization of murine B-B417 and preparation of humanized mAb C242 (huC242)24 have been previously described. mAb-drug conjugates were prepared as described elsewhere.25 An average of 3.5 DM1 molecules were linked per antibody molecule. Activity of a DM1-conjugate against MM cells has been recently demonstrated.26
Cell lines and patient cells
CD138+ dexamethasone (Dex)–sensitive MM.1S and Dex-resistant MM.1R, Ocy-My5, and OPM1 and OPM2 human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL), Dr H. A. Messner (Ontario Cancer Institute, Toronto, ON, Canada), and Dr Edward I. B. Thompson (University of Texas Medical Branch, Galveston, TX), respectively. CD138- Waldenstrom macroglobulinemia (WM) WSU-WM and the lymphoma (LB) SUDHL4 cell lines were kindly provided by Dr Ayad Al-Katib (Wayne State University, Detroit, MI) and Dr Margaret Shipp (Dana Farber Cancer Institute, Boston, MA), respectively. Cell lines were cultured in RPMI-1640 medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), l-glutamine, penicillin, and streptomycin (GIBCO). Plasma cells (PCs) and bone marrow (BM) cells were isolated using Ficoll-Hypaque density gradient sedimentation from BM aspirates, obtained from MM patients following informed consent. BMSCs were obtained by long-term cultures of BM cells (4-8 weeks) in RPMI-1640 medium supplemented with 20% FBS.
Gene expression analysis
BM aspirate samples from healthy donors and patients with MM were treated with 0.86% ammonium chloride to lyse red blood cells. PCs were then isolated by positive immunomagnetic bead selection using anti-CD138 antibodies and magnet assisted cell sorting (MACS; Miltenyi Biotech, Auburn, CA). Purity of PCs (> 95%) was assessed by flow cytometric (FACSort; Becton Dickinson, San Jose, CA) monitoring for CD38+/CD45lo phenotype as well as forward and side scatter and morphologic characteristics. Total RNA was isolated from 5 × 106 cells using an RNeasy kit (Qiagen, Valencia, CA). Total RNA (10-15 μg) was reverse-transcribed to yield cDNA using the “Superscript II RT kit” (Invitrogen Life Technologies, Carlsbad, CA). cDNA was used in an in vitro transcription reaction to synthesize biotin-labeled cRNA using “ENZO RNA labeling kit” (Enzo Diagnostics, Farmingdale, NY). Labeled cRNA was purified with the RNeasy Mini-kit (Qiagen) and quantitated. Purified cRNA (15 μg) was hybridized to Human Genome U133 (HG-U133) GeneChip arrays (Affymetrix, Santa Clara, CA) representing approximately 33 000 human genes, and GeneChip arrays were scanned on a GeneArray Scanner (Affymetrix).
Microarray data analysis
Normalization of arrays and calculation of expression values were performed using DNA-Chip Analyzer (dChip) program (C. Li and W. Hung Wong, DFCI, Boston, MA). Arrays were normalized based on relative signal produced for an invariant subset of genes. This model-based method was used for probe selection and computing expression values. By pooling hybridization information across multiple arrays, it is possible to assess standard errors for the expression level indexes. This approach also allows automatic probe selection in the analysis stage to reduce errors due to cross-hybridizing probes and image contamination.
Immunohistochemistry
Paraffin multitissue arrays were purchased from Biochain Institute (catalog no. T8234708; Lot A603500, Hayward, CA) and Zymed Labs (“Max-arrays”; South San Francisco, CA). Normal frozen organ tissues were cryostat cut at 5-μm sections and fixed in cold 2% paraformaldehyde/phosphate-buffered saline (PBS) prior to staining, which was performed using the avidin-biotin-peroxidase (ABC) technique.
Colorimetric survival assay
Cell survival was examined using a tetrazolium colorimetric assay (CellTiter 96 Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI), as previously described.27 Cells (1 × 104) were plated in 24-well plates in 1 mL RPMI medium supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics, and then treated as indicated. At the end of each treatment, cells were incubated with 150 μL Dye Solution and then incubated for 4 hours at 37°C. A solubilization/stop solution was then added to each well under vigorous pipetting to dissolve the formazan crystals. Absorbance was measured at 570 nm, and cell viability was estimated as percentage of untreated controls. All experiments were repeated 3 times, and each experimental condition was repeated in triplicate wells in each experiment. Data reported are average values ± SD of 3 representative experiments.
Cell proliferation assay
Cell proliferation was measured by [3H]-thymidine (NEN Life Science Products, Boston, MA) incorporation. Cells (2 × 104 cells/well) were incubated in 96-well culture plates in the presence of 70% to 80% confluent BMSCs at 37°C with or without a test-agent (in triplicate wells). [3H]-thymidine (0.5 μCi [0.0185 MBq]) was then added to each well for the last 8 hours. Cells were harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA) and counted using a Micro-Beta Trilux counter (Wallac, Gaithersburg, MD).
Detection of apoptosis
Dual staining with fluorescein isothiocyanate (FITC)–labeled annexin V and propidium iodide (PI) was carried out to detect induction of apoptotic cell death. After treatment of 1 × 106 tumor cells for 48 hours, cells were washed with PBS and resuspended in 100 μL HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer containing annexin V–FITC and propidium iodide (PI) (annexin V–FLUOS staining kit; Roche Diagnostic, Indianapolis, IN). Following 15 minutes of incubation at room temperature, cells were analyzed using a Coulter Epics XL flow cytometer (Coulter, Birmingham, United Kingdom) for the presence of an annexin V–FITC–positive/PI-negative apoptotic cell population.
Cell-cycle analysis
MM cells (1 × 106) were incubated with or without a test-agent for 48 hours, washed with PBS, permeabilized by a 30-minute exposure to 70% ethanol at 4°C, incubated with PI (50 μg/mL) in 0.5 mL PBS containing 20 U/mL Rnase A (Roche Diagnostics) for 30 minutes at room temperature, and analyzed for DNA content by cell-associated fluorescence using a flow cytometer and CellQuest software (BD Biosciences Immunocytometry Systems, San Jose, CA).
In vivo activity
Animals. Male CB-17 SCID mice (6- to 8-weeks old; Taconic, German-town, NY) were housed and monitored in our Animal Research Facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee (VA Boston Healthcare System). In accordance with institutional guidelines, mice were killed when their tumors reached 2 cm in diameter or in the event of paralysis or major compromise in their quality of life, to prevent unnecessary suffering.
Human MM xenograft murine model. In this model, mice were subcutaneously inoculated in the interscapular area with 5 × 106 OPM1 or OPM2 cells in 100 μL RPMI-1640 medium. Treatment was initiated after the detection of palpable tumors, approximately 3 weeks following injection of MM cells. Tumor size was measured weekly in 2 dimensions using a caliper, and volume was calculated using the formula: V = 0.5a × b2, where a and b are the long and short diameter of the tumor, respectively. The survival time is defined as the time interval between the start of the experiment and either death or the day of killing. Mice were treated intravenously with vehicle alone (PBS), unconjugated B-B4 (13.3 μg/kg), B-B4–DM1 (conjugate containing 75 or 150 μg DM1/kg per day), or control huC242-DM1 (150 μg DM1/kg per day), for a total of 3 days. In addition, 2 mice bearing large tumors (average size, 1309 ± 60 mm3) were treated with B-B4–DM1 (150 μg DM1/kg per day) for a total of 3 days and observed for changes in the tumor size.
Autofluorescent green fluorescent protein–positive (GFP+) human MM xenograft model
OPM1 cells were transfected with green fluorescent protein (OPM1GFP+) using a lentiviral vector, as previously described.28 Mice were injected subcutaneously with 5 × 106 OPM1GFP+ cells and were monitored by whole-body fluorescence imaging using Illumatool Bright Light System LT-9900 (Lightools Research, Encinitas, CA), following a cutaneous shave of the tumor area. The images were captured with a Sony DSC-P5 digital camera (Boston, MA) and analyzed using Image-Pro Discovery software (MediaCybernetics, Silver Spring, MD).
SCID-hu mouse model
Human fetal long bone grafts were implanted into SCID mice (SCID-hu mice) as previously described.29-36 Approximately 8 weeks following bone implantation, 2 to 5 × 106 BM cells from an MM patient in 50 μL PBS were injected directly into human bone of SCID-hu hosts. Production of human paraprotein in mouse serum was monitored as an indicator of myeloma engraftment and growth. At least 2 consecutive measurements of increasing levels of circulating human immunoglobulin (huIg) signified human MM cell growth.
Measurement of serum paraprotein concentration
Blood (50-100 μL) was drawn from the tail vein for measurement of human paraprotein in murine serum using enzyme-linked immunosorbent assay (ELISA; Bethyl, Montgomery, TX). Goat antihuman λ and κ antisera were used for capture, and goat antihuman λ or κ horseradish peroxidase (HRP) conjugates were used for detection.
Statistical analysis
Statistical significance of differences was determined using Student's t test. Differences were considered significant when P < .05.
Results
Expression of CD138 in MM patients
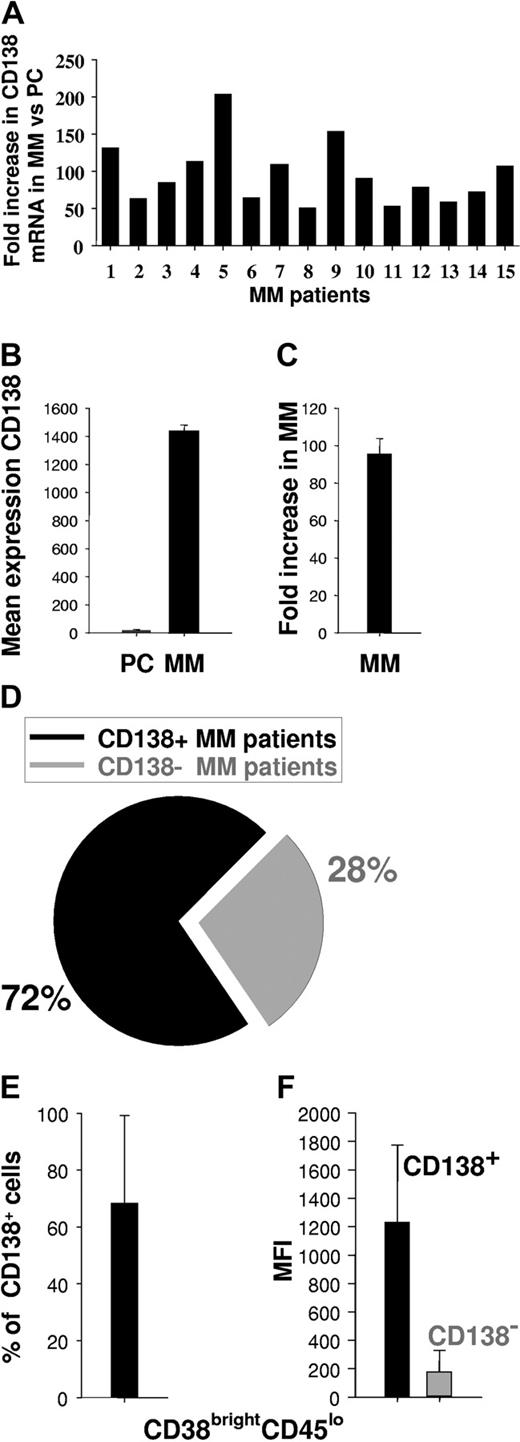
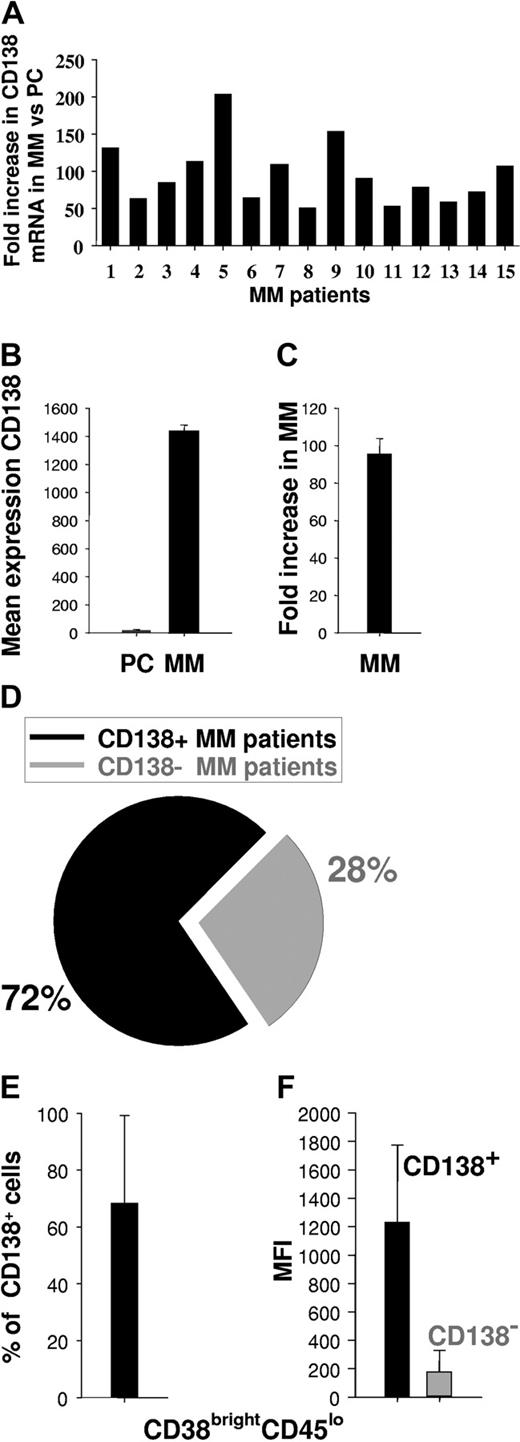
Since CD138 may be heterogeneously expressed among various MM patients or within MM cell populations,17,37-40 we first measured CD138 expression on patient MM cells as a potential target for mAb-based therapy. We analyzed CD138 gene expression profiles of patient MM cells (n = 15) versus normal PCs (n = 3) using HG-U133 GeneChip array (Affymetrix) data. CD138 mRNA was expressed in MM specimens examined at a 95 ± 8-fold mean increase in intensity relative to normal PCs (Figure 1A-C). We next used flow cytometry to assess cell-surface expression of CD138 on MM cells from 25 patients. Expression of CD138 on the CD38brightCD45lo cell population was assessed both by percentage of positive cells and by mean fluorescence intensity (MFI). Of 25 patients, 18 (72%) (Figure 1D) expressed CD138, with a mean of 68 ± 31% CD138+ cells (Figure 1E) and MFI of 1234 ± 539 (range, 166-2208) (Figure 1F). Taken together, these results indicate that CD138 is expressed at high density in a majority of MM patients.
Expression of CD138 in MM. (A) Individual fold increase in intensity of CD138 gene expression (transcription) in patient MM cells (15 patients) compared with normal PCs (n = 3 individuals). (B) Mean intensity of CD138 gene expression in normal PCs (n = 3) and patient MM cells (n = 15). (C) Mean fold increase intensity of CD138 gene expression in MM cells (n = 15) versus normal PCs (n = 3). (D) Percentage of patients expressing CD138+ MM cells, as determined by flow cytometry on fresh BM aspirate samples. (E) Percentage of CD138+ MM cells in CD138+ patients as determined by flow cytometry on fresh BM aspirates. (F) MFI of CD138+ or CD138- MM cells within CD38brightCD45lo population, as determined by flow cytometry. Error bars indicate standard deviation (SD).
Expression of CD138 in MM. (A) Individual fold increase in intensity of CD138 gene expression (transcription) in patient MM cells (15 patients) compared with normal PCs (n = 3 individuals). (B) Mean intensity of CD138 gene expression in normal PCs (n = 3) and patient MM cells (n = 15). (C) Mean fold increase intensity of CD138 gene expression in MM cells (n = 15) versus normal PCs (n = 3). (D) Percentage of patients expressing CD138+ MM cells, as determined by flow cytometry on fresh BM aspirate samples. (E) Percentage of CD138+ MM cells in CD138+ patients as determined by flow cytometry on fresh BM aspirates. (F) MFI of CD138+ or CD138- MM cells within CD38brightCD45lo population, as determined by flow cytometry. Error bars indicate standard deviation (SD).
Distribution of CD138 antigen in normal human tissues
We next evaluated the reactivity of mAb B-B4 on a large panel of human tissues by immunohistochemistry (IHC). Freshly frozen tissue sections and paraffin multitissue arrays were analyzed, in conjunction with assay controls OPM2 and Namalwa cell sections, using the ABC immunoperoxidase method. The slide evaluation used a 4-tiered scoring system and included the staining intensity (scored 0, 1, 2, or 3), uniformity (scattered, focal, heterogeneous, or homogeneous), site of reactivity (structure), and main type of reactive cell in test samples (Table 1). B-B4 staining was not observed in heart, brain, stomach, umbilical cord, and brain. No staining was seen in 4 lung specimens, specifically in bronchiolar epithelium, alveoli, or endothelial cells, with strong focal staining seen in scattered macrophages. No staining was seen in splenic, red, or white pulp, with strong focal staining in macrophages. Moderate, heterogeneous staining was seen in thymus Hassall corpuscle, with strong, focal staining in medullary region. Moderate, homogeneous staining was seen in breast ductal epithelium, with moderate focal staining of connective tissue. Strong staining was seen in surface epithelium of the normal colon, with the submucosa and muscularis showing no staining. We observed strong, homogeneous staining of the skin epidermis, with no dermis staining. Strong staining was seen in the esophagus, specifically the stratified squamous epithelium (mucosa), with the submucosa and muscularis showing no staining. The staining of frozen sections of 4 liver samples produced variable, inconclusive results; therefore, paraffin-embedded liver sections were evaluated. In the paraffin-embedded liver sections, we observed less variation within cell constituents: no staining of hepatocytes was observed, with moderate to strong staining in spaces between the hepatocytes, or sinusoidal lining cells, and stronger staining of the hepatic vein. Strong staining was seen in 3 kidney samples, specifically in tubules in the cortex distal to the main blood supply, and in the medullary region of one sample. The glomeruli present in these samples showed no B-B4 staining.
We also tested whether mAb B-B4 is reactive toward cynomolgus monkey CD138. If reactive, then the monkey could be used for preclinical evaluation of CD138-related systemic toxicity of B-B4–DM1. There was virtually no staining in any of the normal monkey tissues examined, including colon, epidermis, and kidney tubules. These data indicated that mAb B-B4 does not react with cynomolgus monkey CD138.
B-B4–DM1 is selectively cytotoxic to CD138+ MM cell lines in vitro
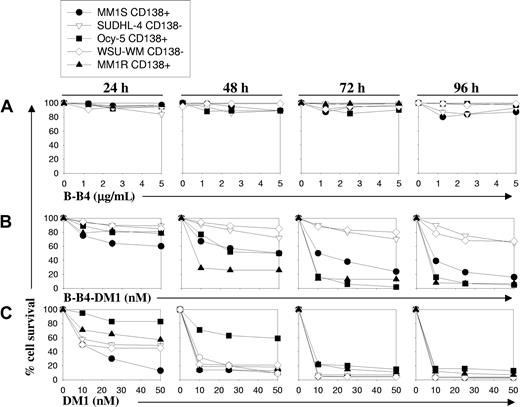
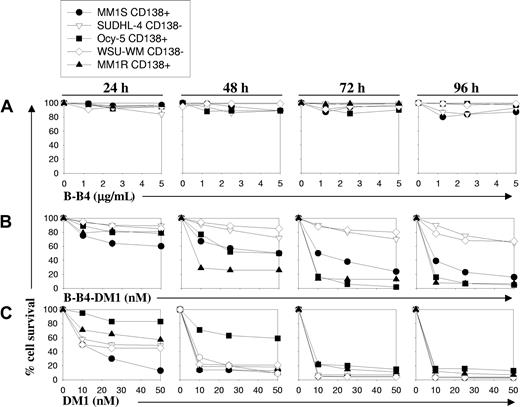
To evaluate the therapeutic potential of B-B4 as an immunoconjugate with DM1, we next examined the effects of B-B4–DM1 on survival of CD138+ (MM.1S, MM.1R, Ocy-My5) and CD138- cells (SUDHL-4 and WSU-WM) using an methyl thiazolyl tetrazolium (MTT) assay. As seen in Figure 2B, treatment with B-B4–DM1 (1-50 nM) induced growth inhibition in CD138+ tumor cells in a time- and dose-dependent manner. This effect was most prominent after 72 hours in all CD138+ cells. B-B4–DM1 treatment of CD138+ OPM1 and OPM2 MM cells further confirmed these observations (data not shown). In contrast, B-B4–DM1 (1-50 nM) was much less toxic to CD138- cells under these conditions. To confirm that the inhibitory activity of the immunoconjugate is specifically related to the mAb-delivered drug, we similarly tested the effect of equimolar concentrations of B-B4 antibody or unconjugated drug, DM1. Even at the highest tested concentrations (5 μg/mL or 31 nM), B-B4 did not affect the growth of cells following a 96-hour exposure (Figure 2A), whereas free DM1 was equally and highly cytotoxic in both CD138+ and CD138- cell lines (Figure 2C). These data indicate that the activity of the immunoconjugate is related to neither the differential sensitivity of cells to the drug, nor the intrinsic properties of the antibody.
Effect of B-B4–DM1 on survival of CD138+ and CD138- MM cells. MM cell lines were exposed to (A) unconjugated B-B4 mAb, (B) immunoconjugate B-B4–DM1, or (C) free DM1 drug at equimolar concentrations. Cell survival was measured using an MTT assay. Data (mean ± SD of triplicate experiments) are expressed as percentage of untreated controls. CD138+ MM cell lines evaluated are MM.1S, MM.1R, and Ocy-My5; CD138- cell lines included the lymphoma cell line SUDHL4 and the Waldenstrom macroglobulinemia cell line WSU-WM.
Effect of B-B4–DM1 on survival of CD138+ and CD138- MM cells. MM cell lines were exposed to (A) unconjugated B-B4 mAb, (B) immunoconjugate B-B4–DM1, or (C) free DM1 drug at equimolar concentrations. Cell survival was measured using an MTT assay. Data (mean ± SD of triplicate experiments) are expressed as percentage of untreated controls. CD138+ MM cell lines evaluated are MM.1S, MM.1R, and Ocy-My5; CD138- cell lines included the lymphoma cell line SUDHL4 and the Waldenstrom macroglobulinemia cell line WSU-WM.
B-B4–DM1 is cytotoxic to CD138+ MM cell lines and patient MM cells adherent to BMSCs
Since adhesion of MM cells to BMSCs induces development of drug resistance in MM cells,41 we next evaluated the effect of B-B4–DM1 on proliferation of CD138+ (Ocy-My5) MM and CD138- (SUDHL-4) LB cells adherent to BMSCs. Proliferation was measured by [3H]thymidine incorporation at 72 hours. As seen in Figure 3A, B-B4–DM1 (10 nM) markedly (8.6-fold) inhibited the proliferation of CD138+ Ocy-My5 cells, but had no significant effect on CD138- SUDHL-4 cells. Unconjugated B-B4 did not exert any inhibitory effect, whereas free DM1 (10 nM) was cytotoxic to both cell lines. We then similarly evaluated the cytotoxic activity of B-B4–DM1 (10 nM) on CD138+/CD56+ patient MM cells cultured with BMSCs (Figure 3B) using flow cytometry. Following 72 hours of treatment with the immunoconjugate, more than 90% reduction in MM cells was observed. Taken together, these results indicate that B-B4–DM1 overcomes cell adhesion–mediated drug resistance (CAM-DR).
Inhibitory effect of B-B4–DM1 on proliferation of CD138+ and CD138- cells adherent to BMSCs. (A) Ocy-My5 or SUDHL-4 cells (2 × 104) were seeded on 70% to 80% confluent BMSCs for 24 hours. Cell proliferation was measured by [3H]thymidine ([3H]-TdR) incorporation following 72 hours of treatment with 10 nM B-B4–DM1. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures. Error bars indicate SD. (B) Patient MM cells were cultured on BMSC layers and exposed for 72 hours to the 10 nM B-B4–DM1 immunoconjugate. CD56 and CD138 expression was evaluated by flow cytometry. We have previously established that B-B4–DM1, even at a concentration as high as 240 nM, did not affect binding of PE-labeled anti-CD138 antibody (Syndecan-1 DL-101; Santa Cruz Biotechnology, Santa Cruz, CA) to CD138-expressing cells. Figure is representative of 2 experiments.
Inhibitory effect of B-B4–DM1 on proliferation of CD138+ and CD138- cells adherent to BMSCs. (A) Ocy-My5 or SUDHL-4 cells (2 × 104) were seeded on 70% to 80% confluent BMSCs for 24 hours. Cell proliferation was measured by [3H]thymidine ([3H]-TdR) incorporation following 72 hours of treatment with 10 nM B-B4–DM1. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures. Error bars indicate SD. (B) Patient MM cells were cultured on BMSC layers and exposed for 72 hours to the 10 nM B-B4–DM1 immunoconjugate. CD56 and CD138 expression was evaluated by flow cytometry. We have previously established that B-B4–DM1, even at a concentration as high as 240 nM, did not affect binding of PE-labeled anti-CD138 antibody (Syndecan-1 DL-101; Santa Cruz Biotechnology, Santa Cruz, CA) to CD138-expressing cells. Figure is representative of 2 experiments.
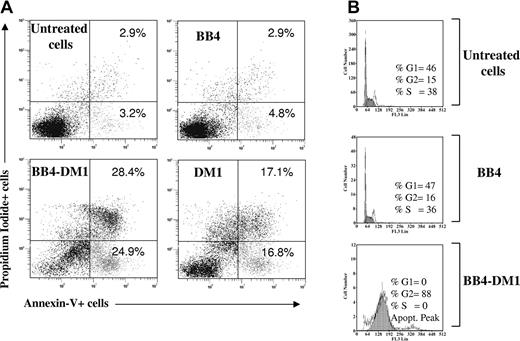
B-B4–DM1 induces apoptosis and G2/M cell-cycle arrest in CD138+ Ocy-My5 MM cells
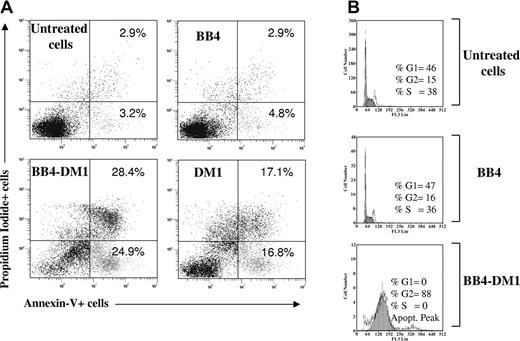
To determine whether apoptotic cell death occurs in cells exposed to the immunoconjugate, CD138+ Ocy-My5 MM cells were incubated with B-B4–DM1 (10 nM) for 72 hours. Apoptotic cell death was then measured by staining with annexin V and PI and analysis using flow cytometry. As shown in Figure 4A, a significant increase in both annexin V+/PI- and annexin V+/PI+ fractions was observed in CD138+ cells exposed to B-B4–DM1 and free DM1, whereas no significant differences were detected in cells treated with unconjugated mAb alone. We next analyzed cell-cycle changes induced by an exposure of MM cells to B-B4–DM1. Cells were treated with the immunoconjugate for 48 hours, labeled with PI, and analyzed using flow cytometry. As shown in Figure 4B, B-B4 mAb alone had no significant effect on the proportion of cells in G2/M-phase compared with untreated cells (15% vs 16%), whereas exposure of MM cells to B-B4–DM1 resulted in accumulation of the majority (89%) of cells in G2/M.
Survival and cell-cycle effects. (A) Induction of apoptotic cell death in CD138+ Ocy-My5 MM cells after 48 hours of exposure to 10 nM B-B4–DM1. Cells were stained with PI and annexin V and then analyzed by flow cytometry. Percentages of stained cells are reported in each quadrant. (B) Effects of B-B4–DM1 treatment on the cell cycle. Ocy-My5 MM cells were exposed to 13.3 μg/mL (83 nM) B-B4 mAb or 10 nM B-B4–DM1 for 48 hours. Cell-cycle profile was analyzed using PI staining, and percentages of cells in S phase (S) and G2/M phase are indicated in each quadrant. Data are representative of 3 experiments.
Survival and cell-cycle effects. (A) Induction of apoptotic cell death in CD138+ Ocy-My5 MM cells after 48 hours of exposure to 10 nM B-B4–DM1. Cells were stained with PI and annexin V and then analyzed by flow cytometry. Percentages of stained cells are reported in each quadrant. (B) Effects of B-B4–DM1 treatment on the cell cycle. Ocy-My5 MM cells were exposed to 13.3 μg/mL (83 nM) B-B4 mAb or 10 nM B-B4–DM1 for 48 hours. Cell-cycle profile was analyzed using PI staining, and percentages of cells in S phase (S) and G2/M phase are indicated in each quadrant. Data are representative of 3 experiments.
B-B4–DM1 inhibits tumor growth in a human MM xenograft model in SCID mice
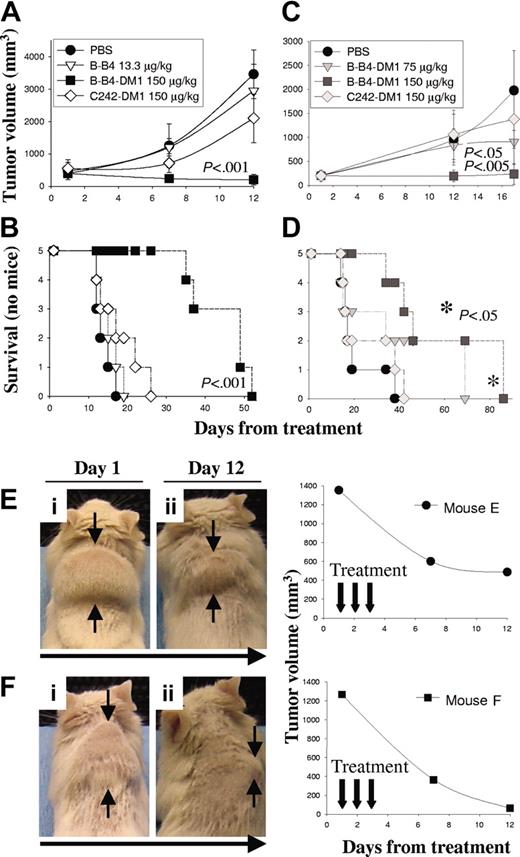
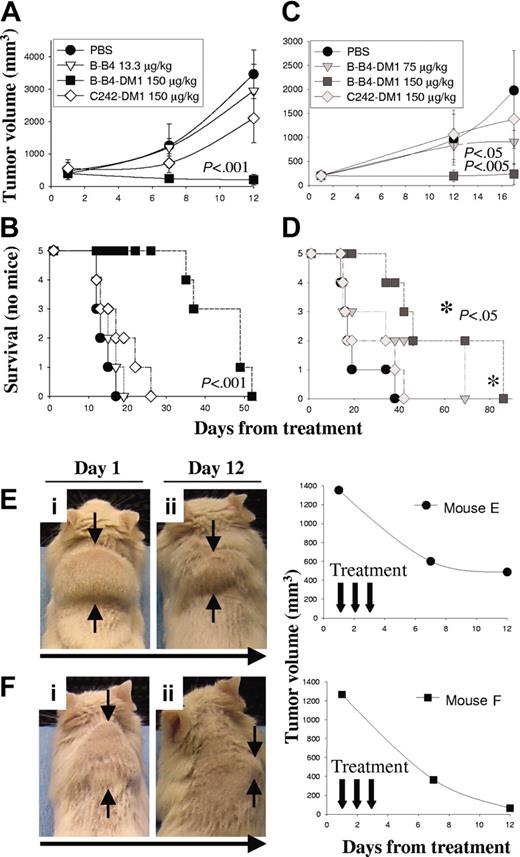
We first used a human MM subcutaneous xenograft model in SCID mice to study the in vivo activity of B-B4–DM1 against CD138+ OPM1 cells. In this model, the therapeutic efficacy of B-B4–DM1 was measured in mice bearing large palpable tumors (average size, 453 ± 74 mm3). Animals were treated daily intravenously for 3 consecutive days with either vehicle alone (PBS; n = 5), unconjugated B-B4 (13.3 μg/kg; n = 5), B-B4–DM1 (150 μg DM1/kg; n = 5), or control huC242-DM1 (150 μg DM1/kg; n = 5), which does not bind OPM1 cells. Tumor size and overall survival were monitored in these cohorts. As shown in Figure 5A-B, vehicle alone, unconjugated B-B4, and a nontargeting conjugate huC242-DM1 had no significant effect on tumor growth (panel A) or survival (panel B). In contrast, treatment with 150 μg DM1/kg B-B4–DM1 induced significant tumor regression (P < .001) and increase in survival (P < .001). We also studied the effect induced by B-B4–DM1 (75 or 150 μg DM1/kg; n = 10) against OPM2 MM cells. As shown in Figure 5C-D, treatment with 75 μg DM1/kg B-B4–DM1 induced a significant delay in tumor growth (P < .05), and 150 μg DM1/kg B-B4–DM1 completely inhibited tumor growth (P < .005). A significant increase (P < .05) in survival was also observed in mice treated with 150 μg DM1/kg B-B4–DM1. We also tested the antitumor activity of B-B4–DM1 (150 μg DM1/kg) in animals bearing larger (1309 ± 60 mm3) tumors. As shown in Figure 5E-F, marked tumor regression was induced by B-B4–DM1 treatment (150 μg DM1/kg) in these animals. Taken together, these results indicate that B-B4–DM1 is active in suppressing tumor growth in the murine model of human MM.
Activity of B-B4–DM1 in a tumor xenograft model of human CD138+ MM. CB-17 SCID mice were inoculated subcutaneously in the interscapular area with 5 × 106 OPM1 (A-B) or OPM2 (C-D) MM cells. Mice were treated intravenously with B-B4–DM1 or control mAbs for 3 consecutive days. Tumor volume was assessed in 2 dimensions using an electronic caliper, and the volume was expressed in mm3 using the formula: V = 0.5a × b2, where a and b are the long and short diameters of the tumor, respectively. Tumor volume and survival were calculated as described in “Materials and methods.” * indicates that P value is related to the curve with ▪. (E-F) Mice bearing large OPM1 tumors (1309 ± 60 mm3) were treated intravenously with B-B4–DM1 (150 μg DM1/kg) for a total of 3 consecutive days. Figure shows a significant tumor regression 12 days following the beginning of treatment.
Activity of B-B4–DM1 in a tumor xenograft model of human CD138+ MM. CB-17 SCID mice were inoculated subcutaneously in the interscapular area with 5 × 106 OPM1 (A-B) or OPM2 (C-D) MM cells. Mice were treated intravenously with B-B4–DM1 or control mAbs for 3 consecutive days. Tumor volume was assessed in 2 dimensions using an electronic caliper, and the volume was expressed in mm3 using the formula: V = 0.5a × b2, where a and b are the long and short diameters of the tumor, respectively. Tumor volume and survival were calculated as described in “Materials and methods.” * indicates that P value is related to the curve with ▪. (E-F) Mice bearing large OPM1 tumors (1309 ± 60 mm3) were treated intravenously with B-B4–DM1 (150 μg DM1/kg) for a total of 3 consecutive days. Figure shows a significant tumor regression 12 days following the beginning of treatment.
B-B4–DM1 inhibits tumor growth in a GFP+ human MM model in SCID mice
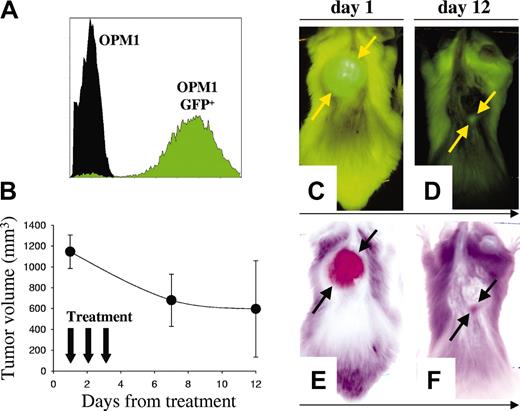
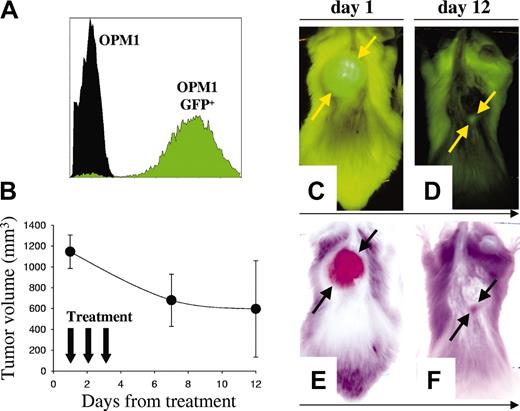
Labeling of tumor cells with reporter genes encoding fluorescent proteins42 is a sensitive method for in vivo detection of localized tumor growth43 as well as distant metastasis.42 We established a highly fluorescent clone of OPM1 MM cells (OPM1GFP+) (Figure 6A) and examined B-B4–DM1 antitumor in vivo activity with these cells. Mice were injected subcutaneously with OPM1GFP+ cells, followed by serial whole-body fluorescence imaging to assess development of GFP+ tumors. Mice were then treated intravenously for 3 consecutive days with B-B4–DM1 (150 μg DM1/kg; n = 5). As shown in Figure 6B-F, B-B4–DM1 induced regressions of GFP+ tumors.
Activity of B-B4–DM1 on GFP+ human MM xenografts. (A) Flow cytometric analysis of OPM1GFP+ cells indicates that these cells are highly fluorescent. (B) There were 5 animals injected with 5 × 106 OPM1GFP+ cells, monitored with whole-body fluorescence imaging for tumor development, and then treated with B-B4–DM1 (150 μg DM1/kg) for 3 consecutive days. Tumor sizes were determined directly by the fluorescent imaging of the GFP-expressing tumors. Error bars indicate SD. (C-D) Representative whole-body fluorescence images from a mouse treated with B-B4–DM1. (E-F) Negative images of the representative mouse. Figure shows a marked tumor regression 12 days after treatment.
Activity of B-B4–DM1 on GFP+ human MM xenografts. (A) Flow cytometric analysis of OPM1GFP+ cells indicates that these cells are highly fluorescent. (B) There were 5 animals injected with 5 × 106 OPM1GFP+ cells, monitored with whole-body fluorescence imaging for tumor development, and then treated with B-B4–DM1 (150 μg DM1/kg) for 3 consecutive days. Tumor sizes were determined directly by the fluorescent imaging of the GFP-expressing tumors. Error bars indicate SD. (C-D) Representative whole-body fluorescence images from a mouse treated with B-B4–DM1. (E-F) Negative images of the representative mouse. Figure shows a marked tumor regression 12 days after treatment.
B-B4–DM1 inhibits MM growth in SCID-hu hosts
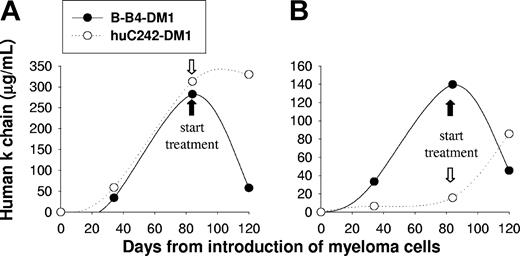
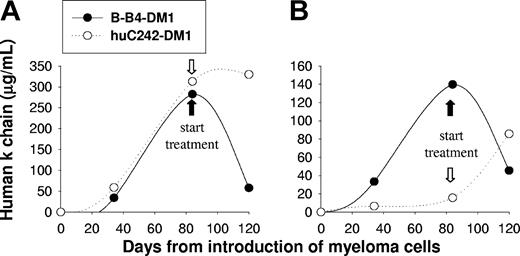
Since the SCID-hu model of MM mimics the pathologic behavior of the disease,36 we next studied the efficacy of B-B4–DM1 treatment in SCID-hu mice injected with patient MM cells. In these experiments, we studied the activity of the immunoconjugate on disease confined to the human fetal bone chip implanted subcutaneously in mice. There were 4 SCID-hu mice injected with patient MM cells (IgGκ), and tumor growth in the human bone environment was reflected by increasing serum κ chain levels. Mice were treated with either B-B4–DM1 (150 μg DM1/kg) or control huC242-DM1 (150 μg DM1/kg) for 3 consecutive days. As seen in Figure 7A-B, treatment with B-B4–DM1 induced a significant reduction of κ chain, whereas the human paraprotein continued to rise in mice treated with control antibody. These results confirm activity of B-B4–DM1 as an antitumor agent in SCID-hu models of human MM.
B-B4–DM1 reduces MM tumor burden in SCID-hu mice. Mice were bled at the indicated time points after inoculation of 5 × 106 cells from 2 different IgGk MM patients (A-B), monitored for changes in levels of human κ chain as an indicator of disease burden, and then treated with B-B4–DM1 (150 μg DM1/kg; n = 2) or control huC242-DM1 (150 μg DM1/kg; n = 2) for 3 consecutive days. Figure shows a marked reduction of κ levels after treatment with B-B4–DM1.
B-B4–DM1 reduces MM tumor burden in SCID-hu mice. Mice were bled at the indicated time points after inoculation of 5 × 106 cells from 2 different IgGk MM patients (A-B), monitored for changes in levels of human κ chain as an indicator of disease burden, and then treated with B-B4–DM1 (150 μg DM1/kg; n = 2) or control huC242-DM1 (150 μg DM1/kg; n = 2) for 3 consecutive days. Figure shows a marked reduction of κ levels after treatment with B-B4–DM1.
Discussion
Despite numerous advances in systemic and supportive treatments,44-47 few, if any, patients with MM are cured. There is therefore an urgent need for new treatment strategies. The recent approval of mAbs for clinical use against cancer3,4 has renewed interest in mAb-based therapies for hematologic, as well as solid tumor, malignancies. However, to date few studies have been reported for MM.
Since CD138 has been reported to be heterogeneously expressed in MM,17,37-40 we first re-examined CD138 expression on patient MM cells. By gene profiling, we found high CD138 mRNA expression in patient MM cells. Flow cytometric analysis of patient MM cells showed CD138 expression in approximately 72% of cases, consistent with previous reports showing CD138 expression between 60% and 100% of cases.17,21 By immunohistochemistry, CD138 has been reported to be a highly sensitive and specific marker of MM cells in BM biopsies.20 These data therefore support the potential value of CD138 as a target for immunotherapeutic approaches in MM.
An important issue is the pattern of CD138 expression in normal cells: within the hematopoietic compartment, CD138 is restricted to normal PCs and is not expressed on peripheral blood lymphocytes, monocytes, granulocytes, and red blood cells. Importantly, CD138 is not expressed on CD34+ hematopoietic stem cells and anti-CD138 mAbs do not affect colony formation in hematopoietic stem cell cultures.17 Treatment of BM cells with B-B4 conjugated to a highly cytotoxic ribosome-inactivating protein saporin did not decrease colony formation in hematopoietic precursor cells.48 In addition, immunohistochemical analysis of BM sections from MM patients49 showed CD138 expression exclusively on malignant PCs, but not on endothelial cells, stromal cells, or other hematopoietic elements, suggesting that mAb-based strategies can be used to deplete MM cells while sparing cells of the hematopoietic system for autografting.
It has been recently shown that clonogenic cells obtained from bone marrow of MM patients are CD138-/CD20+,50 while MM cells of a majority of patients are CD138+.17 These clonogenic cells are, possibly, precursors of MM cells, although the origin of MM cells has not been firmly established. These data suggest that, in addition to targeting CD138+ cells in MM patients, it may also be of benefit to target CD20+ cells. In this context, it is of interest that serotherapy with anti-CD20 mAb (Rituximab) achieved responses in 32% of previously treated patients with CD20+ MM cells.51
CD138 expression on MM cells was significantly higher than that on normal plasma cells. In nonhematopoietic compartments, syndecan-1 had been previously reported to be expressed on epithelia of many organs and weakly expressed on endothelial cells.48,52 We therefore performed an extensive IHC analysis, using paraffin slides and frozen sections, of multiple independent tissue samples from critical organs. We found that CD138 expression is restricted in normal tissues. Tissues staining with B-B4 included colon and esophageal surface epithelium, breast ductal epithelium, skin epidermis, and renal tubules. Due to the lack of reactivity of B-B4 with murine or cynomolgus monkey CD138, the issue of its systemic toxicity cannot be addressed in these models. B-B4–DM1 toxicity in humans can therefore be examined only in a dose-escalation phase 1 clinical trial. Several mAbs directed against epithelial antigens have been used in vivo to target malignancies of epithelial origin, and uptake of these antibodies by the tumors was higher than that by normal epithelia,53-55 indicating that target cell accessibility56 may vary in normal versus neoplastic tissues. In a clonogenic assay, a B-B4 immunotoxin was not effective in killing the CD138+ HepG2 hepatocyte cell line or normal endothelial cells, but was potent against the MM cell line RPMI8226.48
Early attempts at using mAbs to deliver conventional cytotoxic drugs were not successful, likely due to the low inherent cytotoxic potency of these drugs.57 Recently, a new generation of antibody-drug conjugates has been developed, which uses highly cytotoxic small drugs that are 100- to 1000-fold more potent than conventional chemotherapeutic agents.57 The FDA approval of gemtuzumab ozogamicin, the first antibody-drug conjugate of this type, has led to a resurgence of interest in developing mAb-based cancer treatments. The use of mAbs conjugated to another highly potent cytotoxic drug, the maytansine derivative DM1 used in this study, has the advantage of significant cytotoxic activity and limited systemic toxicity. Recently, immunoconjugates of DM1 with huC242 (huC242-DM1)25,58 or huN901 (huN901-DM1), reactive against a glycotope on MUC1 and against CD56 (NCAM), respectively, have been evaluated in phase 1 clinical studies14-16 in cancer patients. Both immunoconjugates showed biologic activity and limited side effects. These studies provide further support for clinical evaluation of DM1 immunoconjugates in MM.
In summary, B-B4–DM1 showed both in vitro and in vivo activity against CD138+ MM cell lines and patient MM cells. B-B4–DM1 was active in a SCID-hu mouse model, in which human MM cells proliferate in the human bone microenvironment. These data suggest that B-B4–DM1 can overcome MM cell growth, survival, and drug resistance within the BM milieu.41 B-B4–DM1 therefore represents a new generation of immunoconjugates with activity in both in vitro and in vivo preclinical models of MM, providing a preclinical rationale for clinical trials of B-B4–DM1 to improve patient outcome in MM.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-03-0963.
Supported by Multiple Myeloma Research Foundation Awards (M.A.S., N.C.M., and K.C.A.); National Institutes of Health (NIH) grants P50-100707, PO1-78378, and RO1-50947; the Leukemia and Lymphoma Society Scholar in Translational Research Award (N.C.M.); and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to colleagues at ImmunoGen, especially to Kate Connors and Barbara Leece for technical assistance, to Hongsheng Xie for helpful discussions, and to Walter Blättler for the critical reading of the manuscript.

![Figure 3. Inhibitory effect of B-B4–DM1 on proliferation of CD138+ and CD138- cells adherent to BMSCs. (A) Ocy-My5 or SUDHL-4 cells (2 × 104) were seeded on 70% to 80% confluent BMSCs for 24 hours. Cell proliferation was measured by [3H]thymidine ([3H]-TdR) incorporation following 72 hours of treatment with 10 nM B-B4–DM1. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures. Error bars indicate SD. (B) Patient MM cells were cultured on BMSC layers and exposed for 72 hours to the 10 nM B-B4–DM1 immunoconjugate. CD56 and CD138 expression was evaluated by flow cytometry. We have previously established that B-B4–DM1, even at a concentration as high as 240 nM, did not affect binding of PE-labeled anti-CD138 antibody (Syndecan-1 DL-101; Santa Cruz Biotechnology, Santa Cruz, CA) to CD138-expressing cells. Figure is representative of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-03-0963/5/m_zh80230470460003.jpeg?Expires=1769281813&Signature=4x3FATZ2cv8lEBaDQuPbV-j0AZJF7n5UNYn1xdWTQRhxg3~EuZkDGR4zqnI-D9FhVPSxwdVK-E1XcAGA4vPYUhrsUaPZPNmR0bZ0gGZiVE8Tq1xhw0ojpfKL19Ljx-LFuzGqAw-veqncYQWX6r6qKik3lpeZE87o27hJYVGAQ8NXkZ~nGJaaGcanx2qFMnSc5UGj4y7fA4JGTute9PvwYHWCRbv7C6F979NAB3mIWDTLfvARkGvvhDAvptIhDWO2F4MUIv5YMjJnDo-y3vZPI4A3hUyslttcHS9W-PDa-luBveSH0Ocsl9LdoxZBSsS6mScf8xlcJEJnxLUg61r4cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Inhibitory effect of B-B4–DM1 on proliferation of CD138+ and CD138- cells adherent to BMSCs. (A) Ocy-My5 or SUDHL-4 cells (2 × 104) were seeded on 70% to 80% confluent BMSCs for 24 hours. Cell proliferation was measured by [3H]thymidine ([3H]-TdR) incorporation following 72 hours of treatment with 10 nM B-B4–DM1. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures. Error bars indicate SD. (B) Patient MM cells were cultured on BMSC layers and exposed for 72 hours to the 10 nM B-B4–DM1 immunoconjugate. CD56 and CD138 expression was evaluated by flow cytometry. We have previously established that B-B4–DM1, even at a concentration as high as 240 nM, did not affect binding of PE-labeled anti-CD138 antibody (Syndecan-1 DL-101; Santa Cruz Biotechnology, Santa Cruz, CA) to CD138-expressing cells. Figure is representative of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-03-0963/5/m_zh80230470460003.jpeg?Expires=1769281814&Signature=BlrYqz7FE5ragGSRSsKtcaFxTzc5D1xIaqwZ6jcn-I2L-HxnCkf53se9elGsz3WyqdNOy31igiL-ZV4RD6gZ5binbkfDjUVh5NjitIpyOfSV55YsoZModyrw71763JuZizfr5r1TndNS3f9R54u6Qe5Gc0lz4YaLfksP1FaARAQCHqTM9gfAGd6~ktwtIR5JTwEaOwW18xD8qSWna0WMcPuzRpKWjcwpylMozALHN4lQ1KciB~Rw1cqSSGor-nGlDL5DYGshlO3dq8jc5aSAjQpV~lxPs1jxJ4vpCpAOsaKL0iki97ydx~WY9kHvEemabVQgl1uc12YKVB78JQLBcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)