Bone marrow (BM) cells are reported to contribute to the process of regeneration following myocardial infarction. However, the responsible BM cells have not been fully identified. Here, we used 2 independent clonal studies to determine the origin of bone marrow (BM)–derived cardiomyocytes. First, we transplanted single CD34– c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells or whole BM cells from mice ubiquitously expressing enhanced green fluorescent protein (EGFP) into lethally irradiated mice, induced myocardial infarction (MI), and treated the animals with granulocyte colony-stimulating factor (G-CSF) to mobilize stem cells to the damaged myocardium. At 8 weeks after MI, from 100 specimens we counted only 3 EGFP+ actinin+ cells in myocardium of CD34– KSL-SP cells in mice that received transplants, but more than 5000 EGFP+ actinin+ cells in whole BM cell in mice that received transplants, suggesting that most of EGFP+ actinin+ cells were derived from nonhematopoietic BM cells. Next, clonally purified nonhematopoietic mesenchymal stem cells (MSCs), cardiomyogenic (CMG) cells, that expressed EGFP in the cardiomyocyte-specific manner were transplanted directly into BM of lethally irradiated mice, MI was induced, and they were treated with G-CSF. EGFP+ actinin+ cells were observed in the ischemic myocardium, indicating that CMG cells had been mobilized and differentiated into cardiomyocytes. Together, these results suggest that the origin of the vast majority of BM-derived cardiomyocytes is MSCs.
Introduction
Recent studies have suggested that bone marrow (BM) cells can contribute to regeneration processes in various tissues.1,2 Cardiomyocytes derived from BM cells have been observed after myocardial infarction (MI),3,4 and BM-derived cells mobilized by cytokines were capable of regenerating the myocardial tissue, leading to an improvement in survival and cardiac function after MI.5 BM contains both hematopoietic and nonhematopoietic cells, and the origin of the BM cells with the ability to repair damaged myocardial tissue remains unknown. The identification of specific cell types involved in myocardial repair is a crucial step toward the development of effective stem cell–based therapies for MI.
The most likely candidates for the BM-derived stem cells with the ability to regenerate myocardial tissue are hematopoietic stem cells (HSCs)3-5 and nonhematopoietic mesenchymal stem cells (MSCs).6-10 Alvarez-Dolado et al recently demonstrated that BM-derived cardiomyocytes sporadically detected in noninfarcted mice were exclusively generated by fusion with donor CD45+ cells, possibly hematopoietic cells,11 suggesting that the so-called phenomenon of HSC “plasticity” might result from the fusion of HSCs with cells residing in the target tissue. Two recent studies reported that HSCs are unable to differentiate into cardiomyocytes in vivo even after MI.12,13 MSCs also are candidates for the regeneration of cardiomyocytes in vivo; we previously reported that BM-derived MSCs could differentiate into spontaneously beating cardiomyocytes in vitro,6-8 and other groups have repaired the myocardium using MSC transplantation in vivo.9,10
The aim of this study is to determine the precise origin of the BM cells mobilized by cytokines to repair infarcted myocardium. We transplanted genetically marked single HSCs or clonal MSCs into lethally irradiated recipient mice, induced MI, treated the mice with granulocyte colony-stimulating factor (G-CSF), and analyzed the cardiac tissues. Our results suggest that MSCs are the predominant source of regenerated cardiomyocytes.
Materials and methods
Mice
C57BL/6 (B6) mice and C3H/He (C3H) mice were purchased at 6 to 8 weeks of age from Japan CLEA (Tokyo, Japan). B6 transgenic mice that ubiquitously expressed enhanced green fluorescent protein (EGFP) under the control of the CAG promoter14 were used as the BM donor in the transplantation studies using B6 mice. All the transgenic EGFP mice used in this study were heterozygous for the transgene.
Isolation of CD34–c-kit+Sca-1+ lineage– SP cells (CD34–KSL-SP cells)
The isolation of the CD34–c-kit+Sca-1+ lineage– SP cells was previously described.15 Briefly, BM cells suspended at 1 × 106 cells/mL in HBSS+ (calcium- and magnesium-free Hanks balanced salt solution supplemented with 2% fetal calf serum [FCS], 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 100 U/mL penicillin, and 100 μ/mL streptomycin) were incubated with 5 mg/mL Hoechst 33342 (Sigma Aldrich, St Louis, MO) for 60 minutes at 37°C. After washing, the cells were resuspended in ice cold HBSS+ at a cell density of 107 cells/mL and then stained for 30 minutes on ice with various monoclonal antibodies, including biotinylated CD34, allophycocyanin (APC)–conjugated c-kit, phycoerythrin (PE)–conjugated Sca-1, and PE-Cy5–conjugated lineage markers (Gr-1, Mac-1, B220, CD3, TER119). The biotinylated antibodies were visualized using PharRed (APC-Cy7)–conjugated streptavidin. All of these reagents were purchased from BD PharMingen (San Diego, CA). Cell sorting was performed using a triple laser MoFlo (Cytomation, CO) using Summit software. Hoechst 33342 was excited at 350 nm, and the fluorescence emission was detected using 405/BP30 and 570/BP20 optical filters against Hoechst blue and Hoechst red, respectively, and a 555-nm long-pass dichroic mirror (Omega Optical, Brattleboro, VT) to separate the emission wavelengths. Both Hoechst blue and red fluorescence were shown on a linear scale. After collecting 105 events, the side population (SP) was defined as described previously, and additional gates were defined as positive for Sca-1 and c-kit and negative for CD34 and lineage markers according to the isotype control fluorescence intensity. Populations of CD34–KSL-SP cells with 99% purity were routinely prepared using this method. Single CD34–KSL-SP cells derived from transgenic EGFP mice were sorted directly into separate wells of a 96-well plate containing 100 μL HBSS+ using a CyClone automated cell deposition unit.
HSC transplantation
Single CD34–KSL-SP cells or 5 × 106 whole BM cells from transgenic EGFP mice were injected intravenously into the retro-orbital plexus of etherized recipient B6 mice that had been lethally irradiated with a dose of 10.5 Gy (Figure 1A). For radioprotection, the single CD34–KSL-SP cells were transplanted along with 2 × 105 whole BM cells as radioprotective cells from B6 mice that did not carry the CAG-EGFP transgene. Three months after BM transplantation, peripheral blood samples were collected from the recipient mice, and the erythrocytes in the samples were depleted using Ficoll-Paque. Dual-laser fluorescence-activated cell-sorter scanner (FACS) analysis was performed using a FACS Calibur (Becton Dickinson, San Jose, CA). Donor BM–derived cells were determined by the fluorescent intensity of the EGFP, compared with wild-type cells, and mice engrafted with more than 50% of the EGFP+ cells were used for the experiments.
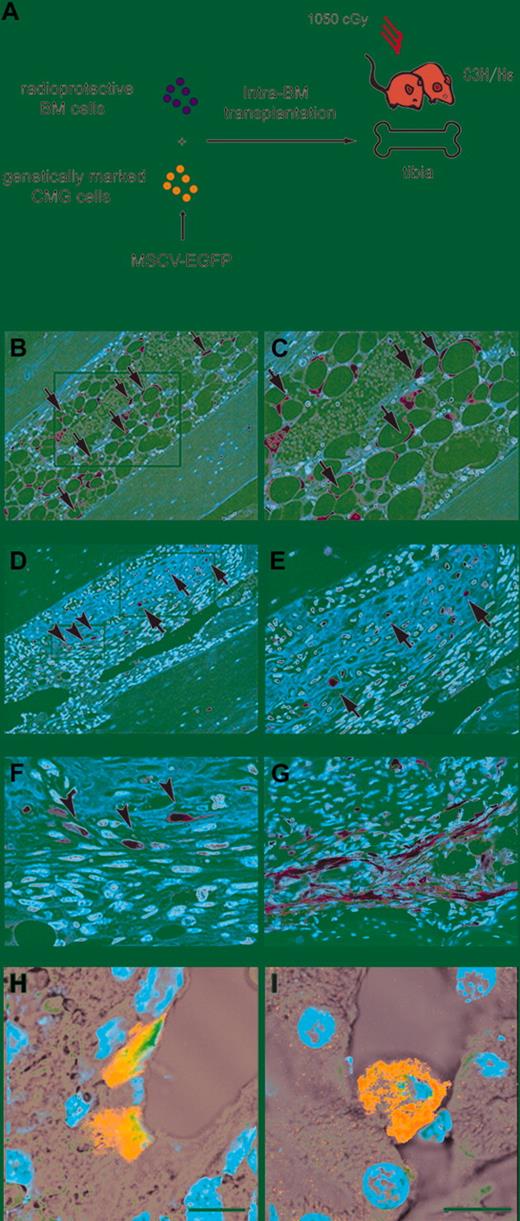
G-CSF–induced mobilization of BM cells in mice receiving transplants of single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells or whole BM cells following MI. (A) Experimental protocol. Single EGFP+ CD34–KSL-SP cells and radioprotective EGFP– BM cells were transplanted into the recipient mice. (B) The nucleated cell counts (NCCs) for peripheral blood samples obtained 24 hours after the last injection of G-CSF (n = 10) or saline (n = 10) are shown. ▪ indicates EGFP+ cells; ▨, EGFP– cells. NS indicates not significant; *P < .0001. Bars indicate the standard error. (C-F) Panels show representative results for immunofluorescent analysis using anti–GFP antibody and TOTO-3 dye in the hearts of mice receiving translplants of w-BM cells or single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells separated from the BM of transgenic EGFP mice. The green and blue signals indicate EGFP and nuclei, respectively. Saline-treated mice (G-CSF[–] mice) (C) and G-CSF–treated mice (G-CSF (+) mice) (D) in the w-BM group. G-CSF(–) mice (E) and G-CSF (+) mice (F) in the CD34–KSL-SP group. Bars indicate 200 μm. (G) The control experiment using C57BL/6J mice as donors revealed that no green fluorescence was detected in the whole heart. Bar indicates 50 μm. (H-J) Coimmunostaining with anti-GFP and anti–actinin antibodies in the infarcted myocardium of G-CSF(+) mice transplanted with w-BM (H, I) or single CD34–KSL-SP cells (J). The green, red, and blue signals indicate EGFP, actinin, and nuclei, respectively. (H, I) In the w-BM group, some of the EGFP+ cells in the infarcted area were positive also for actinin and showed striation, indicating that they had differentiated into cardiomyocytes. (J) In contrast, very few EGFP+ actinin+ cells were seen in the infarcted area in the CD34–KSL-SP group. Bars indicate 50 μm (H, I) and 20 μm (J).
G-CSF–induced mobilization of BM cells in mice receiving transplants of single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells or whole BM cells following MI. (A) Experimental protocol. Single EGFP+ CD34–KSL-SP cells and radioprotective EGFP– BM cells were transplanted into the recipient mice. (B) The nucleated cell counts (NCCs) for peripheral blood samples obtained 24 hours after the last injection of G-CSF (n = 10) or saline (n = 10) are shown. ▪ indicates EGFP+ cells; ▨, EGFP– cells. NS indicates not significant; *P < .0001. Bars indicate the standard error. (C-F) Panels show representative results for immunofluorescent analysis using anti–GFP antibody and TOTO-3 dye in the hearts of mice receiving translplants of w-BM cells or single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells separated from the BM of transgenic EGFP mice. The green and blue signals indicate EGFP and nuclei, respectively. Saline-treated mice (G-CSF[–] mice) (C) and G-CSF–treated mice (G-CSF (+) mice) (D) in the w-BM group. G-CSF(–) mice (E) and G-CSF (+) mice (F) in the CD34–KSL-SP group. Bars indicate 200 μm. (G) The control experiment using C57BL/6J mice as donors revealed that no green fluorescence was detected in the whole heart. Bar indicates 50 μm. (H-J) Coimmunostaining with anti-GFP and anti–actinin antibodies in the infarcted myocardium of G-CSF(+) mice transplanted with w-BM (H, I) or single CD34–KSL-SP cells (J). The green, red, and blue signals indicate EGFP, actinin, and nuclei, respectively. (H, I) In the w-BM group, some of the EGFP+ cells in the infarcted area were positive also for actinin and showed striation, indicating that they had differentiated into cardiomyocytes. (J) In contrast, very few EGFP+ actinin+ cells were seen in the infarcted area in the CD34–KSL-SP group. Bars indicate 50 μm (H, I) and 20 μm (J).
Myocardial infarction and mobilization of BM cells
The recipient mice were intubated and anesthetized with 0.5% isofluorane gas. After left thoracotomy, the left ventricle was exposed and the left coronary artery was ligated. Twenty-four hours later, the mice were subcutaneously injected with 300 μg/kg of recombinant human G-CSF dissolved in saline solution once a day for 10 consecutive days. Control mice received a saline solution without G-CSF.
Immunohistological studies
Hearts were perfused from the apex with phosphate-buffered saline (PBS) and perfusion fixed with 4% paraformaldehyde/PBS. They were then dissected, immersion fixed overnight at 4°C in 4% paraformaldehyde, embedded in Optimal Cutting Temperature (OCT) Compound (TED PELLA, Redding, CA), and quickly frozen in liquid nitrogen. Cryostat sections (6 μm thick) were stained overnight at 4°C using specific antibodies. Anti-GFP (MBL, Nagoya, Japan), anti–α-actinin (Sigma Aldrich), anti–sarcomeric myosin (MF20), anti–cardiac troponin I (Santa Cruz Biotechnology, Santa Cruz, CA), anti-GATA4 (Santa Cruz), and anti-CD45 (BD Pharmingen, San Diego, CA) were used to identify EGFP+ cells, cardiomyocytes, and hematopoietic cells. The sections were incubated with secondary antibodies conjugated with Alexa 488 or 594 (Molecular Probes, Eugene, OR). Nuclei were stained with TOTO-3 (Molecular Probes). Slides were observed under a confocal laser scanning microscope (LSM 510 META; Carl Zeiss, Jena, Germany). In some experiments, the liver, kidney, brain, skeletal muscle, and spleen of recipient mice also were analyzed by immunofluorescence photography. To observe the bone marrow cavity, bone tissue was fixed with 10% formaldehyde and decalcified in K-CX solution (Falma, Tokyo, Japan) at 4°C for 2 days. Sections (3 μm) were incubated with anti–GFP antibody overnight at 4°C. A specific signal was visualized as a brown reaction product of peroxidase substrate 3,3′-diaminobenzidine (Sigma). Samples were counterstained with hematoxylin and eosin.
Adherent cell culture and immunocytochemistry
Twelve weeks after the transplantation of the single CD34–KSL-SP cells or w-BM cells from transgenic mice that ubiquitously express EGFP,14 peripheral blood samples were collected from the retro-orbital plexus of the recipients. If more than 80% of the donor-derived EGFP+ cells were found in the peripheral blood, the mice were killed and the BM cells were flushed from the femurs and tibiae to prepare single-cell suspensions. After most of the erythrocytes were removed with Ficoll-Paque, 3 × 105 BM mononuclear cells were plated on the fibronectin-coated wells of 96-well plates (Costar, Cambridge, United Kingdom) and incubated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a fully humidified atmosphere of 5% CO2. The medium was exchanged every 2 to 3 days, and nonadherent cells were removed. The cultures were terminated 2 weeks after seeding by removal of the culture medium. The samples were flushed 3 times with PBS and fixed with 4% paraformaldehyde, then stained with rabbit anti–GFP antibody (1:1000 dilution, Molecular Probes) and visualized using a secondary antibody of Alexa Fluor 488–conjugated goat anti–rabbit IgG (1:1000 dilution, Molecular Probes). The specimens were also stained with PE-conjugated rat anti–mouse CD45 (1:1000 dilution; eBioscience, San Diego, CA) and Hoechst 33342 (Sigma Aldrich). The adherent cells in each plate were counted under a fluorescent microscope.
Differentiation cultures for osteoblasts and adipocytes
To induce osteocyte differentiation, the adherent cells were cultured on slides in DMEM with 10 mM β-glycerophosphate, 10–7 M dexamethasone, and 0.2 mM ascorbic acid (all from Sigma Aldrich); the media was changed every 3 to 4 days. After 14 days, osteoblast differentiation was confirmed using alkaline phosphatase enzymatic staining. For the alkaline phosphatase staining, cells were fixed with methanol at –20°C for 2 minutes and then washed in 100 mM Tris [tris(hydroxymethyl)aminomethane]-HCl (pH 9.5), 100 mM NaCl, and 10 mM MgCl2 buffer (all from Sigma Aldrich) for 10 minutes. The slides were then stained with fast 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium alkaline phosphatase substrate (both from Sigma Aldrich) for 10 minutes and rinsed in distilled water. To induce adipocyte differentiation, the adherent cells were cultured with 10% horse serum or 100 ng/mL insulin and were maintained for 14 days on the slides, with medium exchanges every 3 to 4 days. After 14 days, we confirmed the differentiation of the cells to lipid-laden adipocytes by staining with oil-red. For the oil-red staining, the cells were fixed with methanol at –20°C for 2 minutes and then rinsed in 50% alcohol. Slides were stained in oil-red-O (Sigma Aldrich) for 10 minutes and rinsed in 50% alcohol. After rinsing in distilled water, slides were counterstained with Mayer hematoxylin (Sigma Aldrich) for 1 minute.
Transduction of CMG cells
CMG cells derived from C3H mice were cultured in Iscoves modified Dulbecco medium supplemented with 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 85 μg/mL amphotericin B on fibronectin-coated dishes at 33°C in 5% CO2, as previously described.6 In the experiments shown in Figure 2A, the CMG cells were transduced with the recombinant ecotropic retrovirus murine stem cell virus (MSCV)-EGFP16 at multiplicity of infection (MOI) 10, and the EGFP+ cells were sorted using a FACS Vantage (Becton Dickinson, Cockeysville, MD), as previously described.17 In the experiments shown in Figure 3A, an expression vector, pMLC2v-EGFP, was constructed by cloning a 2.7-kb HindIII-EcoRI fragment of the rat MLC-2v promoter region18,19 into the HindIII-EcoRI site of pEGFP-1 (Clontech, Palo Alto, CA) so that EGFP would be expressed under the control of the MLC-2v promoter. The MLC2v-EGFP plasmid was then transfected into the CMG cells by liposomal transfection. After culturing with 1000 μg/mL of G418 for 4 weeks, permanently transfected single cell–derived colonies were cloned and pooled (CMG-ME cells). For in vitro differentiation, CMG-ME cells were plated onto fibronectin-coated dishes and treated with 3 μmol/L of 5-azacytidine (Sigma Aldrich) for 24 hours.
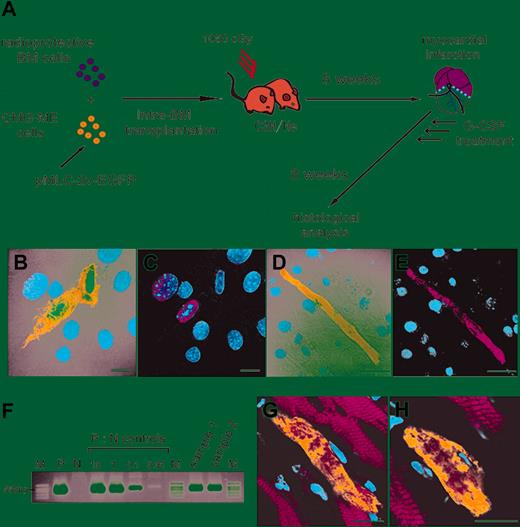
Engraftment and multilineage differentiation of clonal mesenchymal stem cells (CMG cells). (A) The experimental protocol for the intra-BM transplantation of CMG cells transfected with MSCV-EGFP is shown. (B-G) Transplanted CMG cells were observed in the recipients' BM. CMG cells were recognized as brown cells by immunohistochemistry. (B) CMG cells differentiated into adipocytes (arrows) (magnification, × 100). The rectangle in panel B is shown at a higher magnification (× 200) in panel C. (D) CMG cells also differentiated into osteocytes (arrows). Undifferentiated CMG cells exhibiting spindlelike shapes were also observed (arrowheads) (magnification, × 100). The rectangles in panel D are shown at higher magnifications in panels E (× 200) and F (× 400). (G) Undifferentiated CMG cells in other part (× 200). (H, I) CMG cells also were found in the liver (H) and kidney (I) by immunofluorescence photography. Bars indicate 10 μm. The green and blue signals indicate EGFP and nuclei, respectively.
Engraftment and multilineage differentiation of clonal mesenchymal stem cells (CMG cells). (A) The experimental protocol for the intra-BM transplantation of CMG cells transfected with MSCV-EGFP is shown. (B-G) Transplanted CMG cells were observed in the recipients' BM. CMG cells were recognized as brown cells by immunohistochemistry. (B) CMG cells differentiated into adipocytes (arrows) (magnification, × 100). The rectangle in panel B is shown at a higher magnification (× 200) in panel C. (D) CMG cells also differentiated into osteocytes (arrows). Undifferentiated CMG cells exhibiting spindlelike shapes were also observed (arrowheads) (magnification, × 100). The rectangles in panel D are shown at higher magnifications in panels E (× 200) and F (× 400). (G) Undifferentiated CMG cells in other part (× 200). (H, I) CMG cells also were found in the liver (H) and kidney (I) by immunofluorescence photography. Bars indicate 10 μm. The green and blue signals indicate EGFP and nuclei, respectively.
Mobilization and differentiation of mesenchymal stem cells (CMG cells) into cardiomyocytes in vitro and in vivo. (A) The experimental protocol is shown. The CMG-ME cells are CMG cells that have been permanently transfected with a plasmid-encoding EGFP driven by the myosin light chain promoter (pMLC2v-EGFP). (B,C) Cultured CMG-ME cells were treated with 5-azacytidine and immunoassayed with anti-GATA4 (red) antibody on the seventh day of culture. GATA4 was expressed in the CMG-ME cells. Some of the CMG-ME cells became EGFP+ (green). (D,E) At 3 weeks, CMG-ME cells that were positive for both EGFP and actinin were observed, indicating that the cells had differentiated into cardiomyocytes. (F) The engraftment of CMG-ME cells into the recipients' BM was confirmed by PCR. Representative results using BM samples collected from recipient mice (samples 1 and 2) are shown. The transgene was clearly detected in the 2 samples. P indicates positive control (CMG-ME cells); N, negative control (CMG cells); M, marker. P:N controls are shown as the percentage with respect to the positive control. (G,H) The myocardium of infarcted mice that received transplants of CMG-ME cells was analyzed using immunofluorescent microscopy. The green, red, and blue signals indicate EGFP, actinin, and nuclei, respectively. EGFP+ actinin+ CMG-ME cells were observed in the myocardium, indicating that the CMG-ME cells had been mobilized into the ischemic myocardium. Actinin and EGFP expression, driven by the MLC-2v promoter, was detected, indicating that the cells had differentiated into cardiomyocytes. Bars indicate 10 μm.
Mobilization and differentiation of mesenchymal stem cells (CMG cells) into cardiomyocytes in vitro and in vivo. (A) The experimental protocol is shown. The CMG-ME cells are CMG cells that have been permanently transfected with a plasmid-encoding EGFP driven by the myosin light chain promoter (pMLC2v-EGFP). (B,C) Cultured CMG-ME cells were treated with 5-azacytidine and immunoassayed with anti-GATA4 (red) antibody on the seventh day of culture. GATA4 was expressed in the CMG-ME cells. Some of the CMG-ME cells became EGFP+ (green). (D,E) At 3 weeks, CMG-ME cells that were positive for both EGFP and actinin were observed, indicating that the cells had differentiated into cardiomyocytes. (F) The engraftment of CMG-ME cells into the recipients' BM was confirmed by PCR. Representative results using BM samples collected from recipient mice (samples 1 and 2) are shown. The transgene was clearly detected in the 2 samples. P indicates positive control (CMG-ME cells); N, negative control (CMG cells); M, marker. P:N controls are shown as the percentage with respect to the positive control. (G,H) The myocardium of infarcted mice that received transplants of CMG-ME cells was analyzed using immunofluorescent microscopy. The green, red, and blue signals indicate EGFP, actinin, and nuclei, respectively. EGFP+ actinin+ CMG-ME cells were observed in the myocardium, indicating that the CMG-ME cells had been mobilized into the ischemic myocardium. Actinin and EGFP expression, driven by the MLC-2v promoter, was detected, indicating that the cells had differentiated into cardiomyocytes. Bars indicate 10 μm.
Intra-BM transplantation of CMG cells
One-hundred thousand CMG cells transduced with MSCV-GFP or CMG-ME cells were transplanted into the right tibia of lethally irradiated C3H mice by intra-BM injection using a previously described method.20 Two-hundred thousand whole BM cells separated from C3H mice also were injected into the recipient mice to serve as radioprotective cells. When the mice were killed, the engraftment of the CMG-ME cells and the CMG cells that were transduced with MSCV-GFP was confirmed by polymerase chain reaction (PCR) and enzyme immunohistochemistry, respectively.
PCR analysis
The presence of the transgene was detected by PCR. The following primers were designed against the EGFP cDNA (5′ primer: 5′-CCAGTTCAGCGTGTCCGGCG-3′; 3′ primer: 5′-GGGGTCTTTGCTCAGGGCGG-3′). The PCR conditions have been previously described.17
Statistics
All values are presented as the mean ± SEM. Statistical significance was evaluated using unpaired Student t tests for comparisons between 2 mean values. Multiple comparisons between more than 3 groups were performed using an ANOVA. A value of P less than .05 was considered significant.
Results
Single HSC progeny are not a major source of new cardiomyocytes after cytokine-enhanced post-MI repair
In a previous report, we demonstrated that a single-cell transplantation analysis using cells that had the strongest dye-efflux activity (“Tip”-SP cells) with a phenotype of CD34–c-Kit+Sca-1+Lin– (CD34–KSL-SP) exhibited nearly complete (∼ 96%) level of hematopoietic engraftment activity.15 To first examine the contribution of BM-derived HSCs to the regeneration of cardiomyocytes after MI, we transplanted either whole BM cells (w-BM) or single CD34–KSL-SP cells from transgenic EGFP mice into irradiated mice, induced MI, treated the mice with G-CSF daily for 10 days to mobilize the BM cells to the heart, and then examined the hearts using histologic methods (Figure 1A). Eight weeks after transplantation, the mean percentages of EGFP+ cells among the peripheral blood nucleated cells of the mice transplanted with w-BM and that of the mice transplanted with single CD34–KSL-SP cells were 87% ± 3% (n = 10) and 63% ± 10% (n = 10), respectively. The peripheral blood nucleated cell counts (NCCs), measured 24 hours after the last injection of G-CSF, were significantly higher in the G-CSF–treated (G-CSF[+]) mice than in saline-injected (G-CSF[–]) mice (Figure 1B). No significant difference in the NCC of G-CSF(+) mice was observed between the w-BM group and the CD34–KSL-SP group.
A histologic analysis performed 8 weeks after the MI showed a markedly higher degree of EGFP+ cell infiltration into the infarcted area in G-CSF(+) mice than in G-CSF(–) mice (Figure 1C-F; Table 1). Among the G-CSF(+) mice, a greater degree of infiltration was seen in the w-BM group than in the CD34–KSL-SP group. Nonspecific autofluorescence was distinguished as reported previously.21 As a control, the same experiment was performed using wild C57BL/6 mice–derived BM cells as donor cells for BM transplantation. Figure 1G shows that no green fluorescence was observed in the infarct area of control mice, thus, G-CSF treatment–induced augmentation of green fluorescence was not due to nonspecific fluorescence. The presence of the EGFP gene in the infarcted area also was confirmed by PCR analysis (data not shown).
In the w-BM group, some of the EGFP+ cells in the ischemic area expressed actinin and showed striations, suggesting that they were cardiomyocytes (Figure 1H-I; Table 1). Some EGFP+ cells also expressed sarcomeric myosin or cardiac troponin I (data not shown). Very few EGFP+ actinin+ cells were seen in the 2 CD34–KSL-SP groups (Figure 1J; Table 1), which was consistent with previous reports.12,13 The marked increase in EGFP+ actinin+ cells in the w-BM groups cannot be explained simply by the superiority of hematopoietic chimerism in the w-BM group (87% ± 3% vs 63% ± 10%) if regenerated cardiomyocytes were derived from HSCs. Collectively, these results suggest that the major population of cells mobilized from the BM and active in the regeneration of cardiomyocytes was nonhematopoietic in origin.
HSCs have little potency to differentiate into a mesenchymal lineage
Next, we compared the nonhematopoietic BM cell populations of mice transplanted with single CD34–KSL-SP cells or w-BM cells from transgenic EGFP mice. We cultured the BM cells from both groups of mice (n = 5 per group) with a high chimerism (> 80%) of EGFP+ donor cells using fibronectin-coated, 96-well plates. After 14 days of culture, the adherent cells were stained with anti-GFP and anti-CD45, and the frequency of donor-derived nonhematopoietic cells was determined using 3 specimens from each mouse BM. A significant proportion of the adherent cells from the BM of the w-BM group was positive for EGFP (EGFP+CD45– mononuclear cells = 22.38%).
On the other hand, most of the adherent cells from the BM of the CD34–KSL-SP group were negative for EGFP (EGFP+CD45– mononuclear cells = 0.8%, P < .001). These adherent cells were further cultured in either adipocyte- or osteocyte-inducing conditions for another 14 days; 200 adipocytes and 200 osteocytes among the adherent cells of the CD34–KSL-SP group were counted, but no EGFP+ cells were found (data not shown). These results suggest that purified HSCs have very little potency to differentiate into a mesenchymal lineage, while MSCs are feasible candidates for the source of regenerated cardiomyocytes among nonhematopoietic BM cells.6-10 Based on these results, we focused on the contribution of MSCs in w-BM in the regeneration of cardiomyocytes after MI.
Clonal MSC progeny contribute to the regeneration of cardiomyocytes after cytokine-enhanced post-MI repair
Unlike HSCc, MSCs are difficult to purify prospectively according to surface phenotypes. Therefore, to assess the contribution of BM-derived MSCs to the regeneration of cardiomyocytes after MI, we designed a series of experiments using cardiomyogenic (CMG) cells,6 a clonally isolated cell line of MSCs (Figures 2A and 3A). CMG cells can differentiate into spontaneously beating cardiomyocytes in vitro after exposure to 5-azacytidine. Since cultured MSCs are known to lose their BM homing ability,22 we first confirmed that the CMG cells, tagged by transfection with the MSCV-EGFP retrovirus vector,16 could be engrafted into the recipients' BM (n = 5) by intra-BM injection,20 rather than intravenous injection, and differentiate into multiple lineages in a manner similar to that of authentic MSCs (Figure 2A). We detected EGFP+ cells in the BM, most of which had differentiated into adipocytes and osteocytes (Figure 2B-F), and we also found a small number of cells residing among the stromal cells (Figure 2D, F, G). We detected EGFP+ cells in several other organs, although in very low numbers (4, 5, 0, 0, 0, and 0 EGFP+ cells per 100 specimens [20 specimens per mouse] in the liver, kidney, heart, brain, skeletal muscle, and spleen, respectively) (Figure 2H-I).
To detect cardiomyocytes derived from CMG cells with a higher sensitivity and specificity, cells transfected with a pMLC2v-EGFP plasmid encoding EGFP driven by the myosin light chain promoter (CMG-ME cells) were prepared by selection with G418 and then treated with 5-azacytidine. Ten days after treatment, 6% of the cells were positive for EGFP and negative for actinin but expressed GATA4 (Figure 3B-C). After 3 weeks, most of the EGFP+ cells became actinin positive and developed a rod-like appearance (Figure 3D-E); at 4 weeks, they exhibited spontaneous beating. These findings demonstrate that the CMG-ME cells that were committed to cardiomyocytes could be detected by the expression of EGFP in this system.
Uncommitted CMG-ME cells then were transplanted directly into the BM of lethally irradiated C3H/He (C3H) mice, MI was induced, and the mice were treated with G-CSF (Figure 3A). Eight weeks after induction of MI, the mice (n = 5) were killed, and PCR analysis confirmed the engraftment of CMG-ME cells in the BM of all recipients (Figure 3F). The myocardium of the 5 mice was analyzed using immunofluorescence, and a total of 1034 EGFP+ actinin+ cells in 100 samples (20 samples per mouse) were observed. The number of EGFP+ actinin+ cells from each group of 20 samples from each mouse was 253, 92, 135, 327, and 227. This result indicated that CMG-ME cells mobilized from the BM contributed to the regeneration of the myocardium after MI (Figure 3G-H). The presence of the EGFP gene in the infarcted area also was confirmed by PCR analysis (data not shown). Although the number of detected cells was lower than that observed in mice transplanted with whole BM, CMG-derived cardiomyocytes were clearly visible in the ischemic myocardium. We did not detect EGFP+ cells in any other organs such as liver, kidney, skeletal muscle, and BM (data not shown). These results suggested that the myosin light chain promoter restricted EGFP expression to cardiac tissue.
Discussion
Although BM-derived cells were reported to differentiate into cardiomyocytes after MI,3-5 the identity of specific cell types involved has not been determined. There are 2 possibilities: first, BM-derived HSCs trans-differentiated into cardiomyocytes. Second, other cell types, such as MSCs, regenerated cardiomyocytes. To address this issue, we performed 2 independent clonal analyses to test which cell types contribute to cardiomyocyte regeneration. The results suggest that MSCs, but not HSCs, in BM play a major role in the regeneration of myocardial tissue after MI.
Purified HSCs and their progeny rarely contributed to the regeneration of cardiomyocytes, even with G-CSF treatment after single-cell transplantation following MI induction. On the other hand, w-BM transplantation resulted in marked increase of the donor-derived cardiomyocytes after MI and subsequent treatment with G-CSF. The results suggest that the repopulation of cardiomyocytes was achieved by nonhematopoietic cells in BM rather than HSCs and their progeny. In addition, we did not detect any EGFP+ MSCs in the CD34–KSL-SP group, while a precise analysis indicated that around 90% of the MSCs, assayed as fibroblastic colony-forming units (CFU-Fs), were derived from donor mice in the w-BM transplantation model when the recipient mice received more than 7 Gy of total body irradiation.22 These results led us to focus on the nonhematopoietic population of MSCs in BM as a candidate for the donor-derived cardiomyocytes.
While HSCs can be purified prospectively according to their surface phenotypes, such purification is difficult to achieve for MSCs. Therefore, we used a cell line of MSCs to clonally identify the origin of BM-derived cardiomyocytes after MI. CMG cells, previously established from MSCs in the BM of C3H mice, can differentiate into spontaneously beating cells with a fetal ventricular cardiomyocyte phenotype in vitro after exposure to 5-azacytidine.6 Since only a small fraction of these cells differentiate into cardiomyocytes and CMG cells were sporadically found in other organs after intra-BM injection (Figure 2H-I), we labeled the CMG cells to improve specific detection. Although the number of detected cells was smaller than that observed in mice that received transplants of whole BM, CMG-derived cardiomyocytes were clearly detected in the ischemic myocardium; these results are the first evidence that CMG cells can differentiate into cardiomyocytes in the cardiac niche without 5-azacytidine treatment. Most of the CMG cells that were transplanted directly into the BM differentiated into bone tissue (Figure 2D-E). This is most likely because insertion of the needle into the BM cavity stimulated osteogenesis23 ; such activity may account for the small number of CMG-derived cardiomyocytes. Systemic intravenous delivery of CMG may obviate this loss of CMG cells to osteogenesis and improve cardiomyocyte differentiation.24 However, the intravenous delivery of cultured MSCs to BM is limited by either a defect in the homing ability of the cells22 or the entrapment of the cells in the lungs.25 A limitation of these experiments is that the CMG cell population isolated in vitro might not be a natural cell lineage. An important future experiment is to determine whether authentic MSCs purified from the BM can regenerate cardiomyocytes using the protocol outlined in Figure 3A.
Human MSCs were reported to mobilize into the systemic circulation as a result of G-CSF treatment.26 The present study also shows that G-CSF treatment induces mobilization of MSCs from the BM into the systemic circulation leading to engraftment at peripheral organ sites. Matrix metalloproteinases and elastase, released mainly from granulocytes, are thought to be the major mediators of HSC mobilization induced by G-CSF.27 The role of these factors in mediating the mobilization of MSC is unknown and provides an important avenue for future research. As G-CSF treatment following MI is reported to improve cardiac function and survival,5 MSC mobilization may provide a new strategy for regenerative treatment. Furthermore, the data presented here support the concept that one function of MSC in BM is to contribute to the repair of injured tissue by being mobilizing to the site of injury and differentiating according to the niche.25,26,28,29
While this study focused on cardiomyocytes derived from BM, the reported improvement of infarcted tissue by G-CSF cannot be solely attributed to these cells. Most of the donor-derived cells in the infarcted area were actinin-negative fibroblastic cells and endothelial cells derived from HSCs. It is possible that these cells are f-macrophages30 or are generated by fusion.11 The role of these cells in remodeling is now under investigation. Neovascularization by endothelial progenitor cells (EPCs) mobilized from BM by G-CSF is also reported to contribute to an improved outcome.27 In addition, EPC are reported to transdifferentiate into cardiomyocytes.31 Adult cardiac stem cells in the heart32,33 and multipotent adult progenitor cells34 also may be affected by G-CSF. Therefore, the clinical significance of cardiomyocyte regeneration by BM-derived cells must take into account the role of different populations of cells in the regeneration process. The outcome of this research should be the development of novel cytokine and cell-based therapies for MI.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-04-1488.
Supported by grants from the Ministry of Education, Science and Culture; and the Ministry of Welfare and Labor, Japan; as well as grants from the 21st Century Center of Excellence Program of the Ministry of Education, Science and Culture of Japan to Keio University and Tokai University.
H. K., J. F., and K. K. contributed equally to this paper.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Tadayuki Sato, Hideyuki Matsuzawa, and Takashi Yahata for their technical assistance.

![Figure 1. G-CSF–induced mobilization of BM cells in mice receiving transplants of single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells or whole BM cells following MI. (A) Experimental protocol. Single EGFP+ CD34–KSL-SP cells and radioprotective EGFP– BM cells were transplanted into the recipient mice. (B) The nucleated cell counts (NCCs) for peripheral blood samples obtained 24 hours after the last injection of G-CSF (n = 10) or saline (n = 10) are shown. ▪ indicates EGFP+ cells; ▨, EGFP– cells. NS indicates not significant; *P < .0001. Bars indicate the standard error. (C-F) Panels show representative results for immunofluorescent analysis using anti–GFP antibody and TOTO-3 dye in the hearts of mice receiving translplants of w-BM cells or single CD34–c-kit+Sca-1+ lineage– side population (CD34–KSL-SP) cells separated from the BM of transgenic EGFP mice. The green and blue signals indicate EGFP and nuclei, respectively. Saline-treated mice (G-CSF[–] mice) (C) and G-CSF–treated mice (G-CSF (+) mice) (D) in the w-BM group. G-CSF(–) mice (E) and G-CSF (+) mice (F) in the CD34–KSL-SP group. Bars indicate 200 μm. (G) The control experiment using C57BL/6J mice as donors revealed that no green fluorescence was detected in the whole heart. Bar indicates 50 μm. (H-J) Coimmunostaining with anti-GFP and anti–actinin antibodies in the infarcted myocardium of G-CSF(+) mice transplanted with w-BM (H, I) or single CD34–KSL-SP cells (J). The green, red, and blue signals indicate EGFP, actinin, and nuclei, respectively. (H, I) In the w-BM group, some of the EGFP+ cells in the infarcted area were positive also for actinin and showed striation, indicating that they had differentiated into cardiomyocytes. (J) In contrast, very few EGFP+ actinin+ cells were seen in the infarcted area in the CD34–KSL-SP group. Bars indicate 50 μm (H, I) and 20 μm (J).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-04-1488/5/m_zh80230470140001.jpeg?Expires=1767903992&Signature=S2~csF65FvbumNuxwJuQ5CrDHo2bH55QZTxw5qDI9etWH~Vu1wnIJZcRdJttVtp3jx9xQa8dTiUT99Jo47Kk84PLkaIPDett3iSKt6lRpVAWYBHXbHh0jLNPB20K8xGgzZibMIgrMYl5drJJsL5e0IsMQIzsfMSNYvpyHtZNxnbbl5El0LHfAe1exNLEboe6lBpAl0sQOagwilUsL9Q~YsVgkDZuLL16VPp6n1H-sV1gLlIkEjzsvGazPZkkbdDUqecgfBTSB4f8urcc~eCpXFA6w0pLw7D8CgIErfylwL6B0E0K6Imy2-UvSCrjIJcsQxWzOQdLe9vR9YeyNCtzlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)