Deletion of megakaryocytic-specific regulatory sequences of GATA-1 (Gata1tm2Sho or GATA-1low mutation) results in severe thrombocytopenia, because of defective thrombocytopoiesis, and myelofibrosis. As documented here, the GATA-1low mutation blocks megakaryocytic maturation between stage I and II, resulting in accumulation of defective megakaryocytes (MKs) in the tissues of GATA-1low mice. The block in maturation includes failure to properly organize α granules because von Willebrand factor is barely detectable in mutant MKs, and P-selectin, although normally expressed, is found frequently associated with the demarcation membrane system (DMS) instead of within granules. Conversely, both von Willebrand factor and P-selectin are barely detectable in GATA-1low platelets. Mutant MKs are surrounded by numerous myeloperoxidase-positive neutrophils, some of which appear in the process to establish contact with MKs by fusing their membrane with those of the DMS. As a result, 16% (in spleen) to 34% (in marrow) of GATA-1low MKs contain 1 to 3 neutrophils embedded in a vacuolated cytoplasm. The neutrophil-embedded GATA-1low MKs have morphologic features (high electron density and negativity to TUNEL staining) compatible with those of cells dying from para-apoptosis. We suggest that such an increased and pathologic neutrophil emperipolesis may represent one of the mechanisms leading to myelofibrosis by releasing fibrogenic MK cytokines and neutrophil proteases in the microenvironment.
Introduction
Megakaryocytes (MKs) are specialized cells of the blood responsible for platelet production.1 They originate from committed progenitor cells, usually localized in the marrow, through a complex maturation process, during which MK precursors progressively increase in size, while undergoing extensive synchronous morphologic changes in the cytoplasm and nucleus.2 At the ultrastructural level, the major cytoplasmic modifications are represented by massive compartmentalization into discrete regions, delimited by intrusions of the plasma membranes, bound to give rise to the demarcation membrane system (DMS).2 The DMS will, in turn, internalize platelet-specific α granules, giving rise to proplatelets through a process defined thrombocytopoiesis.3 In the meantime, chromosomes undergo several cycles of endo-duplications. As a result, the nucleus itself appears multilobed.
Despite few differences, the morphologic changes associated with the maturation of MK precursors are similar between mice and humans.4 On the basis of distinct ultrastructural characteristics, murine and human MK precursors are divided into 4 classes2 : the promegakaryoblast, a small mononuclear cell expressing already platelet-specific proteins, such as von Willebrand Factor (VWF); the megakaryoblast (or stage I MK), a cell 15 to 50 μm in diameter with a large, oval or kidney-shaped nucleus and several nucleoli, whose cytoplasm presents abundant ribosomes and a well-developed rough endoplasmic reticulum (RER); the promegakaryocyte (stage II MK), a cell 20 to 80 μm in diameter with an irregularly shaped nucleus and a more abundant cytoplasm, containing a rudimental DMS; and, finally, mature megakaryocytes (stage III MKs) that contain a multilobed nucleus surrounded by abundant cytoplasm divided into a perinuclear (hosting the centrioles, few biosynthetically active organelles, and many α granules), the intermediate (containing a well-developed DMS and platelet territories), and the peripheral (devoid of organelles and enriched of cytoskeletal proteins and microtubules) zone.2
The complex process of MK maturation is controlled by lineage-specific extrinsic and intrinsic factors represented, respectively, by growth factors (such as thrombopoietin5 [TPO]) and transcription factors (such as nuclear factor-E26 [NF-E2] and GATA-17,8 ). Mice harboring experimentally induced mutations in the genes encoding these factors have been instrumental in elucidating their role in the process. In particular, an essential role for GATA-1 in megakaryocytopoiesis has been established by the observation that mice lacking regulatory regions of the GATA-1 gene specifically required for its expression in MK cells (Gata1tm2Sho or GATA-1low mutation)9,10 or lacking the gene for the GATA-1 obligatory partner FOG-1,11 are thrombocytopenic. The defects induced by the GATA-1low mutation in MK precursors include in vivo and in vitro hyperproliferation,9 markedly reduced expression of lineage-specific genes (eg, the TPO receptor, c-mpl, glycoprotein [GP] Ibα and Ibβ, and platelet factor 4 [PF4])9 and ultrastructural abnormalities, suggesting retarded cytoplasm maturation (eg, underdeveloped or disorganized DMS, presence of excessive RER, and a wide peripheral zone).10 Consequently, GATA-1low MKs generate reduced numbers of megathrombocytes, whose ultrastructural abnormalities include presence of excessive RER and paucity of organelles, especially of α granules.9 It is not clear how the number of hyperproliferating MK precursors in the tissues of the mutant animals is restrained. In fact, although the mutation increases apoptosis of the erythroblasts,12 the number of TUNEL+ MK precursors in the tissues of these animals is not apparently increased12 (R.A.R. and A.R.M., unpublished observations, June 2004).
Alterations in the MK population from patients with idiopathic myelofibrosis (IM), a myeloproliferative disorder of clonal origin characterized by marrow fibrosis, increased progenitor cell mobilization, and extramedullary hemopoiesis that may eventually evolve in leukemia,13-15 have been described since 1974.16 The observation that the MK defects induced by the GATA-1low mutation9,10 are similar to those observed in MKs from these patients16,17 led us to discover that mice carrying this mutation present an IM-like pathology (development of marrow fibrosis and increased progenitor cell mobilization by 8 months and presence of anemia and teardrop poikilocytes in the blood and of extramedullary hemopoiesis in the liver by 15 months18 ). The MK abnormalities observed in patients with IM also include increased emperipolesis of neutrophils within MKs.19,20 The significance, however, of this observation is not clear, because emperipolesis of neutrophils within MKs increases in other stress-related conditions not necessarily associated with marrow fibrosis, such as recovery after sublethal irradiation,21 aging,22 and altered MK maturation associated with the gunmetal mutation.23
The aim of this study is to define at the ultrastructural level the stage of MK maturation blocked by the GATA-1low mutation and to clarify whether this block is associated with increased emperipolesis of neutrophils within the MKs. The results obtained indicate that the mutation blocks MK maturation between stage I and II and halts proper assembly of lineage-specific proteins into the α granules either because, as in the case of VWF, they are weakly expressed by the mutant cells, or because, as in the case of P-selectin, they become abnormally associated with the DMS. Apoptotic MKs are not recognized in the tissues of the GATA-1low mice. However, 50% of the MKs present in these tissues are heavily electron dense and have a shrunken and damaged morphology. Many of them (16%-34%) contain 1 to 3 degranulated neutrophils engulfed in an extensively vacuolated cytoplasm. We propose that this pathologic neutrophil-MK interaction, although instrumental to destroy the hyperproliferating mutant MKs, releases in the microenvironment of the GATA-1low mice fibrogenic MK proteins and neutrophil proteases that result, respectively, in fibrosis and abnormal stem/progenitor cell trafficking and extramedullary hemopoiesis.
Materials and methods
Mice
Male wild-type and GATA-1low mice were generated in the animal facility of Istituto Superiore di Sanità as described.12,24 The mutants are also available from Jackson Laboratories (Bar Harbor, ME; JAX@Mice DATAbase-STOCK Gata1<tm2Sho>). Because the mutant mice develop myelofibrosis while aging,18 they were divided into 3 age classes: disease-free (1-6 months old), early myelofibrotic (8-12 months old, when presence of the disease is detectable only in bone marrow), and myelofibrotic (15 months old to natural death, when the complete clinical picture of the human disease is manifested). To avoid the possibility that the MK alterations eventually observed might be due to an ongoing disease progression, most of the experiments were performed on organs (both marrow and spleen) from animals killed at age 4 to 6 months. Control experiments were also performed on early and myelofibrotic mice, and age-matched littermates, as indicated. All the experiments were performed according to protocols approved by the institutional animal care committee.
Purification of platelets
Blood was collected from the retro-orbital plexus in 500-μL tubes containing 50 μL 3.8% sodium citrate (Sigma, Milan, Italy), diluted with 1.5 mL Ca++- and Mg++-free phosphate-buffered saline (PBS; Sigma) and gently centrifuged at 800 rpm for 5 minutes. This platelet-enriched plasma was centrifuged again at 3000 rpm for 15 minutes and the resulting pellet processed for electron microscopy.
Transmission electron microscopy
Spleen and marrow (carefully flushed from the femoral cavity) samples for transmission electron microscopy (TEM) were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, for 2 hours at 4°C and postfixed in osmium tetroxide for 60 minutes at 4°C. The samples were then dehydrated in alcohol at progressively higher concentrations and embedded in Spurr resin (Poliscience, Warrington, PA). Consecutive thin and ultrathin sections were cut using a Reichert ultramicrotome. Ultrathin sections were collected on 200-mesh copper grids counterstained with uranyl acetate and lead citrate and observed under vacuum with an EM 109 Zeiss microscope equipped with built-in electromagnetic objective lenses and camera (Oberkochen, Germany). Photographs were taken with Kodak Technical Pan film (Kodak, Rochester, NY), developed with Kodak D19 1+4 automatic developer and scanned with an EPSON Perfection 3200 photoscanner (Seiko EPSON, Nagano-ken, Japan). A minimum of 50 MKs was analyzed per experimental point and each experiment was repeated on tissues obtained from at least 3 separate mice per experimental group.
Immunoelectron microscopy
Spleen and isolated platelets were fixed for 3 hours at 4°C in a mixture of 2% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6. They were dehydrated in alcohol at progressively higher concentrations and embedded in Bioacryl resin (British Biocell, Cardiff, United Kingdom), followed by UV polymerization, according to standard procedures.25 Ultrathin sections were cut and mounted on 300-mesh nickel grids. To block nonspecific binding sites, these grids were treated with a blocking buffer made of PBS supplemented with 0.1% Tween-20, 0.1% bovine serum albumin, and 4% normal rabbit serum, and incubated overnight in the presence of goat anti–P-selectin, anti-VWF, and antimyeloperoxidase antibodies (catalog nos. sc-8068, sc-6941, and sc-12128, respectively, from Santa Cruz Biotechnology, Santa Cruz, CA). All of the antibodies recognize antigens expressed on the human, mouse, and rat proteins. The grids were then incubated for 1 hour with rabbit antigoat IgG conjugated with 15-nm colloidal gold particles (British Biocell), counterstained in uranyl acetate to show the cell morphology, and observed with EM 109 Zeiss. Negative controls were represented by cells treated as described, but not exposed to the primary antibody. Semiquantitative observations were obtained on 20 to 50 individual cells. Twenty to 50 cells were visually analyzed for each experimental point and the number of immunogold particles counted at × 30 000 magnification in 5 randomly selected cells.
Statistical analysis
Results are presented as the mean (± SD) of 3 separate experiments and statistical analysis was performed by analysis of variance (ANOVA test) using Origin 3.5 software for Windows (Microcal Software, Northampton, MA).
Results
In mice, MK precursors are consistently located both in the marrow and spleen, and no difference has been reported between the morphology of the MKs located in the 2 organs.4 In this species, the spleen is recruited as an additional hematopoietic site in almost all of the stress-related conditions and represents a bona fide hematopoietic organ. Therefore, because it is difficult to properly process for ultrastructural studies the marrow of GATA-1low mice because of bone density,26 the morphologic changes induced by the GATA-1low mutation in the MK precursors were routinely investigated in the spleen. Control experiments were also done on marrow, whenever possible.
As expected,19 spleen sections from wild-type mice reveal low numbers (5 ± 1/cm2) of individual MKs dispersed in the red pulp (Figure 1A-B and results not shown). All stages of MK maturation are recognized by TEM in the spleen of normal animals (data not shown).
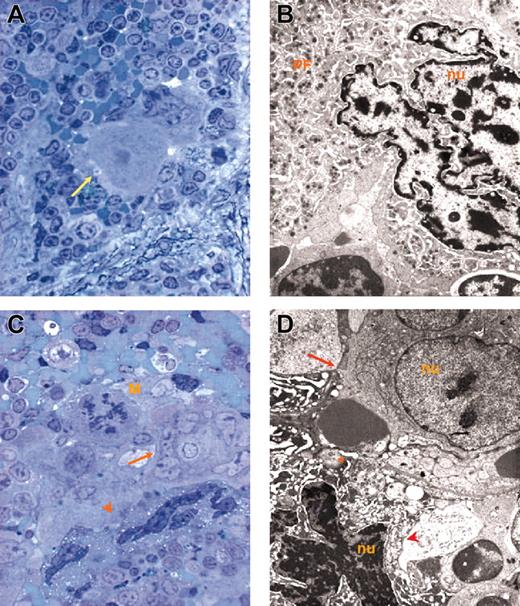
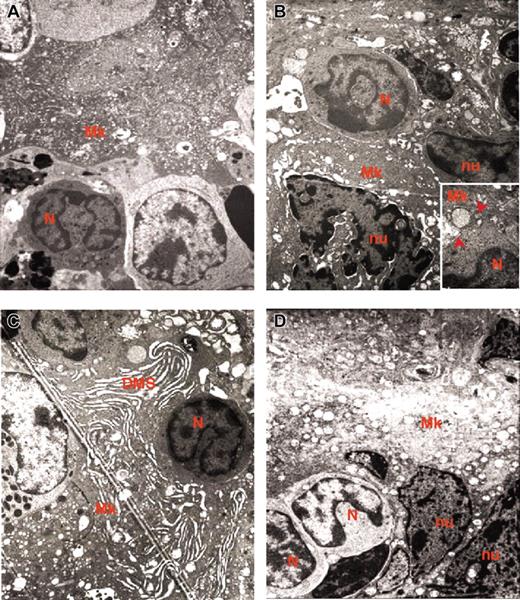
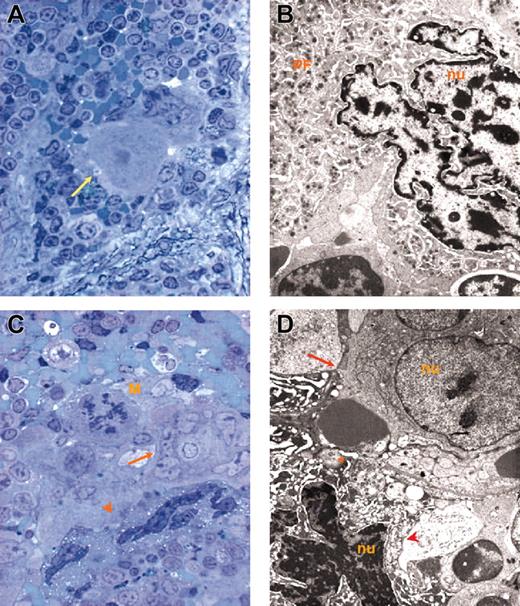
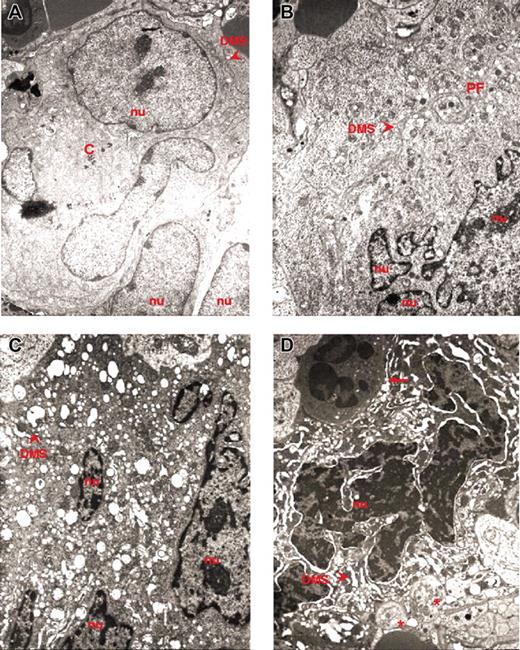
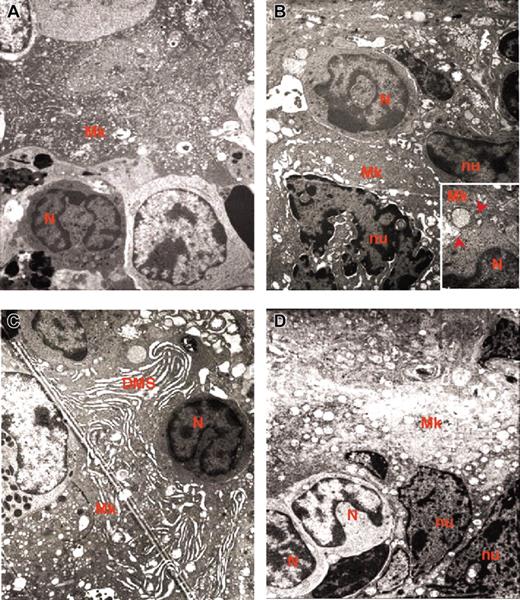
Representative MKs. Representative MKs in consecutive semithin (A,C) and ultrathin (B,D) spleen sections from wild-type (A-B) and GATA-1low (C-D) mice. The arrow in panel A indicates an isolated MK in the semithin section from a wild-type spleen further analyzed by TEM in panel B. The ultrastructural morphology (cytoplasm rich in α granules and platelet territories, PF) characterizes this cell as a stage III MK. The arrow and arrowhead in panel C indicate MKs within a cluster in a semithin spleen section from GATA-1low mice further analyzed by TEM in panel D. Two populations of mutant MKs were identified by TEM, one with normal (arrow) and the other with high (arrowhead) electron density of the nucleus (nu) and cytoplasm. The M in panel C indicates a mitotic MK. The asterisk in panel D indicates structures with plateletlike morphology. Similar observations were obtained with spleens and marrows from 2 additional wild-type and GATA-1low littermates. Original magnifications × 100 (A,C) and × 3000 (B,D).
Representative MKs. Representative MKs in consecutive semithin (A,C) and ultrathin (B,D) spleen sections from wild-type (A-B) and GATA-1low (C-D) mice. The arrow in panel A indicates an isolated MK in the semithin section from a wild-type spleen further analyzed by TEM in panel B. The ultrastructural morphology (cytoplasm rich in α granules and platelet territories, PF) characterizes this cell as a stage III MK. The arrow and arrowhead in panel C indicate MKs within a cluster in a semithin spleen section from GATA-1low mice further analyzed by TEM in panel D. Two populations of mutant MKs were identified by TEM, one with normal (arrow) and the other with high (arrowhead) electron density of the nucleus (nu) and cytoplasm. The M in panel C indicates a mitotic MK. The asterisk in panel D indicates structures with plateletlike morphology. Similar observations were obtained with spleens and marrows from 2 additional wild-type and GATA-1low littermates. Original magnifications × 100 (A,C) and × 3000 (B,D).
In contrast, sections from the area within the white and red pulp of the spleen from GATA-1low mice contain many MK clusters composed of 5 to 10 precursors each (Figure 1C). The frequency of MKs in this region of the spleen from mutant mice is as high as 168 ± 18 MKs/cm2. Each cluster includes MK precursors different in size and morphology, some of which are even in the process of mitosis. The morphology of these MKs does not clearly resemble that of the normal cells. Mutant MK precursors are, instead, strikingly heterogeneous in electron density and both light and heavy electron-dense MKs are identified in the spleen of the mutant mice (Figure 1C-D). The ratio between the 2 cell types is approximately 1:1 both in marrow and spleen and is not affected by the progression of myelofibrosis (Table 1).
Light electron-dense MK precursors range from 25 to 63 to 77 μm in diameter. The smaller light MK precursors resemble stage I MKs. In fact, they contain an indented predominantly euchromatic nucleus and numerous nucleoli (Figure 2A). Their cytoplasm presents up to 5 centrioles, many free and endoplasmic reticulum-associated polyribosomes, a highly developed Golgi apparatus, and numerous mitochondria. Their cytoplasm is also rich in microtubules and presents an onset of vacuolization that ranges from small, empty structures, to medium-sized vacuoles partially filled with electron-dense material, to large vacuoles containing microvesicles resembling multivesicular bodies, the intermediate stage in the formation of the α granules.27 In some cases, an invagination of the cell membrane was observed, indicating beginning of DMS formation.
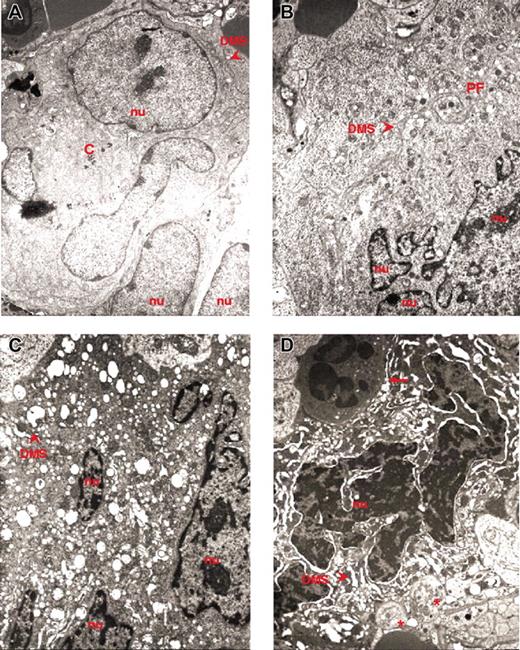
Ultrastructural electron density and heterogeneity of MKs from the spleen of GATA-1low mice. TEM of representative MKs identified in spleen sections from GATA-1low mice. Light and heavy electron-dense MKs are presented in panels A-B and C-D, respectively; nu indicates nuclear area; arrowhead, DMS; C, centrioles; PF, platelet field; arrows, neutrophils; asterisks, platelets. Original magnification × 3000 for all panels.
Ultrastructural electron density and heterogeneity of MKs from the spleen of GATA-1low mice. TEM of representative MKs identified in spleen sections from GATA-1low mice. Light and heavy electron-dense MKs are presented in panels A-B and C-D, respectively; nu indicates nuclear area; arrowhead, DMS; C, centrioles; PF, platelet field; arrows, neutrophils; asterisks, platelets. Original magnification × 3000 for all panels.
The larger light MK precursors are more similar to stage II MKs. They present multilobed nuclei and a cytoplasm with reduced Golgi apparatus and an increased vacuole system, indicating that the DMS is being formed (Figure 2B). The electron density of the large MK is slightly higher than that of the smaller one, due to the presence of many polyribosomes, a dense matrix in the cytoplasm, and heterochromatin in the nucleus. The cytoplasm of these cells, however, rarely contains fields with typical platelet morphology (Figure 2B).
Cells resembling stage III MKs were never observed in the tissues from GATA-1low mice.
High electron-dense MKs are about 24 μm in diameter and contain a multilobed nucleus with a massive heterochromatic area that even surrounds the nucleoli (Figure 2C-D). The fact that such a massive heterochromatic degeneration is, however, not associated with nuclear fragmentation, excludes that these cells are undergoing apoptosis. Indeed, the spleen of GATA-1low mice contains numerous TUNEL+ erythroblasts but very few TUNEL+ MKs (Figure 2 in Vannucchi et al12 and A.M.V., R.A.R., A.R.M., unpublished observation, June 2004). The cytoplasm of a high electron-dense MK presents a large DMS area organized in long hollows, often very dilated and is markedly vacuolated, with a prevalence of completely empty vacuoles. Because high numbers of mitochondria are present in the cytoplasm of these cells, we believe that these vacuoles are not originated, as in classic para-apoptosis,28-30 by lyses of mitochondria, but by dissolution of granules. The intense electron density of their cytoplasm is due to the presence of many polyribosome aggregates and of poorly developed Golgi and RER apparatus.
The abnormal ultrastructural organization of the granules of the mutant GATA-1low Mk (Figure 2) is retained at the level of the megathrombocytes. In fact, large platelets containing numerous atypical granules, but no α granules, are frequently observed in the spleen and in the blood from the mutant animals (Figure 3).
Ultrastructural analysis and immunogold labeling for VWF and P-selectin. Ultrastructural analysis (A,D) and immunogold labeling for VWF (B,E) and P-selectin (C,F) of platelets purified from the blood of wild-type (A-C) and GATA-1low (D-F) mice. Original magnification × 20 000 (A,D) and × 30 000 for all other panels. Arrows indicate representative immunogold particles.
Ultrastructural analysis and immunogold labeling for VWF and P-selectin. Ultrastructural analysis (A,D) and immunogold labeling for VWF (B,E) and P-selectin (C,F) of platelets purified from the blood of wild-type (A-C) and GATA-1low (D-F) mice. Original magnification × 20 000 (A,D) and × 30 000 for all other panels. Arrows indicate representative immunogold particles.
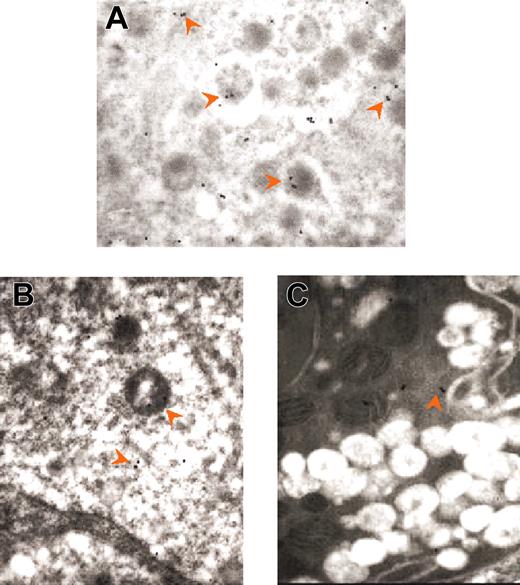
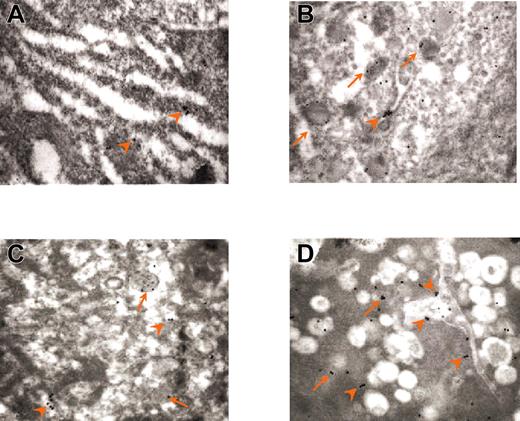
The abnormal organization of the granules in GATA-1low MKs is further documented by immunoelectron microscopy for the localization of VWF and P-selectin. In normal MKs, immunogold labeling for VWF is clearly detectable and is often localized, as reported,19 at one pole of the α granules (Figure 4A). In contrast, GATA-1low MKs contain few VWF immunoparticles (Figure 4B-C; Table 2), consistent with the observation that GATA-1low MKs express less VWF mRNA than normal cells (A.M.V., R.A.R., A.R.M., unpublished observations, 2003). On the other hand, normal stage I MKs react poorly with antibodies for P-selectin, whereas stage III MKs contain numerous P-selectin–specific immunogold particles mainly localized, as expected,19 in the α granules (Figure 5A-B; Table 2). The mutant light electron-dense MKs contain slightly lower P-selectin–related gold particles in their cytoplasm (200 ± 50 versus 331 ± 42, P < .01) and in their granules (188 ± 22 versus 240 ± 30, P < .05) than normal stage I to II MKs (Figure 5C; Table 2). Also heavy electron-dense mutant MKs contain many P-selectin immunogold particles. However, in this case the labeling is mainly present in vacuoles and on the DMS (Figure 5D; Table 2). The presence of P-selectin immunoparticles in apparently empty vacuoles suggests that these structures represent vesicles once containing granules.
Immunogold labeling for wild-type and GATA-1low MKs. Immunogold labeling for VWF of the cytoplasm from a stage III wild-type MK (A) and from representative light (B) and heavy (C) electron-dense GATA-1low MKs. Representative immunogold particles are indicated by arrowheads. In normal MKs, VWF-immunoparticles are often localized at one pole of typical α granule structures. In comparison, the cytoplasm of GATA-1low MKs contains lower numbers of gold particles localized both in α granule–like structures and in the cytoplasm (B). Note how few VWF particles are present in the cytoplasm of a heavy electron-dense GATA-1low MKs. Original magnification × 30 000 for all panels.
Immunogold labeling for wild-type and GATA-1low MKs. Immunogold labeling for VWF of the cytoplasm from a stage III wild-type MK (A) and from representative light (B) and heavy (C) electron-dense GATA-1low MKs. Representative immunogold particles are indicated by arrowheads. In normal MKs, VWF-immunoparticles are often localized at one pole of typical α granule structures. In comparison, the cytoplasm of GATA-1low MKs contains lower numbers of gold particles localized both in α granule–like structures and in the cytoplasm (B). Note how few VWF particles are present in the cytoplasm of a heavy electron-dense GATA-1low MKs. Original magnification × 30 000 for all panels.
Immunogold labeling for P-selectin of the cytoplasm from stage I and III wild-type MKs and from light and heavy electron-dense GATA-1low MKs. (A) Normal stage I MKs are weakly labeled for P-selectin. (B) Normal stage III MKs present many P-selectin gold particles mainly in association with α granules and rarely with the DMS. In contrast, light electron-dense GATA-1low MKs present few gold particles localized both on the membrane of granulelike structures and on the initial DMS (C), whereas high electron-dense Gata-1low MKs show numerous gold particles mostly associated with DMS, rarely on vacuoles or dispersed in the cytoplasm (D). Arrows and arrowheads indicate gold particles associated with granules (either α or atypical) and with DMS, respectively. Original magnification × 30 000 for all panels.
Immunogold labeling for P-selectin of the cytoplasm from stage I and III wild-type MKs and from light and heavy electron-dense GATA-1low MKs. (A) Normal stage I MKs are weakly labeled for P-selectin. (B) Normal stage III MKs present many P-selectin gold particles mainly in association with α granules and rarely with the DMS. In contrast, light electron-dense GATA-1low MKs present few gold particles localized both on the membrane of granulelike structures and on the initial DMS (C), whereas high electron-dense Gata-1low MKs show numerous gold particles mostly associated with DMS, rarely on vacuoles or dispersed in the cytoplasm (D). Arrows and arrowheads indicate gold particles associated with granules (either α or atypical) and with DMS, respectively. Original magnification × 30 000 for all panels.
The reduced presence of VWF and P-selectin–related gold particles in the granules of the MKs from GATA-1low mice is reflected by the extreme paucity with which both these proteins are detected in their platelets (Figure 3; Table 2). The observation that P-selectin is detectable in MKs from GATA-1low mice but not in their platelets suggests that appropriate P-selectin localization in the MK α granules might be dependent on the presence of a partner protein. Analogy with the endothelial system where appropriate localization of P-selectin on the cell surface is dependent on appropriate expression of VWF31,32 and our data suggest that VWF might also be required for localization of P-selectin on the α granules of the MKs.
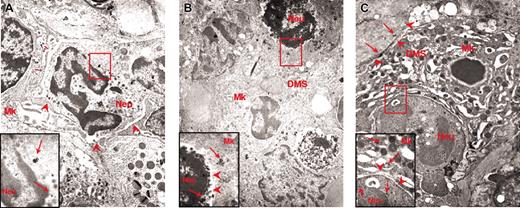
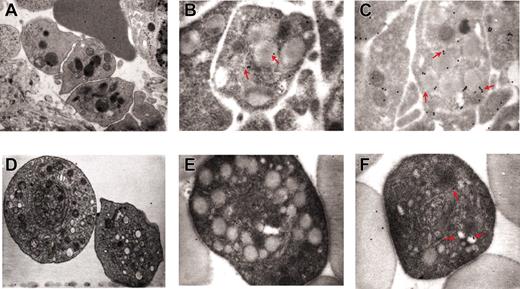
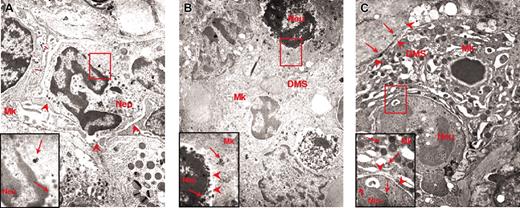
The abnormal P-selectin localization on the DMS of the heavy electron-dense mutant MKs prompted us to analyze the reciprocal distribution of MKs and neutrophils in the spleen from normal and GATA-1low littermates. As expected, very few (0.07 × 103/cm2) neutrophils are recognized on spleen sections from normal animals.18,19 In contrast, as many as 103/cm2 neutrophils are identified on sections from the same region within the white and red pulp of the GATA-1low spleen where are also found high numbers of MKs. Furthermore, as many as 40% of the heavy electron-dense GATA-1low MKs are engulfed with other cell types. More precisely, 16% to 19% (in the spleen) and 32% to 34% (in the marrow) of heavy electron-dense MKs contain neutrophils (Table 1). All the steps of neutrophils establishing contacts with MKs can be identified in sections from the spleen of the mutant mice. Examples of a neutrophil approaching an MK, touching the MK plasma membrane, and fusing its membrane with that of the MK, starting to enter the MK via the dysmorphic DMS and completely enclosed in the MK cytoplasm are shown in Figure 6. Up to 3 neutrophils are found embedded into heavy electron-dense MKs.
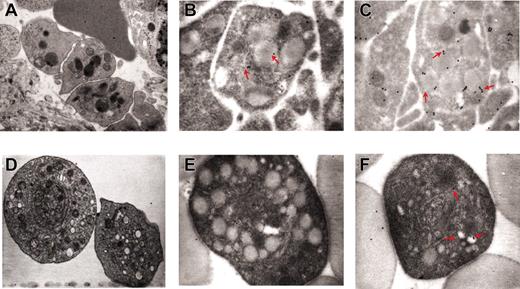
Sequential phases of neutrophil emperipolesis in MKs from the spleen of GATA-1low mouse. The different panels present a neutrophil (N) approaching the MK (A); establishing contacts with the MK (B) and fusing its own membrane with that of the MK (arrowheads in the insert in panel B); penetrating the MK through the DMS (C). Finally, panel D shows 2 neutrophils enclosed inside the MK cytoplasm. Note that in this last case, the MK cytoplasm appears highly vacuolated, a sign of cell damage, whereas the cytoplasm of the neutrophil appears degranulated. Nu indicates nuclear area of the MK. Original magnification × 3000 for all panels and × 30 000 for the insert.
Sequential phases of neutrophil emperipolesis in MKs from the spleen of GATA-1low mouse. The different panels present a neutrophil (N) approaching the MK (A); establishing contacts with the MK (B) and fusing its own membrane with that of the MK (arrowheads in the insert in panel B); penetrating the MK through the DMS (C). Finally, panel D shows 2 neutrophils enclosed inside the MK cytoplasm. Note that in this last case, the MK cytoplasm appears highly vacuolated, a sign of cell damage, whereas the cytoplasm of the neutrophil appears degranulated. Nu indicates nuclear area of the MK. Original magnification × 3000 for all panels and × 30 000 for the insert.
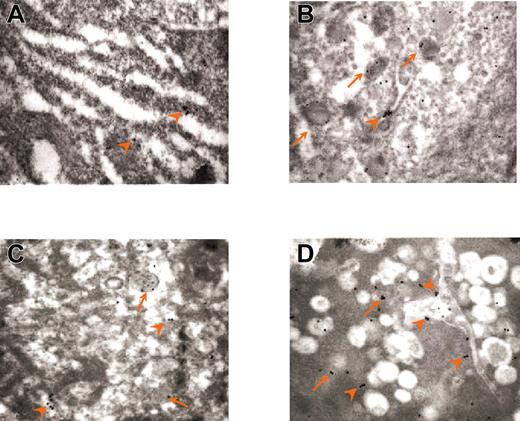
To clarify the nature of the cells engulfed in the GATA-1low MKs, the presence of myeloperoxidase in neutrophils from normal as well as from GATA-1low spleens was analyzed by immunoelectron microscopy (Figure 7; Table 3 and data not shown). Immunogold particles for myeloperoxidase staining are clearly detectable in neutrophils from both normal and GATA-1low mice (data not shown). On average, neutrophils from GATA-1 mice contain more myeloperoxidase-related gold particles that the corresponding normal cells (46.7 ± 21.7 versus 11.8 ± 3.9, P < .01; Table 3). During the process of embedding in the mutant MKs, the number of gold particles in the neutrophils progressively decreases down to 14.2 ± 4.5 (P < .01), whereas that in the cytoplasm of the MKs increases from less than 2.3 ± 0.5 to 25.6 ± 19.7 (Figure 7; Table 3). In some of these MKs, the gold particles are found localized just on the extracellular side of the cell membrane (Figure 7C).
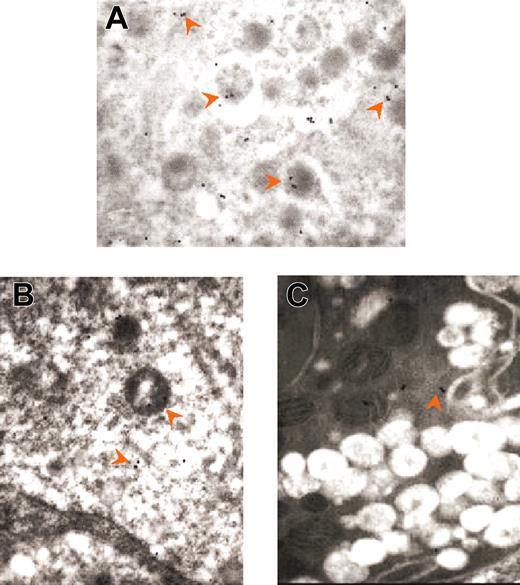
Immunogold labeling for myeloperoxidase of neutrophils at different stages of their emperipolesis within GATA-1low MKs. Examples of neutrophils approaching the MK (A), penetrating the MK through the DMS (B), and embedded in the MK cytoplasm (C). The rectangle in each panel indicates the area shown at higher magnification in the insert. The arrows indicate isolated as well as clustered gold particles. The arrowheads in the panels indicate the extracellular membrane of the MK; those in the inserts, the neutrophil/MK border. Immunogold particles are localized only in the neutrophil in panel A, on both sides of the neutrophil-MK border in panel B, and on both sides of the neutrophil/MK border and on the extracellular side of the MK membrane in panel C. Original magnification × 3000 for all panels and × 30 000 for the inserts.
Immunogold labeling for myeloperoxidase of neutrophils at different stages of their emperipolesis within GATA-1low MKs. Examples of neutrophils approaching the MK (A), penetrating the MK through the DMS (B), and embedded in the MK cytoplasm (C). The rectangle in each panel indicates the area shown at higher magnification in the insert. The arrows indicate isolated as well as clustered gold particles. The arrowheads in the panels indicate the extracellular membrane of the MK; those in the inserts, the neutrophil/MK border. Immunogold particles are localized only in the neutrophil in panel A, on both sides of the neutrophil-MK border in panel B, and on both sides of the neutrophil/MK border and on the extracellular side of the MK membrane in panel C. Original magnification × 3000 for all panels and × 30 000 for the inserts.
Discussion
High electron-dense GATA-1low MKs present many signs of cytoplasm damage, such as swollen mitochondria with disrupted crests, numerous empty vacuoles, and occasional remnants of DMS. Their extensive heterochromatic nuclear organization and the shrunken and degraded appearance of their cytoplasm are reminiscent of the alterations observed in MKs undergoing paraapoptosis, a TUNEL– process of cell death involved in destruction of MKs in idiopathic thrombocytopenic purpura.30 Para-apoptosis, first described by Asher et al,28 is characterized by condensed chromatin and cytoplasmic vacuolization due to swollen mitochondria and endoplasmic reticulum distinct from necrosis because of absence of membrane disruption.29 In the case of idiopathic thrombocytopenic purpura,30 thrombocytopenia is the result of an immunoglobulin-mediated inflammatory response of neutrophils and macrophages against all the stages of MK maturation. Such a response results in massive cell death of stage III (from both apoptosis and para-apoptosis) and stage I (from para-apoptosis only) MKs. Naked MK-specific nuclei have been observed in the marrow from IM patients and have been interpreted as a sign of MKs undergoing para-apoptosis.33 The deeply abnormal morphology of the heavy electron-dense MKs identified in the GATA-1low mice does not resemble that of para-apoptotic MKs in idiopathic thrombocytopenic purpura (they do not retain an euchromatic perinuclear region and their cytoplasmic vacuoles probably derive from lyses of granules rather than from that of mitochondria; Figure 2). Because the pathologic neutrophil-MK interactions in IM are mediated by P-selectin through its abnormal localization on the DMS19,20 (and this manuscript), rather than by antibodies against MK-specific antigens as in idiopathic thrombocytopenic purpura,30 it is possible that neutrophil-mediated MK degradation may occur through at least partially different morphologic changes, depending on the mechanism involved in the initial recognition between the 2 cells.
Emperipolesis is a general cell feature originally defined as a random passage of one cell through the cytoplasm of another one with no physiologic consequence for either of them.2 Emperipolesis is particularly frequent in MKs, 5% of which are normally found engulfed with other cell types, most frequently neutrophils or red blood cells (Zucker-Franklin2 and data not shown). Increased frequency of neutrophil emperipolesis within the MKs is observed in the majority of patients who experience extreme thrombocytosis, whether myeloproliferative or reactive,34 and is not necessarily associated with marrow fibrosis. However, in all the cases in which thrombocytosis is also associated with fibrosis (ie, human IM and the IM-like syndrome developed by mice either given transplants of stem cells infected with a retrovirus containing the TPO gene19,20 or carrying the GATA-1low18 or the Batch1 mutation35 ), neutrophils interact with MKs through abnormal P-selectin localization on the DMS. The specific pathologic consequences of this interaction might be due to the release of neutrophil enzymes in close proximity to the atypical granules within the MK cytoplasm, causing the granules themselves to release their content, including growth factors such as transforming growth factor β (TGF-β), in the microenvironment through the DMS. TGF-β would then stimulate the fibroblasts to produce reticulin and collagen fibers.36 On the other hand, neutrophil proteases similarly released in the microenvironment through the DMS (Figure 7) might cleave adhesion receptors from the surface of stem/progenitor cells leading to their abnormal trafficking and extramedullary hemopoiesis.37 As such, abnormal P-selectin localization on the DMS should represent an element upstream to TGF-β in the pathobiologic mechanism leading to myelofibrosis. The role of TGF-β in the development of this disease has been univocally established by the observation that mice given transplants of TPO-overexpressing normal bone marrow, but not those given transplants of TPO-overexpressing TGF-βnull stem cells, develop myelofibrosis.36 Similarly, the role of abnormal P-selectin localization on the DMS in the development of myelofibrosis should be formally established by demonstrating that mice carrying both the GATA-1low18 and the P-selectinnull38 mutation are free of disease. These experiments are underway and will clarify whether pharmacologic inhibition of P-selectin binding to its receptor, P-selectin ligand 1, represents a candidate targeted therapy for marrow fibrosis.
In conclusion, the GATA-1low mutation blocks MK maturation between stage I and stage II and hampers proper organization of the α granules both by reducing the levels of VWF expression and by inducing abnormal P-selectin localization on the DMS. Abnormal P-selectin localization on the DMS triggers a mechanism of pathologic neutrophil emperipolesis within the MK, which may cause marrow fibrosis and extramedullary hemopoiesis in these mutants.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-01-0193.
Supported by Progetto Strategico Oncologia CNR MIUR legge 449/99, Progetti di Ricerca di Interesse Nazionale 2000, 2001, 2002, and 2003 no. 2003064888_3 from the Ministry of Health, Associazione Italiana Ricerca sul Cancro, PRIN 2003/064888, National Project on Stem Cells and institutional funds from Istituto Superiore Sanità.
L.C. and Angela di Baldassarre contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully thank Dr William Vainchenker for first suggesting this ultrastructural study and Dr Elisabeth Cramer for advice in the interpretation of the results. The editorial assistance of Maria Rosaria Broggi is highly appreciated.