Therapeutic results in elderly patients with acute promyelocytic leukemia (APL) have been generally reported as less effective than for younger patients. Patients 60 years or older with APL who were enrolled in 2 successive multicenter PETHEMA studies received induction therapy with all-trans retinoic acid (ATRA) and idarubicin, consolidation with 3 anthracycline monochemotherapy courses with or without ATRA, and maintenance with ATRA and low-dose chemotherapy. Eighty-seven of 104 patients achieved complete remission (84%). Eighty-six proceeded to consolidation therapy (2 withdrew after the first and second courses). Deaths in remission occurred during consolidation and maintenance therapy in 3 and 4 patients, respectively. One patient showed molecular persistence after consolidation and 5 had a relapse. The 6-year cumulative incidence of relapse, leukemia-free survival, and disease-free survival were 8.5%, 91%, and 79%, respectively. A significantly higher incidence of low-risk patients found among the elderly, as compared to younger patients, may partially account for the low relapse rate observed. This study confirms the high antileukemic efficacy, low toxicity, and high degree of compliance of protocols using ATRA and anthracycline monochemotherapy for induction and consolidation therapy in elderly patients.
Introduction
The outcome for patients with acute promyelocytic leukemia (APL) has dramatically improved with the combination of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy. However, this improvement has been generally less impressive for the elderly than for younger patients. Due to the vulnerability to treatment toxicity in elderly patients, a reduction of chemotherapy intensity has been proposed for this age group.1-4 However, whether the favorable impact of this reduction of chemotherapy on treatment-related mortality is counterbalanced by a lower antileukemic effect remains a matter of debate.
Based on the excellent tolerance and high degree of compliance observed in the PETHEMA (Programa de Estudio y Tratamiento de las Hemopatías Malignas) studies using anthracycline/anthraquinone monochemotherapy for consolidation,5,6 the dose and intensity of postremission therapy was not reduced for elderly patients. By contrast, consolidation therapy has been reinforced since 1999, regardless of age, for those patients with a higher risk.
We report here the results from 104 consecutive patients with newly diagnosed APL who were 60 years or older and enrolled in 2 successive studies of the PETHEMA Group (LPA96 and LPA99).
Patients, materials, and methods
Patients aged 60 years or older with de novo APL with demonstration of the t(15;17) or PML/RARα rearrangements were included in the present study. Other eligibility criteria were: (1) normal hepatic and renal function, (2) no cardiac contraindications to anthracycline chemotherapy, and (3) Eastern Cooperative Oncology Group (ECOG) performance status less than 4. Informed consent was obtained from all patients.
The induction regimen consisted of oral ATRA (45 mg/m2/d) until complete remission (CR) and intravenous idarubicin (12 mg/m2/d) on days 2, 4, 6, and 8. From November 1999, the idarubicin on day 8 was omitted for patients older than 70 years. Patients in CR received 3 monthly consolidation courses. The first course consisted of idarubicin (5 mg/m2/d for 4 days), the second of mitoxantrone (10 mg/m2/d for 5 days), and the third of idarubicin (12 mg/m2/d for 1 day). From November 1, 1999 (LPA99 study), intermediate- and high-risk patients, as previously defined,7 receivedATRA(45 mg/m2/d for 15 days) combined with the reinforced single-agent chemotherapy courses.6 Patients who tested negative for PML/RARα at the end of consolidation were started on maintenance therapy with oral mercaptopurine (50 mg/m2/d), intramuscular methotrexate (15 mg/m2/wk), and oral ATRA (45 mg/m2/d for 15 days every 3 months) over 2 years. Details of the supportive therapy have been described elsewhere.5,6 Response criteria were defined according to the recently revised criteria by Cheson et al.8 Unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate9 and, for comparisons, log-rank tests.10 The probability of relapse was also estimated by the cumulative incidence method (for marginal probability).11,12
Results and discussion
Between November 1, 1996, and December 31, 2003, 127 consecutive patients aged 60 or older with morphologic diagnosis of APL were registered from 43 institutions from Spain, The Netherlands, Argentina, and the Czech Republic (see “Appendix”). A total of 21 patients (16.5%) were considered not eligible because of diagnosis not confirmed at the genetic level (3 patients), poor performance status (10 patients), or death before starting therapy (8 patients). Thus, 106 patients met the previously defined entry criteria and were enrolled in 2 consecutive studies (LPA96 and LPA99). Two of these 106 patients were not evaluated because of protocol violations during induction therapy (addition of cytarabine in one patient and omission of idarubicin in one 75-year-old patient). The main clinical and biologic characteristics of the remaining 104 patients are shown in Table 1. Compared with the GIMEMA (Gruppo Italiano Malattie Ematologiche dell' Adulto) report,2 the only major study on elderly patients reported so far, the present series showed a greater proportion of evaluable patients (98% versus 92%), more patients older than 70 years (33% versus 14%), and a higher upper age limit. The distribution of other presenting features appears to be similar in both series.
Eighty-seven of the 104 evaluable patients achieved hematologic CR (84%; 95% CI, 77%-91%). The remaining 17 were considered as failures because of early death or resistance (one patient). Nine deaths were attributable to infection (56%), 6 to cerebral or pulmonary hemorrhage (37.5%), and one to retinoic acid syndrome. These proportions for infections and lethal hemorrhages were significantly different from those observed in patients younger than 60 (16% and 72%, respectively; P = .01). Univariate analysis demonstrated a significant relationship between sex and presenting white blood cell (WBC) count with response to induction therapy (Table 1). A trend for a poorer response rate was observed in patients with platelet counts lower than 40 × 109/L (77% versus 93%; P = .076) and in those older than 70 (74% versus 89%; P = .096), being 60% (6 of 10) and 79% (19 of 24) for those receiving 4 (LPA96) and 3 doses (LPA99) of idarubicin, respectively (P = .46). Interestingly, the lower failure and mortality rates in women (P = .021 and P = .01, respectively), although not statistically significant, were also observed in the GIMEMA study2 (11% versus 17% and 8% versus 17%, respectively). This may simply reflect a better tolerance to the side effects of chemotherapy linked to better organ function in female patients, which accords with the greater life expectancy of women in general.
Except for one 81-year-old patient who died in remission before starting consolidation, all the remaining 86 patients who achieved CR proceeded to receive consolidation therapy. All 4 deaths during consolidation occurred in the LPA99 study in patients aged 64, 69, 72, and 76, caused by pulmonary aspergillosis, cardiac dysfunction, cerebral hemorrhage, and pulmonary infection, respectively. Due to severe toxicity, 2 patients received one and another received 2 courses of consolidation. The remaining 81 patients completed the 3 scheduled consolidation courses. After completing consolidation therapy, all patients proceeded to maintenance therapy. Three deaths in remission occurred during maintenance in patients aged 73, 75, and 78 because of accidental trauma in one and an unknown cause in the other 2. Two of these were in the LPA99 study and one was in the LPA96 study. We call attention to the high degree of compliance of postremission therapy in the elderly population using the same dose and schedule as younger patients. However, whereas the mortality rate during remission was relatively low for patients younger than 70 years (2 of 62), it increased significantly in older patients (6 of 25).
In addition to one case of molecular persistence among 74 tested at the end of consolidation, 5 patients had clinical relapse (1 in the central nervous system) at 13 to 36 months from the achievement of CR, 2 of 25 in the LPA96 study and 3 of 62 in the LPA99 study. Molecular relapse was documented in one patient 1 month prior to clinical relapse. Seven additional patients died after developing other malignancies, 2 in the LPA96 study (1 pancreatic carcinoma and 1 myelodysplastic syndrome [MDS]) and 5 in the LPA99 study (2 colon carcinoma, 2 acute myeloid leukemia [AML], and 1 non-Hodgkin lymphoma). Secondary MDS/AML occurred in patients aged 65, 67, and 68 at 33, 39, and 43 months from APL diagnosis, respectively. Cytogenetics revealed in the 2 AML patients one normal and one complex karyotype (45,XX,–5, add(17)).
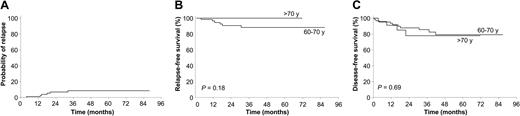
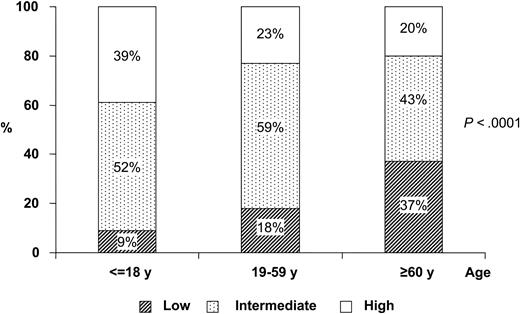
After a median follow-up of 36 months for surviving patients, the 6-year cumulative incidence of relapse (CIR) rate was 8.5% (Figure 1), being 11.2% for patients between 60 and 70 years of age and 0% for those older than 70 years (P = .19). The 4-year CIR rate was 7.2% and 12.5% for patients in the LPA99 and LPA96, respectively (P = .4). For patients who achieved CR, the 6-year estimates of disease-free survival (DFS) and relapse-free survival (RFS) were 79% ± 10% and 91% ± 8%, respectively. For patients in the LPA99 study, the 4-year DFS and RFS rates were 84% ± 10% and 92% ± 8%, respectively, whereas in the LPA96 study, they were 75% ± 18% and 87% ± 8% (P = .6 and P = .4). The high efficacy of treatment observed in these series in noteworthy. Unfortunately, informative estimates regarding the impact of relapse (ie, CIR and RFS) are not available from other reports. However, the GIMEMA study2 reported that 23 of 106 patients at risk underwent relapse, whereas only 5 of 87 patients had relapses in the PETHEMA study. Finally, it is worth noting that the results hereby reported for elderly patients are similar to those achieved by our group for younger patients (data not shown). With respect to this finding, we observed a distinct distribution of relapse risk categories according to age group among 602 patients included in the PETHEMA studies and a significantly higher proportion of low-risk cases among the elderly (P < .0001; Figure 2). A similar distribution of relapse risk groups was found when patients ineligible due to poor performance status or death before starting therapy were included in the analysis. This observation may at least partially explain the very low relapse rate observed in elderly patients receiving ATRA and anthracycline monochemotherapy.
CIR, RFS, and DFS results. Cumulative incidence of relapse from the time of complete remission (A), Kaplan-Meier product-limit estimate of RFS (B), and DFS (C) according to age group.
CIR, RFS, and DFS results. Cumulative incidence of relapse from the time of complete remission (A), Kaplan-Meier product-limit estimate of RFS (B), and DFS (C) according to age group.
Distribution of relapse risk groups among 3 age groups. Distribution is shown for among pediatric, adult, and elderly patients from the PETHEMA studies (n = 602) (according to Sanz et al7 ).
Distribution of relapse risk groups among 3 age groups. Distribution is shown for among pediatric, adult, and elderly patients from the PETHEMA studies (n = 602) (according to Sanz et al7 ).
Appendix
The following institutions and clinicians participated in the study: Argentina (Grupo Argentino de Tratamiento de la Leucemia Aguda): Hospital General San Martín de Paraná, Entre Ríos, P. Negri; Instituto de Trasplante de Médula Ósea, La Plata, V. Prates; Czech Republic: Brno Faculty Hospital, Brno, M. Protivankova; Spain (Programa de Estudio y Tratamiento de las Hemopatías Malignas): Basurtuko Ospitalea, Bilbao, J. M. Beltrán de Heredia; Complexo Hospitalario Xeral-Calde, Lugo, J. Arias; Complejo Hospitalario, León, F. Ramos; Fundación Jiménez Díaz, Madrid, J. F. Tomás; Hospital 12 de Octubre, Madrid, J. de la Serna, J. Martínez; Hospital Carlos Haya, Málaga, S. Negri; Hospital Central de Asturias, Oviedo, C. Rayón; Hospital Clinic, Barcelona, J. Esteve, D. Colomer; Hospital Clínico San Carlos, Madrid, J. Díaz Mediavilla; Hospital Clínico Universitario, Santiago de Compostela, M. Pérez; Hospital Clínico Universitario, Valencia, M. Tormo, I. Marugán; Hospital Clínico Universitario Lozano Blesa, Zaragoza, L. Palomera; Hospital de Cruces, Baracaldo, E. Amutio; Hospital del Mar, Barcelona, C. Pedro; Hospital de Navarra, Pamplona, K. Pérez-Equiza; Hospital Dr Negrín, Las Palmas, T. Molero, M. T. Gómez; Hospital Dr Peset, Valencia, M. J. Sayas; Hospital Dr Trueta, Girona, R. Guardia; Hospital General de Alicante, C. Rivas; Hospital General de Especialidades Ciudad de Jaén, A. Alcalá; Hospital General de Jerez de la Frontera, A. León, L. Hermosín; Hospital Germans Trias i Pujol, Badalona, J. M. Ribera; Hospital Insular de Las Palmas, J. D. González San Miguel; Hospital Juan Canalejo, A Coruña, G. Debén; Hospital Joan XXIII, Tarragona, L. Escoda; Hospital La Princesa, Madrid, R. de la Cámara; Hospital Río Hortega, Valladolid, M. J. Peñarrubia; Hospital San Rafael, Madrid, B. López-Ibor; Hospital Sant Pau, Barcelona, S. Brunet; Hospital San Pedro de Alcántara, Cáceres, J. M. Bergua; Hospital Severo Ochoa, Leganés, P. Sánchez; Hospital Son Dureta, Palma de Mallorca, A. Novo; Hospital de Tortosa, L. L. Font; Hospital Universitario del Aire, Madrid, A. Montero; Hospital Universitario La Fe, Valencia, M. A. Sanz, G. Martín, P. Bolufer, E. Barragán; Hospital Universitario Príncipe de Asturias, Alcalá de Henares, J. García; Hospital Universitario Puerta del Mar, Cádiz, F. J. Capote; Hospital Universitario Vall D'Hebron, Barcelona, J. Bueno; Hospital Universitario Virgen de la Arrixaca, Murcia, P. Rosique; Hospital Universitario Virgen del Rocío, Sevilla, R. Parody; The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON): VU Medical Center Amsterdam, The Netherlands, G. J. Ossenkoppele; Academic Medical Center, University of Amsterdam, The Netherlands, M. H. van Oers; Erasmus MC Rotterdam, The Netherlands, B. Lowenberg; University Hospital K.U. Leuven, Belgium, G. E. G. Verhoef; University Hospital Groningen, The Netherlands, E. Vellenga.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-04-1642.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Luis Benlloch for data collection and management.