Abstract
The initial steps of primitive hematopoiesis and endothelial vascular formation in the human embryo remain to be defined. Here, we report the identification of a novel marker, namely the nonclassical HLA-G class I molecule, which targets both primitive erythroid cells of the yolk sac and endothelial cells from developing vessels. Moreover, HLA-G was present in its soluble form in the erythropoietic lineage in all organs sustaining primitive to definitive erythropoiesis (ie, aorta-gonad-mesonephros, liver, spleen, and bone marrow). The alternatively spliced transcript coding the soluble HLA-G5 molecule was detected in erythroid cells. The corresponding intron 4–retaining 37-kDa HLA-G5 isoform was secreted from the erythroid progenitor stage to the reticulocyte but was lost in mature erythrocytes and in endothelial cells from differentiated vessels. This study constitutes the first description of an HLA class I antigen expression on the primitive erythroid lineage and provides a way of seeking both primitive and definitive erythropoiesis using HLA-G5. This new marker, previously known by its immunotolerogeneic properties, may be involved in erythroid differentiation, angiogenesis, or both.
Introduction
Over the past few years, studies have been carried out aimed at understanding the spatial and temporal generation and organization of the human hematopoietic system during ontogeny. Current research has identified 2 independent generations of hematopoietic progenitor cells which differ in their potential for self-renewal and differentiation, and which are produced in the embryo at distinct times and in specific locations.1 The first hematopoietic precursors originate from the extraembryonic yolk sac (YS) in the first stages of development and only lead to short-term erythromyeloid reconstitution, referred to as primitive hematopoiesis. Later, the intraembryonic para-aortic splanchnopleura/aorta-gonad-mesonephros (P-Sp/AGM) region produces the hematopoietic stem cells (HSCs), corresponding to the self-renewing, multipotent precursors that further colonize the fetal liver (FL) and thymus. This later process is referred to as the definitive hematopoiesis, which starts in the bone marrow (BM) from the second trimester of intraembryonic life and continues throughout adult life. However, works in mice, for which ontogeny of hematopoiesis is similar to that of humans, has demonstrated that HSC activity also arises from the yolk sac.2-4
We here identify a novel pattern of distribution of the nonclassical HLA class I molecule, HLA-G, namely the erythroid cells in all organs sustaining primitive and definitive hematopoiesis and the endothelial cells forming developing vessels. This double expression of HLA-G in both endothelial and erythroid cells is of particular interest in the context of the proposed precursor common to both lineages, called the hemangioblast.5
The nonclassical HLA class I molecule, HLA-G, plays a major role in human reproduction because it is expressed from oocyte to blastocyst stages in the human embryo6 in which it constitutes a fundamental prerequisite for the success of implantation and subsequent pregnancy in humans.7 HLA-G is also important along the pregnancy because its expression on invasive trophoblast8 has been shown to protect the fetal semiallograft from maternal immune rejection.9 These findings have pointed out some of the specific characteristics of this HLA molecule that differ from the other classical HLA class I molecules by (1) its restricted distribution to immune privileged sites such as trophoblast, thymus, and cornea; (2) its limited polymorphism; and (3) the alternative splicing of its primary transcript creating at least 7 distinct mRNAs, coding for 4 membrane-bound (HLA-G1 to -G4) and 3 soluble (HLA-G5 to -G7) proteins.10 The immunotolerizing properties of the soluble HLA-G5 isoform are similar to those of the membrane forms, namely the inhibition of natural killer (NK) cell– and cytotoxic T lymphocyte (CTL)–mediated cytolysis11,12 and of alloproliferative T-cell response.13 In addition, the soluble HLA-G5 isoform has been described to display the specific ability to induce apoptosis of T and NK CD8+ cells.14,15 Beside its immunologic properties, the present study suggests a novel role for HLA-G that may be viewed as a novel signal implied into cross talk between erythropoiesis and angiogenesis.
Materials and methods
Tissues
Human embryonic and fetal tissues were obtained from archived slides of extrauterine pregnancies, miscarriages, and elective abortions. These samples were obtained from the Institute of Histo-Cytopathology and the Fetopathology Laboratory of Hospital Pellegrin (Bordeaux, France). Local committee approval was obtained for this study. Embryo developmental age was estimated on the basis of several anatomic criteria according to Carnegie classification (Table 1). Umbilical cord blood (CB) units from normal full-term deliveries were obtained, after informed consent of the mothers, from the Obstetrics Unit of Hospital Robert Debré, Paris, France. BM from children and adults aged 2, 5, 40, and 67 years were obtained from the Pathology Laboratory of Hospital Pellegrin (Bordeaux, France) following normal staging for neoplasia. One sample of BM was obtained from an 82-year-old patient suffering from a fracture without associated neoplasia. A total of 19 embryos, 5 fetuses, and 5 adults were selected for this study from which a total of 56 tissue samples were analyzed.
Monoclonal antibodies
The 5A6G7 monoclonal antibody (mAb) immunoglobulin G1 (IgG1) was made by standard procedures from splenocytes of Balb/c mice immunized with an ovalbumin-bound synthetic 21-mer peptide (SKEGDGGIMSVRESRSLSEDL) derived from the carboxy-terminal sequence of soluble HLA-G5 and HLA-G6 proteins.16 The 5A6G7 mAb used was purified from ascites by using protein A affinity chromatography. Specificity for soluble HLA-G5 and -G6 proteins was demonstrated by using 5 criteria: (1) detection of the 37-kDa HLA-G5 and the 28-kDa HLA-G6 proteins but not of the other HLA-G isoforms and HLA class I molecules present in protein lysates from M8 cells transfected with either the pcDNA vector alone, HLA-G1, -G2, -G3, -G4, -G5, or -G6 cDNA12,17,18 ; (2) no detection of HLA-A, -B, -C, and -E proteins by Western blot analysis of protein lysates from several human cells expressing distinct HLA class I alleles; (3) immunoprecipitation of the 37-kDa HLA-G5 soluble protein from M8-HLA-G5 cell supernatant; (4) specific immunocytochemical staining of the M8-HLA-G5 and M8-HLA-G6 cells but not of the other M8-pcDNA, -HLA-G1, -G2, -G3, and -G4 transfected cells nor of peripheral blood mononuclear cells from several healthy adult donors expressing distinct HLA class I alleles; and (5) immunohistochemical staining of paraffin-embedded trophoblast tissue section but not of paraffin-embedded normal adult tissues sections (ie, skin, liver, kidney, breast, endometrium, duodenum). Notably, this mAb allows us to discriminate between soluble HLA-G protein yielded by shedding and membrane-bound HLA-G forms that do not contain the intron 4–encoded epitope and soluble HLA-G5/-G6 produced from intron 4–retaining alternatively spliced mRNAs.
The monoclonal antibody to the CD71 transferrin receptor (Novocastra Laboratories, Newcastle, United Kingdom) was used to identify erythroid cells. The CD34 (Novocastra Laboratories) and the CD45 (Dako, Trappes, France) mAbs refer to endothelial (for the CD34 only) and hematopoietic progenitors (for both CD34 and CD45).
Immunochemistry analysis
Immunohistochemistry analysis. Deparaffinized tissue sections were subjected to epitope retrieval treatment by high temperature in 10 mM sodium citrate buffer (pH 6), using a commercial microwave to optimize immunoreactivity. Tissue sections were permeabilized by using phosphate-buffered saline (PBS), 1 × 0.1% saponin, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid hemisodium salt) buffer. Endogenous peroxidase activity was quenched by treating sections for 5 minutes at room temperature with 3% hydrogen peroxide in water. Nonspecific binding was prevented by applying 50 mM Tris (tris(hydroxymethyl)aminomethane), 3% bovine serum albumin (BSA); Sigma, Saint Quentin Fallavier, France), 40% human serum for 20 minutes before staining with the primary mAb for 30 minutes at room temperature. Then, HLA-G protein expression was evaluated on serial tissue sections, using the Dako EnVision kit (Dako), according to manufacturer's instructions and as previously described.19
Acquisition of all figure images was performed with an Olympus BX51 microscope equipped with a 40 × /0.75 semi-apochromatic Universal Plan (UPLFL) objective lens and an Olympus DP12 camera (all from Olympus Europa GmbH, Rungis, France). Adobe Photoshop 7.0 software (Adobe, San Jose, CA) was used for image processing.
Immunocytochemistry analysis. Immunocytochemistry analysis on the cryopreserved M8 transfectants was performed, using the UltraTech HRP (horseradish peroxidase) Streptavidin-Biotin Universal Detection system (Immunotech, Marseille, France), as previously described.20
In situ hybridization
Probe preparation. Total RNA was extracted from the HLA-G–positive JEG-3 choriocarcinoma cell line, by RNA-WIZ reagent (Ambion, Austin, TX) according to the manufacturer's instructions. A reverse transcription was realized on 5 μg total RNA by Moloney murine leukemia virus (MMLV)–reverse transcriptase (Gibco-BRL, LIFE Technologies, Cergy-Pontoise, France) for 1 hour at 42°C. Then, a polymerase chain reaction was performed using G1089F (5′-CCCTTTGTGACTTCAAGAAC) and G1252R (5′-AAGTTATAGCTCAGTGGACC) specific primers. This fragment was cloned in a TA vector and used as template to produce digoxigenin-labeled DNA probe. Then, a polymerase chain reaction was performed with Taq polymerase (Applied Biosystems, Courtaboeuf, France) on 12 ng plasmid with a mix containing 0.3 μM of each HLA-G specific primers G1089F and G1252R; and 0.2 mM deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP); 0.19 mM deoxythymidine triphosphate (dTTP); and 10 μM digoxigenin-labeled deoxyuridine triphosphate (DIG-dUTP; Roche Diagnostics, Meylan, France). This amplification provided a 183-bp DNA fragment located in the 3′-untranslated region labeled by DIG-dUTP. Polymerase chain reaction product was purified through Microcon filter 50 (Millipore, Saint Quentin on Yvelines, France) by centrifugation.
Hybridization procedure. After dewaxing and rehydrating, 5-μm paraffin-embedded trophoblastic sections were permeabilized for 30 minutes at 37°C with 0.1% pepsin in 0.2 M HCl. Sections were postfixed for 10 minutes at 4°C with PBS containing 4% paraformaldehyde. Prehybridization was carried out for 2 hours at 37°C in 4 × SSC (standard saline citrate) containing 50% (vol/vol) deionized formamide. Hybridization was performed overnight at 37°C in a moist chamber by using the hybridization solution: 60% deionized formamide, 300 mM NaCl, 30 mM sodium citrate, 10 mM EDTA (ethylenediaminetetraacetic acid), 25 mM NaH2PO4 (pH 7.4), 5% dextran sulfate, and 250 ng/μL sheared salmon DNA, which does or does not contain the denatured DIG-labeled HLA-G DNA probe at a concentration of 2 to 4 ng/μL. After hybridization, sections were washed in 60% formamide, 300 mM NaCl, and 30 mM sodium citrate. Sections were incubated for 30 minutes at room temperature in 100 mM Tris (tris(hydroxymethyl)aminomethane)–HCl and 150 mM NaCl containing 0.1% Triton X-100 and 2% normal sheep serum (Roche Diagnostics). Then they were incubated for 1 hour with 1/1000 dilution of sheep anti-DIG–alkaline phosphatase Fab fragments (Roche). After washes, sections were incubated for 15 minutes in the dark in the substrates of alkaline phosphatase: 2% nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in 100 mM Tris-HCl pH 8.1 and 1 mM EDTA, containing 1 mM Levamisole (Sigma). The reaction was stopped by incubating the sections in a 10 mM Tris-HCl pH 8.1 and 1 mM EDTA solution and rinsing them in distilled water. Sections were counterstained for 5 minutes with 0.02% Fast Green in distilled water. Sections were mounted in Glycergel (Dako).
RT-PCR, cloning, and sequencing
Total RNA was isolated from frozen samples using RNA-WIZ reagent (Ambion, Huntingdon, United Kingdom) according to manufacturer's recommendations. RNA was treated with RNase-free DNase I (Boehringer-Mannheim, Meylan, France) to remove contaminating chromosomal DNA and quantified by spectrophotometry at 260 nm. RNA (5 μg) was reverse-transcribed into cDNA using oligo (dT) primers (Invitrogen, Cergy-Pontoise, France) and MMLV-reverse transcriptase (Life Technologies, Cergy-Pontoise, France) for 1 hour at 42°C. Heating for 8 minutes at 95°C stopped the reaction. For amplification, 35 cycles of polymerase chain reaction (PCR; at 94°C for 30 seconds, 61°C for 30 seconds, and 72°C for 1 minute) were performed. PCR product was run in a 1.5% agarose electrophoresis gel and transferred to a nylon membrane (Hybond-N+; Amersham, Orsay, France) by an alkaline blotting procedure. Soluble HLA-G transcripts were amplified from the promoter with CP5 forward primer (5′-GCTCTAGAGCTCCCCAGACGCCAAGGATG) to the intron 4 with G.2734 reverse primer (5′-CTGGGAAAGGAGGTGAAGGT). The specificity of the amplifications was confirmed by subsequent hybridization with 32P-labeled internal probe located either in exon 2 (G.R, 5′-GGTCTGCAGGTTCATTCTGTC), in exon 3 (G.526R, 5′-CCTTTGTTCAGCCACATTGG), or in exon 4 (G.647F, 5′-CCACCACCCTGTCTTTGACT). β-Actin transcripts were detected by using a forward primer (5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG), a reverse (5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC) primer, and an internal β-actin probe (5′-ATCATGTTTGAGACCTTCAACACCCCAGCC). Soluble HLA-G transcripts amplified from CP5/G.2734 were cloned into pGEM T-Easy vector (Invitrogen) and subsequently sequenced.
Western blot analysis
Nine-week and 12-week fetal livers were homogenized and incubated in ice for 2 hour in 1 mL lysing buffer containing 1% NP40 in 25 mM Tris-HCl pH 7.4, 150 mM NaCl, and Complete (Boehringer Mannheim). Protein extracts (40 μL) solubilized in Laemmli buffer and boiled were applied to 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels and electrophoresed. M8-HLA-G5 and M8-pcDNA transfected cell lysates were used as HLA-G5–positive and –negative controls, respectively. Western blot analysis was performed as previously described.21 The blotted membrane was immunolabeled with the 5A6G7 mAb at 1:1000 dilution. Immunocomplexes were revealed by using a horseradish peroxidase–conjugated goat anti–mouse IgG (Sigma) and enhanced chemiluminescence (ECL) detection system (Amersham).
Erythroid cell cultures
The 2-phase liquid culture was used as previously described.22,23 In short, light density cord blood cells were cultured for 7 days at 37°C under 5% CO2 in air, in the appropriate medium containing 10% of supernatant from cultures of the HLA-G–negative 56.37 bladder carcinoma cell line. After this phase 1 culture, the nonadherent cells were seeded in a new medium containing 1 U/mL human recombinant erythropoietin (Roche) for 5 to 6 days (referred to as phase 2 culture).
Enzyme-linked immunosorbent assays
Soluble HLA-G5 concentrations were measured in cell-free supernatants of 2-phase liquid cultures at the end of the phase 1 and 2, with a specific sandwich enzyme-linked immunosorbent assay (ELISA) according to standard procedure. In short, microtiter plates were coated with the 5A6G7 mAb (5 μg/mL). After extensive washes and saturation, 100 μL sample was added to each well. A biotinylated-W6/32 mAb (Leinco Technologies, St Louis, MI), recognizing a monomorphic determinant of β2-microglobulin associated HLA class I heavy chains, diluted 1/250, was used as detection antibody. Revelation was achieved by using AMDEX streptavidin-conjugated to horseradish peroxidase (Amersham, Orsay, France) followed by the TMB substrate (3,3′,5,5′ Tetramethylbenzidine; Sigma, St Quentin-Fallavier, France). Reaction was stopped by the addition of HCl 1N. Optical densities were measured at 450 nm. Standard curves were performed by using serial dilutions of purified recombinant soluble HLA-G5 protein (HLA-G Technologies, Lyon, France). Soluble HLA-G5 concentration was determined from the value of optical density according to the standard curves. Supernatants cultured from the KG1a cell line and the M8-HLA-G5 transfected cells were used as negative and positive controls, respectively. The HLA-G5 detection limit of the ELISA test was 5 ng/mL.
Results
Detection of soluble HLA-G in cells flowing in chorionic vessels from first-trimester placenta
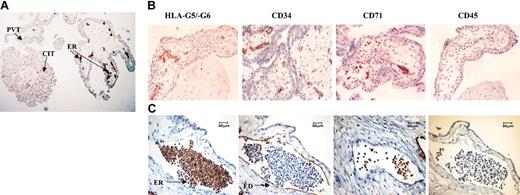
To investigate soluble HLA-G expression in human body fluids and its distribution in various tissues in normal and pathologic conditions, we used a novel monoclonal antibody, named 5A6G7, which recognizes specifically soluble HLA-G5 and -G6 isoform proteins.16 As expected, soluble HLA-G5/-G6 proteins were detected in cell island trophoblast, but not in perivillous trophoblast (Figure 1A). In addition and unexpectedly, these soluble HLA-G proteins were found in cells either in close contact with cytotrophoblast-associated budding vessels or in the lumen of differentiated vessels (Figure 1A-C).
Localization of HLA-G proteins in erythroid cells from first-trimester trophoblast by immunohistochemistry. (A) Expression of soluble HLA-G5/-G6 by cell island trophoblast (CIT) and by erythroid (ER) cells but not by perivillous trophoblast (PVT) was determined by using the 5A6G7 mAb. (B) Analysis of HLA-G5/-G6, CD34, CD71, and CD45 expression on the developing vessels present in first-trimester trophoblast tissue. (C) Immunohistochemical staining of serial trophoblast sections of a differentiated vessel from a 32-day embryo. Analysis was performed by using antibodies to HLA-G5/-G6 (5A6G7 mAb), CD34 which marks endothelial (ED) cells lining vessel, CD71 which identifies erythroid (ER) cells, and CD45 which targets both lymphoid and myeloid cells. Original magnifications: × 100 (A), × 200 (B), and × 400 (C).
Localization of HLA-G proteins in erythroid cells from first-trimester trophoblast by immunohistochemistry. (A) Expression of soluble HLA-G5/-G6 by cell island trophoblast (CIT) and by erythroid (ER) cells but not by perivillous trophoblast (PVT) was determined by using the 5A6G7 mAb. (B) Analysis of HLA-G5/-G6, CD34, CD71, and CD45 expression on the developing vessels present in first-trimester trophoblast tissue. (C) Immunohistochemical staining of serial trophoblast sections of a differentiated vessel from a 32-day embryo. Analysis was performed by using antibodies to HLA-G5/-G6 (5A6G7 mAb), CD34 which marks endothelial (ED) cells lining vessel, CD71 which identifies erythroid (ER) cells, and CD45 which targets both lymphoid and myeloid cells. Original magnifications: × 100 (A), × 200 (B), and × 400 (C).
To precisely identify these HLA-G–positive cells which morphologically resemble erythroblasts, we performed an immunohistochemistry analysis on trophoblast sections from a 32-day embryo by using mAbs staining either HLA-G5/-G6 soluble proteins, or CD34, or CD71, or CD45 antigens. HLA-G5, HLA-G6, or both isoforms were present in the entire CD71-expressing erythroid cell subpopulation, whereas they appeared only in a few CD34-expressing stem cells (Figure 1B-C). On the contrary, endothelial cells in mature chorionic vessels were positive for CD34 but not for soluble HLA-G (Figure 1C). Notably, these erythroid cells were not stained by using the other known anti–HLA-G mAbs, namely 4H8424 and MEM-G/01,21 which both recognize an epitope present into the α1 extracellular domain common to all HLA-G isoforms (data not shown). This discrepancy may be explained by a different conformation and/or glycosylation of the HLA-G protein in these cells, or by the expression of a novel alternative splicing form of HLA-G generating an intron 4–encoding HLA-G isoform that lacks the region containing the epitope recognized by 4H84 and MEM-G/01 mAbs. Unfortunately, the 16G1 mAb which shares the same antigenic specificity with the 5A6G7 mAb25 could not be tested in our experiments because of its current unavailability.
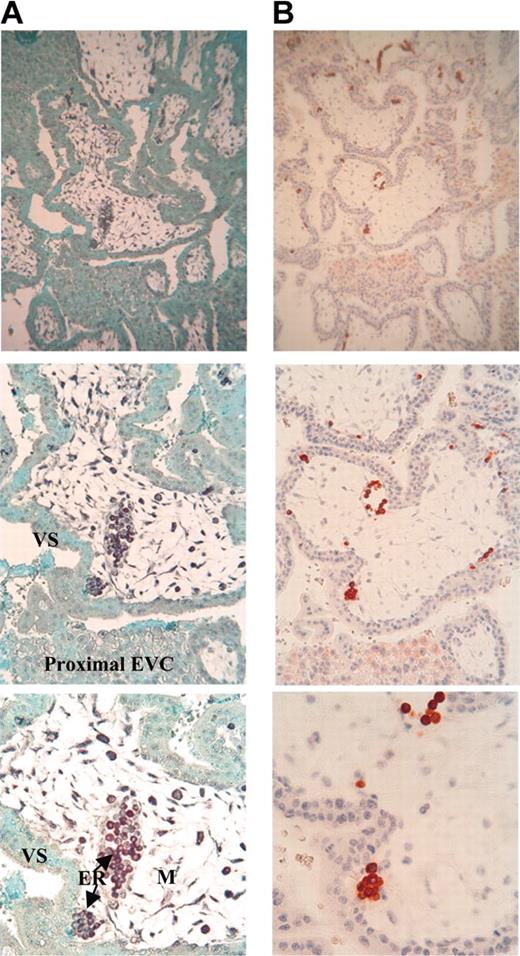
To confirm this result, HLA-G mRNA distribution was studied by in situ hybridization in sections of first-trimester trophoblast by using a pan HLA-G DNA probe. Intense labeling was observed in the erythroid cells flowing in trophoblastic vessels, showing the presence of HLA-G transcripts in erythroblasts (Figure 2A). These erythroid cells were those expressing HLA-G5, -G6, or both, as they were highly stained by 5A6G7 mAb on a serial trophoblastic section (Figure 2B). A lower positive signal was detected in cytotrophoblast cells by carrying out both in situ hybridization and immunohistochemistry analyses. As expected, villous syncytiotrophoblast was not stained by the HLA-G probe.26 Of note, as previously described,26 connective tissue was stained with the HLA-G probe. However, no staining with 5A6G7 mAb was observed in this tissue, revealing HLA-G gene transcription without protein translation, as well as described in many fetal and adult tissues.10,27 No labeling was observed in serial trophoblastic section incubated without the HLA-G DNA probe (data not shown).
Colocalization of HLA-G mRNA and HLA-G protein in erythroid cells from first-trimester trophoblast. (A) Detection of either HLA-G mRNA (black) by in situ hybridization with DIG-labeled Pan HLA-G DNA probe or (B) HLA-G5/-G6 proteins (red) by immunohistochemistry by using the 5A6G7 mAb (right panel) on serial sections of first-trimester trophoblast (magnification from the top to the back of the photo: × 100, × 200, × 400). Sections were counterstained with Fast green and hematoxylin to visualize nuclei by in situ hybridization and immunohistochemistry analyses, respectively. EVC indicates extravillous cytotrophoblast; VS, villous syncytiotrophoblast; and M, mesenchymal core.
Colocalization of HLA-G mRNA and HLA-G protein in erythroid cells from first-trimester trophoblast. (A) Detection of either HLA-G mRNA (black) by in situ hybridization with DIG-labeled Pan HLA-G DNA probe or (B) HLA-G5/-G6 proteins (red) by immunohistochemistry by using the 5A6G7 mAb (right panel) on serial sections of first-trimester trophoblast (magnification from the top to the back of the photo: × 100, × 200, × 400). Sections were counterstained with Fast green and hematoxylin to visualize nuclei by in situ hybridization and immunohistochemistry analyses, respectively. EVC indicates extravillous cytotrophoblast; VS, villous syncytiotrophoblast; and M, mesenchymal core.
All of these observations taken together identified the presence of HLA-G5/-G6 on hematopoietic cells belonging to the erythropoietic lineage.
Identification of the HLA-G5 protein as the soluble HLA-G isoform present in fetal erythroblasts
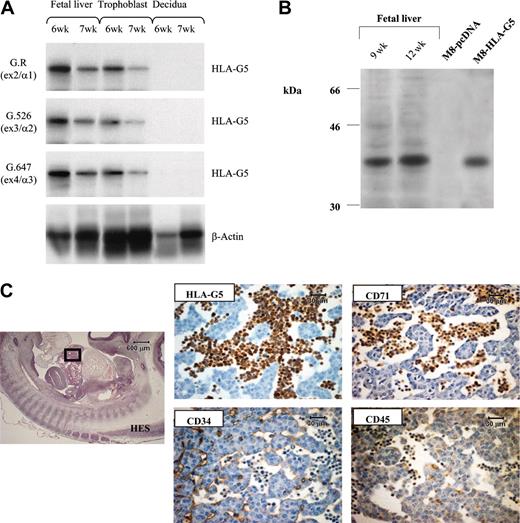
To characterize the HLA-G soluble isoform present in embryonic erythroid cells, we used the fetal liver, because it is the fetal organ in which erythroblasts constitute the major cells. Reverse transcriptase (RT)–PCR was performed by using an intron 4–specific primer on total RNA from 6-week and 7-week fetal livers. Trophoblast villi from the same fetus and decidua from the corresponding mother were used as positive and negative controls of HLA-G gene transcription, respectively (Figure 3A). In both fetal livers, hybridization experiments using probe specific for either exon 2, 3, or 4 showed that in all cases a band is detected at the expected size of the cDNA product encoding HLA-G5 soluble isoform (Figure 3A). The presence of HLA-G5 transcript was confirmed by sequence analysis of the PCR amplification products. By carrying out Western blot analysis of fetal liver extracts, we identified a protein of 37 kDa, which corresponds to the molecular weight of the HLA-G5 heavy chain (Figure 3B). Thus, the soluble HLA-G protein detected on fetal erythroblasts is the HLA-G5 isoform. Supporting this result, numerous HLA-G5–positive cells, of erythroid phenotype, were observed in the lumen of the sinusoids from a fetal liver by immunohistochemistry analysis (Figure 3C).
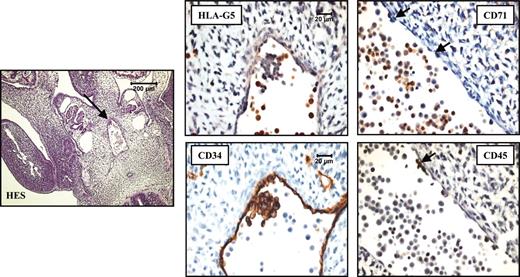
HLA-G5 is the HLA-G isoform present in erythroid cells from fetal liver. (A) Identification of HLA-G5 transcript in liver from 6-week (6w) and 7-week (7w) fetuses was determined by RT-PCR analysis and subsequent hybridizations. Specific amplification of soluble HLA-G was conducted by using forward primer CP5 and G.2734 primer sets. HLA-G products were detected by using either the G.R probe (specific for exon 2 encoding the α1 extracellular domain), the G.526 probe (specific for exon 3 encoding the α2 domain), or the G.647 probe (specific for exon 4 encoding the α3 domain). β-Actin products were amplified in the same reaction and were detected by using aβ-actin probe. Trophoblast villi from the same fetus and decidua from the corresponding mother were used as positive and negative controls of HLA-G gene transcription, respectively. (B) Identification of the 37-kDa HLA-G5 isoform was determined by running 1/25 of the total proteins extracted from 2 fetal livers of 9 weeks (9w) and 12 weeks (12w) on a 12% SDS-PAGE (polyacrylamide gel electrophoresis) gel followed by immunoblotting with the 5A6G7 mAb. The M8-HLA-G5 or M8-pcDNA transfectants were used as HLA-G5–positive and –negative controls, respectively. (C) Hematoxylin Eosin-Safran (HES) coloration of a paraffin-embedded 32-day embryo section (left) localizes the region of the liver for which higher magnifications of the same liver region stained with antibodies to CD34, HLA-G5, CD45, and CD71 are represented (right panel). HLA-G5 is detected in the erythroid cells which originate from the YS and are present in the lumen of sinusoids (embryo capillaries). The CD34 is positive in endothelial cells. Left panel is shown at × 20 original magnification; right panels are shown at × 400.
HLA-G5 is the HLA-G isoform present in erythroid cells from fetal liver. (A) Identification of HLA-G5 transcript in liver from 6-week (6w) and 7-week (7w) fetuses was determined by RT-PCR analysis and subsequent hybridizations. Specific amplification of soluble HLA-G was conducted by using forward primer CP5 and G.2734 primer sets. HLA-G products were detected by using either the G.R probe (specific for exon 2 encoding the α1 extracellular domain), the G.526 probe (specific for exon 3 encoding the α2 domain), or the G.647 probe (specific for exon 4 encoding the α3 domain). β-Actin products were amplified in the same reaction and were detected by using aβ-actin probe. Trophoblast villi from the same fetus and decidua from the corresponding mother were used as positive and negative controls of HLA-G gene transcription, respectively. (B) Identification of the 37-kDa HLA-G5 isoform was determined by running 1/25 of the total proteins extracted from 2 fetal livers of 9 weeks (9w) and 12 weeks (12w) on a 12% SDS-PAGE (polyacrylamide gel electrophoresis) gel followed by immunoblotting with the 5A6G7 mAb. The M8-HLA-G5 or M8-pcDNA transfectants were used as HLA-G5–positive and –negative controls, respectively. (C) Hematoxylin Eosin-Safran (HES) coloration of a paraffin-embedded 32-day embryo section (left) localizes the region of the liver for which higher magnifications of the same liver region stained with antibodies to CD34, HLA-G5, CD45, and CD71 are represented (right panel). HLA-G5 is detected in the erythroid cells which originate from the YS and are present in the lumen of sinusoids (embryo capillaries). The CD34 is positive in endothelial cells. Left panel is shown at × 20 original magnification; right panels are shown at × 400.
Extended distribution of the HLA-G5 soluble isoform to all embryonic and fetal hematopoietic organs
We then analyzed the distribution of HLA-G5 in all embryonic and fetal hematopoietic organs by conducting immunohistochemistry on both embryo and fetal tissue sections.
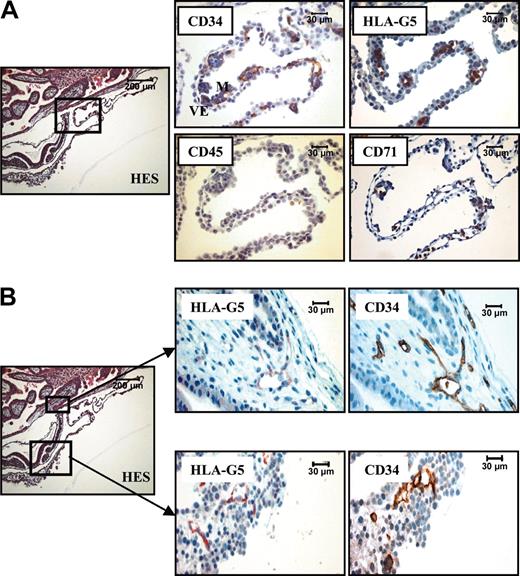
In a 16-day embryo, the CD34 staining appeared to surround the HLA-G5–positive cells in the mesoderm of the extraembryonic yolk sac (YS), allowing the detection of endothelial cells from the first developing vessels (Figure 4A). In contrast to this, the primitive hematopoietic cells present in these budding vessels were costained by monoclonal antibodies to CD71 and HLA-G5 but not by mAb to CD34. At this developmental stage, no cell expressed the CD45 marker. These results are in agreement with previous observations, showing that in a 19-day embryo, the first hematopoietic cells are from the erythroid lineage and do not express the CD34 marker.28 Soluble HLA-G was also identified in endothelial cells in budding vessels from 2 distinct localizations: the juxta-embryonic part of the YS and the mesenchymal core of the chorionic villi (Figure 4B). In both cases, HLA-G colocalized with the CD34 marker. It is of note that endothelial cells in differentiated vessels lost their expression of soluble HLA-G, as illustrated in chorionic vessel (Figure 1C).
HLA-G5 is present in erythroid and endothelial cells from a 16-day embryo. (A) Hematoxylin Eosin-Safran (HES) coloration of a paraffin-embedded 16-day embryo section (left) localizes the region of the YS for which higher magnifications of the same YS region stained with antibodies to CD34, HLA-G5, CD45, and CD71 are represented (right panel). Visceral endoderm and mesoderm are identified by VE and M, respectively, in the CD34 box. (B) The top boxed area is the chorionic plate (left) for which higher magnifications of the same region stained with antibodies to HLA-G5 and CD34 are represented (top right panel). The bottom area is the closed mesoderm (YS) (left) for which higher magnifications of the same region stained with antibodies to HLA-G5 and CD34 are represented (bottom right panel). In both localizations, HLA-G5 stains the CD34-positive endothelial cells. In both A and B, left panels are shown at × 100, and right panels at × 400.
HLA-G5 is present in erythroid and endothelial cells from a 16-day embryo. (A) Hematoxylin Eosin-Safran (HES) coloration of a paraffin-embedded 16-day embryo section (left) localizes the region of the YS for which higher magnifications of the same YS region stained with antibodies to CD34, HLA-G5, CD45, and CD71 are represented (right panel). Visceral endoderm and mesoderm are identified by VE and M, respectively, in the CD34 box. (B) The top boxed area is the chorionic plate (left) for which higher magnifications of the same region stained with antibodies to HLA-G5 and CD34 are represented (top right panel). The bottom area is the closed mesoderm (YS) (left) for which higher magnifications of the same region stained with antibodies to HLA-G5 and CD34 are represented (bottom right panel). In both localizations, HLA-G5 stains the CD34-positive endothelial cells. In both A and B, left panels are shown at × 100, and right panels at × 400.
Later in the embryo development, we revealed the presence of pluripotent stem cells by their coexpression of CD34 and CD45 antigens, and as they cluster to the ventral wall of dorsal aorta29,30 (Figure 5). In a 32-day embryo, neither CD34 nor CD45 staining matched that of HLA-G5 in the intraembryonic P-Sp/AGM (Figure 5), which is evidence of a specific HLA-G5 localization on erythroid cells. We also detected HLA-G5 in circulating erythroblasts that flow in all vessels from early embryo to term fetus (data not shown).
HLA-G5 localizes in the erythroid cells in AGM from a 32-day embryo. HES coloration of a 32-day-paraffin-embedded embryo section (left) permits the localization of the AGM (arrow) for which higher magnifications of the same AGM region stained with antibodies to HLA-G5/-G6, CD71, CD34, and CD45 are represented (right panel). HLA-G5 staining was examined regarding the CD71-expressing erythroid cells that flow in the aorta, the CD34-positive endothelial cells lining the aorta, and the CD34- and CD45-positive pluripotent stem cells which form a cluster associated to the ventral wall of the dorsal aorta. The arrows point to the few residual pluripotent stem cells in the sections stained with the anti-CD71 or anti-CD45 mAb. Left panel is shown at× 100, and right panels at × 600.
HLA-G5 localizes in the erythroid cells in AGM from a 32-day embryo. HES coloration of a 32-day-paraffin-embedded embryo section (left) permits the localization of the AGM (arrow) for which higher magnifications of the same AGM region stained with antibodies to HLA-G5/-G6, CD71, CD34, and CD45 are represented (right panel). HLA-G5 staining was examined regarding the CD71-expressing erythroid cells that flow in the aorta, the CD34-positive endothelial cells lining the aorta, and the CD34- and CD45-positive pluripotent stem cells which form a cluster associated to the ventral wall of the dorsal aorta. The arrows point to the few residual pluripotent stem cells in the sections stained with the anti-CD71 or anti-CD45 mAb. Left panel is shown at× 100, and right panels at × 600.
Erythroid cells from umbilical cord blood and adult bone marrow express HLA-G5
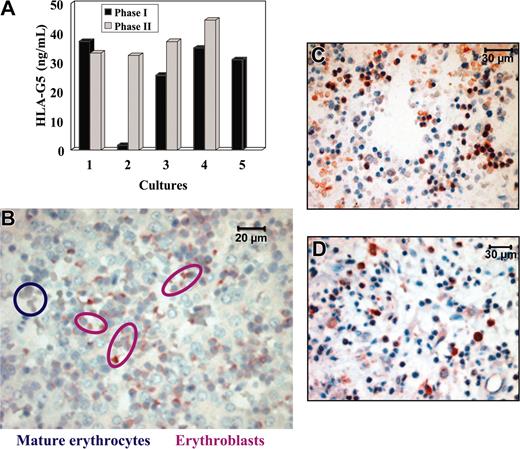
We then asked whether HLA-G5 is produced directly by erythroblasts, or whether it is secreted elsewhere in the body. In this latter case, HLA-G5 would bind to erythroblast cell surface through ligation with specific HLA-G receptors. For this purpose, we examined whether HLA-G5 is secreted during the in vitro erythroid differentiation in a 2-phase liquid culture from light-density cord blood cells.31 First, we checked that the 2-phase liquid culture allowed differentiation of erythroid cells that are positive for CD71 and coexpress the CD36 at the end of the phase 2 culture31 (data not shown). Moreover, we verified that no endothelial cells were present during the entire period of the culture, as no vascular endothelial cadherin-positive cells were detected by immunocytochemistry (data not shown). Second, the concentration of HLA-G5 in supernatants was evaluated by an ELISA which specifically measures soluble HLA-G5/-G6. The culture supernatant of M8-HLA-G5 cells was used as positive control, and HLA-G5 concentration was more than 70 ng/mL. No HLA-G5 protein was detected in the culture supernatant of the HLA-G–negative KG1a leukemic cells. In this experiment, light-density cord blood cell culture supernatants were collected at the end of the phase 1 (which mainly contains erythroid burst-forming unit [BFU-E] and to a lesser extent erythroid colony-forming unit [CFU-E]), and at the end of the phase 2 (which mainly contains reticulocytes). Notably, whatever the stage of culture, ie, from the most primitive to the most differentiated erythroid cell, HLA-G5 was detected in all the supernatants tested, except for one culture (Figure 6A). HLA-G5 concentration ranged between 25.5 and 44.3 ng/mL (mean = 34.4 ng/mL from 4 separate cell culture experiments). It is of note that HLA-G5 concentration in the serum of healthy donors is less than 5 ng/mL using this ELISA.
HLA-G5 is secreted by erythroblasts but not by mature erythrocytes. (A) Detection of HLA-G5 in the supernatants of a 2-phase liquid culture from light-density cord blood cells using an HLA-G5-specific ELISA. The culture supernatant of M8-HLA-G5 cells was used as positive control and HLA-G5 concentration was more than 70 ng/mL. No HLA-G5 proteins were detected in the culture supernatant of KG1a leukemic cells used as negative control. (B) Immunolocalization of HLA-G5 in erythroblasts but not in mature erythrocytes from a 16-week fetal liver. Magnification is × 600. (C) Immunohistochemistry analysis of 16-week fetal BM using the 5A6G7 mAb. (D) Immunohistochemistry analysis of HLA-G5 expression by erythroid cells on paraffin-embedded bone marrow section from an 82-year-old adult. Magnification is × 400 in panels C and D.
HLA-G5 is secreted by erythroblasts but not by mature erythrocytes. (A) Detection of HLA-G5 in the supernatants of a 2-phase liquid culture from light-density cord blood cells using an HLA-G5-specific ELISA. The culture supernatant of M8-HLA-G5 cells was used as positive control and HLA-G5 concentration was more than 70 ng/mL. No HLA-G5 proteins were detected in the culture supernatant of KG1a leukemic cells used as negative control. (B) Immunolocalization of HLA-G5 in erythroblasts but not in mature erythrocytes from a 16-week fetal liver. Magnification is × 600. (C) Immunohistochemistry analysis of 16-week fetal BM using the 5A6G7 mAb. (D) Immunohistochemistry analysis of HLA-G5 expression by erythroid cells on paraffin-embedded bone marrow section from an 82-year-old adult. Magnification is × 400 in panels C and D.
On the basis of these data, we concluded that HLA-G5 is indeed secreted by erythroid cells during all phases of differentiation. Therefore, HLA-G5 appears to be a driven-erythroid lineage marker which is likely to play a role in erythropoiesis.
To investigate whether HLA-G5 is produced in the last stages of erythropoiesis, we performed an immunohistochemistry analysis using the 5A6G7 mAb on a 16-week fetal liver (Figure 6B) and on 16-week fetal BM (Figure 6C). Interestingly, HLA-G5 was detected in erythroblasts but not in mature erythrocytes. In addition, mature erythrocytes flowing into embryo and fetal vessels were not stained by the 5A6G7 mAb (data not shown). Finally, we investigated whether the expression of HLA-G5 is maintained in the erythropoietic lineage during adult life, ie, in the bone marrow, as hematopoiesis is localized in this organ in adults. Results showed that HLA-G5 protein was localized in erythroid cells from BM from an 82-year-old adult, taken as a representative example (Figure 6D). Similar results were obtained from children's BM (data not shown).
Discussion
By studying a total of 56 embryo, fetal, and adult tissue samples, we here identify for the first time a novel pattern of expression of the nonclassical HLA-G class I molecule, as a soluble form, which is a marker of the erythropoietic lineage in all hematopoietic organs in time and space. This constitutes the first description of an HLA molecule expressed by the primitive erythroid lineage. Indeed, no classical HLA class I and II expression has been reported in the YS, whereas these HLA molecules have been described as gradually expressed on the erythroid progenitors and precursors from the fetal liver.32 In our present study, soluble HLA-G5 protein never localizes on CD34+ CD45+ hematopoietic stem cells in the hematopoietic organs from a 32-day embryo. Thus, we concluded that soluble HLA-G5 either is not secreted or does not act on the multipotent stem cells. On the contrary, soluble HLA-G5 is present in the CD71+ cells, the transferrin receptor being expressed at the cell surface of erythroid cells.33 This colocalization exists not only during the entire course of embryo and fetal development in hematopoietic organs but also in children and adult BM. Therefore, although HLA-G5 is not an erythroid-specific marker, because it is produced by other cell types such as trophoblast cells, HLA-G5 appears to be a driven-erythroid lineage marker which is likely to play a role in erythropoiesis. This hypothesis is supported by our results, which show that soluble HLA-G is produced during in vitro erythroid cell differentiation whatever the maturation stage. Thus, HLA-G5 may be implicated in the proliferation, maturation, or both of erythroid precursors.
Moreover, we also show that soluble HLA-G is expressed by endothelial cells localized in the early embryo in the developing vessels from the chorionic villi and the juxta-allantoid part of the YS. Of note, such expression is lost in endothelial cells lining mature vessels. Unfortunately, we could not identify which soluble HLA-G isoform is expressed in endothelial cells from developing vessels because the only available endothelial cell lines or primary cultures correspond to endothelial cells obtained from mature vessels. Indeed, we investigated the expression of HLA-G in human umbilical vein endothelial cells (HUVECs) on treatment or not with a factor inducing HLA-G expression such as interferon γ (IFN-γ). Results showed that no HLA-G transcript and protein was detected by quantitative PCR, Western blot, and ELISA in HUVECs and corresponding cell supernatants in both conditions (data not shown). In agreement with our present description of soluble HLA-G in developing vessels, the presence of such protein had previously been shown in endothelial cells in chorionic vessels from first-trimester placenta by immunohistochemistry using the 16G1 mAb.34 This HLA-G localization highlights its possible implication in vascular development. Indeed, HLA-G may be an angiogenic factor allowing invasion of fetal trophoblast into maternal decidua and myometrium through the remodeling of uterine vascularization during normal pregnancy. With respect to this hypothesis, we had previously demonstrated that an alteration of HLA-G gene transcription in invasive trophoblasts may play a role in genetic susceptibility to, and pathogenesis of, preeclampsia, a gestational disease associated with inadequate or shallow trophoblast invasion, because of widespread endothelial cell damage.35 This potential role of HLA-G in angiogenesis is also emphasized by our recent studies showing that the detection of soluble HLA-G5 in serum following heart or combined liver-kidney transplantation is associated with a reduced incidence of transplantation rejection.36-38 Hence, in addition to its immunologic role, soluble HLA-G5 could favor graft acceptance by neovascularization of the transplant.
In conclusion, this specific HLA-G expression in both erythroid and endothelial cell types in time and space highlights its possible implication in the proliferation, maturation, or both of erythroid precursors and vascular development. The existence of such growth factors that act on the primitive cells of both hematopoietic and endothelial cell lineages has been recently demonstrated by the identification of hemangiopoietin,39 supporting that both types of cells derive from a common precursor, called the hemangioblast.5 In addition, it has been shown that erythroid cells, but not other mature hematopoietic cells, secrete angiogenic factors.40,41 We may, thus, hypothesize that HLA-G5 is involved in cross talk between hematopoiesis and angiogenesis. This cross talk between endothelial cells and erythroid cells might be regulated by the following way: erythroid cells secrete HLA-G5 that might act on their own differentiation/proliferation (autocrine regulation), and that might also bind to endothelial cells inducing a signal of differentiation/proliferation (paracrine regulation). Initially restricted to cytotrophoblasts, the placental expression of HLA-G is here extended to the resident hematopoietic progenitors. Whether human placenta may be the site of hematopoiesis remains to be elucidated. In favor of such hypothesis, mouse placenta has been demonstrated as a major hematopoietic organ that is active during most of pregnancy.42
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2004-03-0809.
Supported by the Commissariat à l'Energie Atomique and the Etablissement Français des Greffes.
C.M. and M.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank I. Krawice-Radanne, M. Landi, S. Bruel, and A. Brionne for their excellent technical assistance and Dr S. Ferlicot of Hospital Kremlin-Bicêtre for her helpful advice for in situ hybridization protocol. We also thank Prof J.F. Oury and Dr O. Siboni of the Obstetric Unit of Hospital Robert Debré for providing us with cord blood samples, and Dr M. MacMaster for the anti-HLA-G mAb. We thank Dr D. Carles and Pr. B. Le Bail from the Pathology Laboratory of Hospital Pellegrin, Bordeaux, France, for providing us with some embryos and fetal tissues and bone marrow samples, respectively.