Abstract
We have investigated the ability of glycoprotein (GP) Ibα, a megakaryocytic gene product, to sequester the signal transduction protein 14-3-3ξ and to influence megakaryocytopoiesis. Using a Gp1ba–/– mouse colony, we compared the rescued phenotypes produced by a wild-type human GP Ibα allele or a similar allele containing a 6-residue cytoplasmic tail truncation that abrogates binding to 14-3-3ξ. The observed phenotypes illustrate an involvement for GP Ibα in thrombopoietin-mediated events of megakaryocyte proliferation, polyploidization, and the expression of apoptotic markers in maturing megakaryocytes. We developed a hypothesis for the involvement of a GP Ibα/14-3-3ξ/PI-3 kinase complex in regulating thrombopoietin-mediated responses. An observed increase in thrombopoietin-mediated Akt phosphorylation in the truncated variant supported the hypothesis and led to the development of a model in which the GP Ibα cytoplasmic tail sequestered signaling proteins during megakaryocytopoiesis and, as such, became a critical regulator in the temporal sequence of events that led to normal megakaryocyte maturation.
Introduction
Megakaryocytopoiesis and thrombopoiesis are highly specialized forms of cell maturation in which bone marrow precursors develop from stem cells, proliferate, and are triggered to differentiate into mature polyploid megakaryocytes.1,2 A hallmark feature of the process is the repeated endomitotic divisions producing mature megakaryocytes with a DNA content that can reach 128n (diploid = 2n). Subsequent to platelet release, the cytoplasm of the mature megakaryocyte partitions to form a demarcation membrane system, leading to the formation of the platelet precursor, the proplatelet.3,4 Thrombopoietin (TPO) is the essential cytokine for megakaryocytopoiesis,5-7 and it stimulates bone marrow precursor cells through a series of signaling pathways, including the Janus kinase/signal transducer and activator of transduction (Jak/STAT), Ras/mitogen-activated protein kinase (Ras/MAPK), and phosphoinositol-3-kinase/Akt (PI3K/Akt) pathways.8-10 Although the evidence is strong for each of these pathways participating in megakaryocytopoiesis, it is unclear what molecular mechanisms switch a proliferating precursor cell to a path of repeated endomitotic divisions to produce the polyploid megakaryocyte.
A hereditary bleeding disorder, Bernard-Soulier syndrome, is associated with abnormal bone marrow megakaryocytes with a poorly developed demarcation membrane system.11,12 These abnormal megakaryocytes mimic the presentation of macrothrombocytopenia, characterized by giant circulating platelets and reduced platelet count. The genetic basis of Bernard-Soulier syndrome is well established. It is caused by mutations that impair expression of a multiple subunit receptor, the glycoprotein (GP) Ib-IX complex.11,13 The molecular basis of macrothrombocytopenia is linked to an absence of the cytoplasmic tail of the GP Ibα subunit of the GP Ib-IX complex.14 However, a role for the extracytoplasmic domains of the complex cannot be excluded because antibodies to GP Ib-IX can alter proplatelet formation in vitro.15,16 In addition, in vitro evidence links the expression of GP Ib-IX to cell proliferation, further suggesting the GP Ib-IX complex might regulate megakaryocyte growth.17
The mouse phenotype mimicking Bernard-Soulier syndrome in humans can be rescued by expression of a human GP Ibα transgene under the control of a megakaryocytic gene promoter.11 We have extended this model, generating a comparable mouse colony expressing a variant human GP Ibα subunit lacking the 6 terminal residues (605-610) on the cytoplasmic tail of GP Ibα critical for binding to the signal transduction protein, 14-3-3ξ.18-21 Using animal models, we tested the hypothesis that sequestering 14-3-3ξ by GP Ibα alters megakaryocytopoiesis and thrombopoiesis. The basis for this hypothesis is the extensive literature on members of the 14-3-3 protein family and their influence in various physiologic processes, including intracellular signaling (Raf, MLK, MEKK, PI3K, IRS-1), cell cycling (Cdc25, Wee1, CDK2, centrosome), apoptosis (BAD, ASK-1), and the regulation of transcription (FKHRL1, DAF-16, p53, TAZ, TLX-2, histone deacetylase).22
Our results identify a link between the GP Ibα/14-3-3ξ interaction and the phosphorylation state of Akt. A model is proposed wherein the PI3K/Akt axis of TPO stimulation is regulated by the cytoplasmic tail of GP Ibα during megakaryocyte differentiation. The model is based on results obtained in several different experimental settings, including an in vivo model of severe thrombocytopenia, in vivo administration of TPO, and in vitro analysis of megakaryocyte proliferation and ploidy.
Materials and methods
Generation of mouse models
In vivo use and characterization of a megakaryocyte-specific promoter has been previously described.23,24 Animals expressing a wild-type human GP Ibα transgene in the absence of mouse GP Ibα [mGp1ba(–/–)–TgN(hGPIbαWT)] and designated, hTgWT, have also been described.11 Briefly, hTgWT animals were generated by breeding a colony of mice expressing a wild-type human transgene into a GP Ibα knockout mouse colony to produce offspring with heterozygous mouse GP Ibα alleles [mGp1ba(+/–)] and a portion of the offspring expressing the transgene. Expression of the human transgene was established by flow cytometry using the fluorescein isothiocyanate (FITC)–labeled anti–human monoclonal antibody LJ-P3. These mice were again bred to mGp1ba(–/–) mice, and the offspring from this cross were characterized by Southern blot analysis identifying mice lacking 2 mouse GP Ibα alleles and by flow cytometry to identify those expressing the human transgene. These mice are referred to as hTgWT throughout this article. Using a similar breeding strategy, mice expressing a truncated GP Ibα subunit were also generated [mGp1ba(–/–)–TgN(hGPIbαY605X)]. These mice express a human transgene with a stop codon in place of the mature subunit Tyr605 codon (Figure 1). After breeding into the mGp1ba(–/–) mouse colony and selecting animals lacking both mouse GP Ibα alleles, these animals were designated hTgY605X. Expression of the hTgY605X transgene is driven by the rat platelet factor 4 promoter, provided by Katya Ravid and Robert Rosenberg (Massachusetts Institute of Technology, Cambridge).25 The hTgY605X transgenic construct contained in a 5′ → 3′ direction the rat platelet factor 4 promoter, the coding sequence of human GP Ibα, and the 3′ untranslated cDNA sequence, including the polyadenylation signal sequence. Subsequent expansion of the hTgWT and hTgY605X colonies was accomplished by breeding into the GP Ibαnull colony, a colony that has been backcrossed into the C57/Bl6 strain since its inception. Transgenic animals carrying human GP Ibα alleles have been backcrossed for approximately 6 generations. All animal experiments were performed with approval from the institutional review board of The Scripps Research Institute, La Jolla, CA.
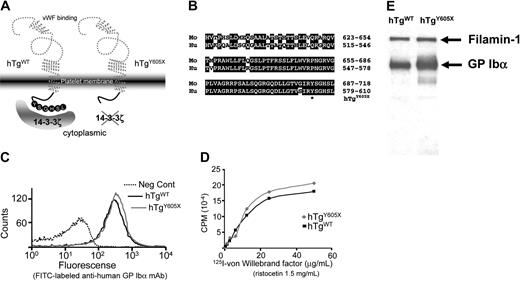
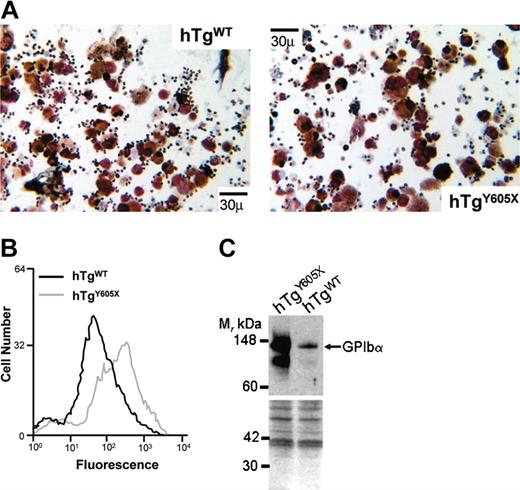
Generation and characterization of mouse models expressing wild-type human GP Ibα (hTgWT) and a truncated form of GP Ibα (hTgY605X). (A) Animal models have been developed lacking the murine GP Ibα genes and expressing normal human GP Ibα (hTgWT) and a truncated human GP Ibα variant (hTgY605X). Both expressed human transgenes were driven by megakaryocytic-specific promoters. (B) Shown are the primary sequence alignments for the cytoplasmic tails of mouse (Mo) and human (Hu) GP Ibα. *The amino acid position (605) where a Tyr605 codon was replaced with a stop codon to truncate the cytoplasmic tail of GP Ibα. (C) Flow cytometry profiles of platelets in whole blood are shown using blood from hTgWT and hTgY605X animals. Fluorescence was produced using an FITC-labeled anti–human GP Ibα monoclonal antibody, LJ-P3. The negative control (dotted line) was fluorescence produced by nontransgenic or normal mice. (D) von Willebrand factor binding isotherms are shown for washed platelets from hTgWT and hTgY605X blood. (E) Mouse platelet lysates were immunoprecipitated with LJ-P3 and immunoblotted with an antifilamin-1 polyclonal antibody and an anti–GP Ibα polyclonal antibody.
Generation and characterization of mouse models expressing wild-type human GP Ibα (hTgWT) and a truncated form of GP Ibα (hTgY605X). (A) Animal models have been developed lacking the murine GP Ibα genes and expressing normal human GP Ibα (hTgWT) and a truncated human GP Ibα variant (hTgY605X). Both expressed human transgenes were driven by megakaryocytic-specific promoters. (B) Shown are the primary sequence alignments for the cytoplasmic tails of mouse (Mo) and human (Hu) GP Ibα. *The amino acid position (605) where a Tyr605 codon was replaced with a stop codon to truncate the cytoplasmic tail of GP Ibα. (C) Flow cytometry profiles of platelets in whole blood are shown using blood from hTgWT and hTgY605X animals. Fluorescence was produced using an FITC-labeled anti–human GP Ibα monoclonal antibody, LJ-P3. The negative control (dotted line) was fluorescence produced by nontransgenic or normal mice. (D) von Willebrand factor binding isotherms are shown for washed platelets from hTgWT and hTgY605X blood. (E) Mouse platelet lysates were immunoprecipitated with LJ-P3 and immunoblotted with an antifilamin-1 polyclonal antibody and an anti–GP Ibα polyclonal antibody.
Immunologic reagents
Anti–human GP Ibα monoclonal antibodies LJ-P3 and LJ-Ibα1 were kindly provided by Dr Zaverio Ruggeri (The Scripps Research Institute, La Jolla, CA). Both antibodies recognize the extracytoplasmic domain of platelet GP Ibα.26 Either denaturation or reduction of human GP Ibα abrogates the epitope of LJ-P3,27 whereas the LJ-Ibα1 epitope is not sensitive to denaturation and is used for Western blot detection of human GP Ibα antigen. FITC-labeling of purified LJ-P3 was performed according to standard procedures.28 An antifilamin-1 polyclonal antibody was provided by Drs Hoffmeister and Stossel (Brigham and Women's Hospital, Boston, MA). Other antibodies were purchased from commercial vendors: an FITC-labeled monoclonal antibody recognizing the mouse integrin αIIb subunit was purchased from PharMingen (La Jolla, CA), an anti–14-3-3ζ (C-16) polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), an antiphospho-Akt (Ser473) antibody was obtained from New England Biolabs (Beverly, MA), an anti-PI3K polyclonal was purchased from Upstate (Charlottesville, VA), and antiphosphotyrosine polyclonal antibodies (py20 and 4G10) were purchased from Chemicon (Temecula, CA).
von Willebrand factor binding
Using ristocetin (1.5 mg/mL; Sigma, St Louis, MO) as a modulator of von Willebrand factor binding to GP Ib, washed platelets were mixed at a final count of 2 × 108/mL with increasing concentrations of sodium iodide I 125 (125I)–labeled von Willebrand factor (VWF).23 The mixtures were incubated at 22°C to 25°C for 15 minutes. At the end of the incubation period, platelet-bound VWF and free VWF were separated by centrifuging the platelets through a sucrose layer, and the platelet-bound radioactivity was measured in a γ-scintillation spectrometer.
Western blot and immunoprecipitation analyses
Platelet pellets prepared from platelet-rich plasma (PRP) were lysed in solubilization buffer (2% Triton X-100, 0.1 M Tris [pH 7.4], 0.01 M EGTA [ethyleneglycotetraacetic acid], 0.15 M NaCl, 2 mM Pefabloc SC [Boehringer Mannheim, Indianapolis, IN]). For sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the samples were mixed with an SDS sample buffer and were boiled (5 minutes) before electrophoresis. After transfer to nitrocellulose and reactivity with the designated antibodies,29 the immunoreactive proteins were visualized using a chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ) and or an iodinated secondary antibody. Immunoreactive signals were identified using Kodak Biomax MR film (Kodak, Rochester, NY).
Immunoprecipitation experiments were performed with washed platelets (3.4 × 108 platelets) resuspended in 250 μL modified Tyrode buffer (137 mM NaCl, 2.7 mM KCl, 2.8 mM dextrose, 0.4 mM NaH2PO4, 5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.4]) and were lysed with an equal volume of solubilization buffer. The mixture was kept on ice for 45 minutes and was centrifuged (10 minutes, 13 000g) to remove the insoluble material. Lysates (500 μL) were mixed with 100 μL (50% vol/vol) protein A beads (IPA-300; Repligen, Cambridge, MA) and 10 μg of the indicated antibody for 90 minutes. The beads were then washed 4 times in an equal volume of modified Tyrode buffer and solubilization buffer. Bound proteins were eluted by boiling in SDS-PAGE sample buffer in the presence of 10 mM dithiothreitol.
In vivo model of thrombocytopenia and TPO administration
To induce thrombocytopenia in mice expressing human GP Ibα, 50 μg purified LJ-P3 immunoglobulin G (IgG) was injected into the tail vein of each mouse. Purified recombinant murine TPO was administered subcutaneously (1.5 μg per mouse) to 6- to 8-week-old animals. The TPO was a gift from Dr Hiroshi Miyazaki (Kirin Brewery, Tokyo, Japan). Circulating blood counts were determined using manual methods (Unopette; Becton Dickinson, Franklin Lakes, NJ) and an automated cell counter (Baker, Allentown, PA). The number of platelets and the DNA content of megakaryocytes were analyzed at the indicated time points.
Purification and analysis of mature megakaryocytes
After the induction of thrombocytopenia or the administration of TPO, mice were killed and whole marrow was flushed from both femurs and tibias using a 25-gauge needle until the bone appeared white. Marrow cells are collected in MK buffer (Ca2+, Mg2+-free phosphate-buffered saline [PBS] containing 3% bovine serum albumin [BSA], 5.5 mM d-glucose, 10.2 mM trisodium citrate, and 10 μM prostaglandin E1 [PGE1]) and were filtered through a sterile nylon mesh (70 μm) to remove small bone fragments. Megakaryocytes were harvested by centrifugation (5 minutes, 220g) and were overlaid on a discontinuous gradient (0%, 2%, 4%) of BSA. By this method, megakaryocytes made up more than 90% of the cells settling to the bottom within 40 minutes at 1g, as evidenced by reactivity with an anti–integrin αIIb monoclonal antibody and acetyl cholinesterase staining.9
In vitro culture of murine bone marrow and megakaryocytes
Isolated cells from the bone marrow of mice were suspended at a concentration of 5 × 106 cells/mL in Iscove modified Dulbecco medium (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (HyClone, Logan, UT), 1 × Pen/Strep Fungizone Mix (BioWhittaker, Walkersville, MD), and 50 ng/mL murine TPO. Cells were cultured at 37°C in a humidified chamber with 5% CO2.
Ploidy and TUNEL analysis
DNA ploidy was assessed by flow cytometry. Bone marrow–derived cells were centrifuged (5 minutes, 220g) washed, and fixed in 70% ethanol (4°C, overnight). Cells were then washed and resuspended in PBS, incubated for 30 minutes at room temperature with 10 μg/mL FITC-conjugated anti-αIIb, 50 μg/mL propidium iodide (Sigma), and 10 U/mL RNase A (Sigma). Cells were analyzed by flow cytometry using a FACScan (Becton Dickinson). In case of antibody injection, megakaryocytes were enriched before staining using Percoll (Sigma) density centrifugation (ρ = 1.07 g/mL) according to the procedure of Tomer et al.30
To assay for DNA degradation (TdT-mediated dUTP nick-end labeling [TUNEL] assay), cells were fixed in 1% paraformaldehyde for 15 minutes on ice and were stored in 70% ethanol (–20°C, 30 minutes). This was followed by incubation with terminal deoxynucleotide transferase and fluorescein isothiocyanate-dUTP according to the manual in the APO-Direct Kit (PharMingen).
CFU-Meg assays
For megakaryocyte colony-forming unit (CFU-Meg) assays, bone marrow cells (2 × 105) were cultured with MegaCult-C media (StemCell Technologies, Vancouver, BC, Canada) in the presence of 10 ng/mL recombinant mouse interleukin-3 (IL-3), 20 ng/mL recombinant human IL-6, and 50 ng/mL recombinant mouse TPO (all cytokines courtesy of Kirin Brewery) according to the manufacturer's suggested conditions. After culturing for 7 days, the colonies were stained with acetylcholine esterase, and a CFU-Meg colony was defined as a colony with at least 3 acetylcholine esterase–positive cells.
Platelet preparation and TPO stimulation
Murine blood was withdrawn from the retro-orbital plexus using heparincoated micro-hematocrit capillaries (Fisher Scientific, Pittsburgh, PA) and was transferred to tubes containing the anticoagulant heparin (Sigma), at a final concentration of 30 U/mL, or acid-citrate-dextrose at a ratio of 1:6. The blood was diluted (1:2) in modified Tyrode buffer, and PRP was prepared by 10-minute centrifugation at 220g. PGE1 [10 μM] and apyrase (5 U/mL; Sigma) were added to the PRP. Platelets were washed with 3 sequential centrifugations (2 minutes, 1000g) at room temperature. Platelet pellets were washed one additional time with modified Tyrode buffer followed by a final centrifugation (3 minutes, 1000g). Final platelet pellets were resuspended in a buffer appropriate for the next procedure, such as immunoprecipitation or Western blot analysis. To analyze the TPO-mediated phosphorylation of Akt, washed platelets were stimulated with TPO (200 ng/mL) at the indicated time points. Phosphorylation was terminated by the addition of an equal volume of 2% SDS, 50 mM Tris-HCl (pH 7.5), 10 mM EGTA, and 2 mM Na3VO4.
Results
Murine platelet model with an ablated GP Ibα/14-3-3ξ interaction
Animals devoid of mouse GP Ibα but expressing either of 2 human transgenes were generated to test the hypothesis that a GP Ibα/14-3-3ξ interaction controls aspects of megakaryocyte differentiation. Animals expressing the normal human GP Ibα subunit were designated hTgWT, and those expressing a truncated human GP Ibα subunit were designated hTgY605X (Figure 1A). The validity of the model was based on a high degree of sequence similarity between human and mouse GP Ibα cytoplasmic domains (Figure 1B) and the ability of human GP Ibα to correct the mouse Bernard-Soulier syndrome.11 Gross characterization of the hematologic parameters from hTgY605X animals, including platelet count and platelet size, gave results indistinguishable from those obtained using platelets from hTgWT animals.11 Tail bleeding time assays, crude measurements of mouse hemostasis and coagulation, were also comparable between the 2 colonies of mice and were indistinguishable from those of healthy mice (data not shown).
Critical for a comparison of the hTgWT and hTgY605X transgenic megakaryocytes or platelets was the level of expressed human antigen. Using a monoclonal antibody that recognized a conformation-specific epitope within the amino terminus of GP Ibα, the expression levels produced by both transgenes were virtually indistinguishable (Figure 1C). Binding isotherms using the radiolabeled ligand, VWF, produced similar binding to hTgWT and hTgY605X platelets (Figure 1D). The similar levels of GP Ibα antigen expressed by hTgWT and hTgY605X reflected the rate-limiting requirement of GP Ibα and GP IX to assemble the multiple subunit GP Ib-IX complex.31,32 Intracytoplasmic levels of GP Ibα antigen were higher in platelet lysates from hTgY605X animals (Figure 1E), even though the surface-expressed levels were similar. In addition, we observed similar levels of filamin-1 associated with each cytoplasmic tail, further validating structural integrity (Figure 1E). Thus, 2 mouse colonies were established expressing similar levels of human GP Ibα antigen and differing only in their cytoplasmic tail lengths.
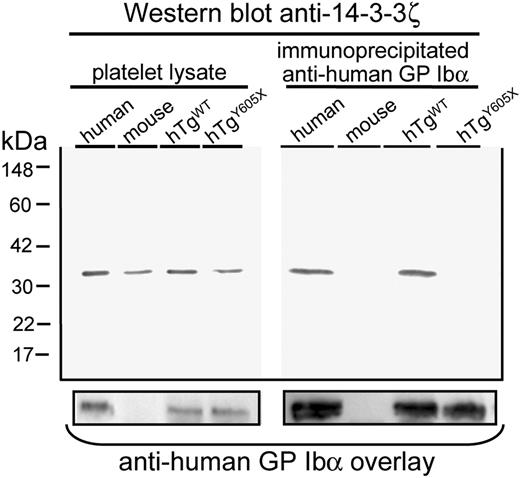
Next we performed immunoprecipitation experiments using an anti–human GP Ibα monoclonal antibody to determine in vivo whether an absence of the 6-terminal residues of GP Ibα residues would lead to loss of interaction with 14-3-3ξ. Indeed, 14-3-3ξ was coimmunoprecipitated from mouse platelets expressing the wild-type human GP Ibα, whereas immunoprecipitation of the truncated GP Ibα molecule failed to copurify 14-3-3ξ, even though comparable levels of human GP Ibα antigen were immunoprecipitated (Figure 2).
Truncation of platelet GP Ibα at Tyr605 ablates the interaction between GP Ibα and 14-3-3ξ. (Left) Western blot of platelet lysates detecting 14-3-3ξ protein at approximately 32 kDa in human platelets, mouse platelets, and mouse platelets expressing human GP Ibα subunits but devoid of mouse GP Ibα (hTgWT and hTgY605X). The experiment demonstrated that total levels of 14-3-3ξ were similar in each of the samples. (Right) Results obtained after immunoprecipitation of human GP Ibα. Bound anti–14-3-3ξ antibody was detected using radiolabeled goat anti–rabbit IgG. Results demonstrate that the truncation of GP Ibα ablated the interaction with 14-3-3ξ, as evidenced by the lack of 14-3-3ξ antigen. Longer exposures of the autoradiograph failed to detect any 14-3-3ξ antigen in the hTgY605X sample. The same nitrocellulose filter was probed a second time with an anti–human GP Ibα monoclonal antibody, LJ-Ibα1, and demonstrated the presence of GP Ibα antigen in those samples in which the human subunit was present (bottom panel).
Truncation of platelet GP Ibα at Tyr605 ablates the interaction between GP Ibα and 14-3-3ξ. (Left) Western blot of platelet lysates detecting 14-3-3ξ protein at approximately 32 kDa in human platelets, mouse platelets, and mouse platelets expressing human GP Ibα subunits but devoid of mouse GP Ibα (hTgWT and hTgY605X). The experiment demonstrated that total levels of 14-3-3ξ were similar in each of the samples. (Right) Results obtained after immunoprecipitation of human GP Ibα. Bound anti–14-3-3ξ antibody was detected using radiolabeled goat anti–rabbit IgG. Results demonstrate that the truncation of GP Ibα ablated the interaction with 14-3-3ξ, as evidenced by the lack of 14-3-3ξ antigen. Longer exposures of the autoradiograph failed to detect any 14-3-3ξ antigen in the hTgY605X sample. The same nitrocellulose filter was probed a second time with an anti–human GP Ibα monoclonal antibody, LJ-Ibα1, and demonstrated the presence of GP Ibα antigen in those samples in which the human subunit was present (bottom panel).
Truncation of GP Ibα altered megakaryocyte ploidy in an acute model of severe thrombocytopenia
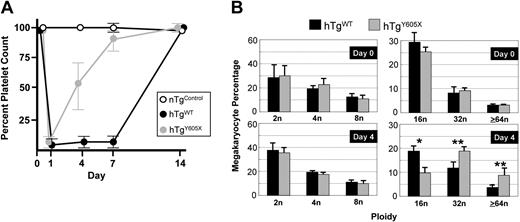
An anti–human GP Ibα mouse monoclonal antibody, LJ-P3, recognized an amino terminal extracytoplasmic domain of human GP Ibα and reacted with platelets from hTgWT and hTgY605X animals (Figure 1C). When the intact IgG of LJ-P3 was injected into the tail veins of mice expressing human GP Ibα, it produced severe thrombocytopenia; circulating platelet counts dropped to 3% to 5% of normal level within 24 hours of injection (Figure 3A). The requirement of human GP Ibα and LJ-P3 to produce thrombocytopenia was evident by the failure of the antibody to produce any thrombocytopenia in nontransgenic animals (Figure 3A). After injections of LJ-P3 into hTgWT animals, platelet counts started to increase after approximately 7 days (Figure 3A). In contrast, when LJ-P3 was injected into hTgY605X animals, platelet counts recovered more quickly, with a return to 50% of normal platelet count 4 days after antibody injection (Figure 3A).
Acute model of severe thrombocytopenia. (A) The anti–human GP Ibα mouse monoclonal antibody, LJ-P3, injected into the tail veins of mice expressing human GP Ibα, produced severe thrombocytopenia. When injected into mice expressing the wild-type human GP Ibα subunit (hTgWT), the platelet count started to return to a normal level after day 7. In mice expressing a truncated form of GP Ibα (hTgY605X), the platelet count began to recover by day 4, and by day 7 the platelet counts were near normal levels. A control LJ-P3 injection into nontransgenic mice (nTgControl) is shown for comparison. (B) The ploidy profile of megakaryocytes is shown before injection of the anti–human monoclonal antibody, LJ-P3 (day 0) and 4 days after injection (day 4). Results were compiled from individual mice, and the mean and SEM are shown (n = 6). *P = .01; **P = .05.
Acute model of severe thrombocytopenia. (A) The anti–human GP Ibα mouse monoclonal antibody, LJ-P3, injected into the tail veins of mice expressing human GP Ibα, produced severe thrombocytopenia. When injected into mice expressing the wild-type human GP Ibα subunit (hTgWT), the platelet count started to return to a normal level after day 7. In mice expressing a truncated form of GP Ibα (hTgY605X), the platelet count began to recover by day 4, and by day 7 the platelet counts were near normal levels. A control LJ-P3 injection into nontransgenic mice (nTgControl) is shown for comparison. (B) The ploidy profile of megakaryocytes is shown before injection of the anti–human monoclonal antibody, LJ-P3 (day 0) and 4 days after injection (day 4). Results were compiled from individual mice, and the mean and SEM are shown (n = 6). *P = .01; **P = .05.
To determine whether the platelet count recovery in the 2 animal models coincided with changes in bone marrow megakaryocytes, we examined megakaryocyte ploidy before and after antibody-induced thrombocytopenia. Before antibody injection, the megakaryocyte ploidy profiles were indistinguishable in hTgWT and hTgY605X marrow (Figure 3B). In addition, the steady-state number of megakaryocytes was the same in the 2 colonies, as determined by the number of αIIb-positive cells identified by flow cytometry of bone marrow aspirates (not shown). However, by day 4 after antibody-induced thrombocytopenia, the marrow from hTgY605X animals contained an increased percentage of megakaryocytes, with a ploidy of 32n or greater (Figure 3B). hTgWT bone marrow by day 4 contained an increased percentage of 16n megakaryocytes. During the recovery from thrombocytopenia, the percentage of megakaryocytes in an 8n or less ploidy class was indistinguishable in the hTgWT and the hTgY605X marrow (Figure 3B).
Truncation of GP Ibα alters the in vivo megakaryocytic response to TPO
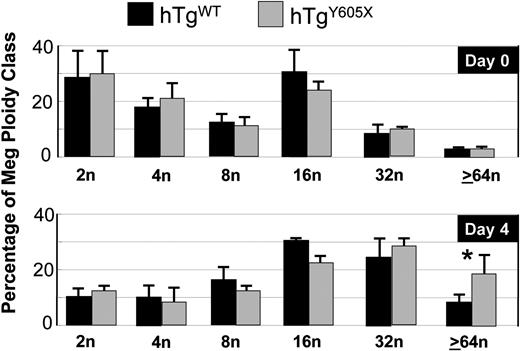
Results from antibody-induced thrombocytopenia suggested a link between the cytoplasmic tail of GP Ibα and megakaryocytopoiesis. We further examined this possibility by testing the hypothesis that TPO-mediated proliferation of megakaryocytes differs in the 2 GP Ibα murine models. First, we injected hTgWT mice and hTgY605X mice with equivalent amounts of TPO. A single subcutaneous injection of TPO (1.5 μg) caused a similar increase in platelet count in both models at days 2, 4, and 6, with a 3-fold increase over baseline as the maximum level of response (not shown). Next, we examined the effects of TPO on megakaryocyte ploidy. At 4 days after TPO injection, the extent of polyploidization was again different in the 2 models (Figure 4). Megakaryocytes of a high ploidy class, 64n or greater; were significantly increased in the marrow of hTgY605X animals, whereas the lower ploidy classes were indistinguishable (Figure 4).
Ploidy analysis of bone marrow megakaryocytes after in vivo administration of TPO. After the injection of TPO, bone marrow megakaryocytes were harvested and analyzed for ploidy classes. Shown are the means and SEM from 6 mice of each genotype. *P = .05.
Ploidy analysis of bone marrow megakaryocytes after in vivo administration of TPO. After the injection of TPO, bone marrow megakaryocytes were harvested and analyzed for ploidy classes. Shown are the means and SEM from 6 mice of each genotype. *P = .05.
Truncation of GP Ibα alters megakaryocytic proliferation and markers of apoptosis
Megakaryocytes from both animal models were tested in vitro for their proliferative potential and the ability to express terminal differentiation markers. CFU-Mk assays revealed no difference in the number of megakaryocytic progenitor cells for each model (hTgWT = 13.8 ± 5.4; hTgY605X = 15.0 ± 3.9). However, after in vitro culturing for 5 days in the presence of TPO, hTgWT cultures were enriched in megakaryocytes, as evidenced by a 2-fold increase in acetyl cholinesterase–positive cells when compared with similar cultures from hTgY605X marrow (Table 1; Figure 5A). Similar to the in vivo results presented in Figure 5, the culture of hTgY605X bone marrow produced an increase in the percentage of higher ploidy cells (not shown). Thus, the number of progenitor cells in each model was similar in the marrow, but the hTgWT cells had a greater proliferative potential whereas the hTgY605X marrow produced megakaryocytes with higher ploidy content.
In vitro bone marrow cultures
. | Total no. cells, × 107 . | . | No. megakaryocytes, × 104 . | . | ||
|---|---|---|---|---|---|---|
| Day . | hTgWT . | hTgY605X . | hTgWT . | hTgY605X . | ||
| 0 | 7.71 ± 0.65 | 7.57 ± 0.99 | — | — | ||
| 5 | 1.48 ± 0.17 | 1.44 ± 0.57 | 13.67 ± 1.58 | 7.54 ± 1.34 | ||
. | Total no. cells, × 107 . | . | No. megakaryocytes, × 104 . | . | ||
|---|---|---|---|---|---|---|
| Day . | hTgWT . | hTgY605X . | hTgWT . | hTgY605X . | ||
| 0 | 7.71 ± 0.65 | 7.57 ± 0.99 | — | — | ||
| 5 | 1.48 ± 0.17 | 1.44 ± 0.57 | 13.67 ± 1.58 | 7.54 ± 1.34 | ||
n = 4.
— indicates undetectable.
In vitro analysis of cultured bone marrow cells. (A) Shown are images from the cultured bone marrow preparations illustrating the increased proliferation of hTgWT cells compared with hTgY605X cells (for comparison, see also Table 1). Images were captured with an Olympus IX71 inverted microscope equipped with a 40 ×/0.95 NA objective (Olympus, Tokyo, Japan). Images were photographed at 400 × original magnification with an Olympus DP70 CCD camera (Olympus) and acquired through Lumina Vision software (Mitani, Fukui, Japan). Cells were stained for acetylcholinesterase using 0.1 mol/L PBS containing 0.05% acetylthiocholine iodide, 0.1 mol/L sodium citrate, 30 mmol/L copper sulfate, and 5 mmol/L potasium ferricyanide (pH 6.0). (B) TUNEL assay on cultured bone marrow cells. Bone marrow cells were harvested and cultured for 5 days in the presence of TPO, and TUNEL assays were performed. Flow cytometry settings were used to gate and provide data for cells with greater than 4n ploidy. (C) Western blot from the same cultures depicted in the center panel was blotted for GP Ibα antigen, a marker of late-stage megakaryocytopoiesis. Ten micrograms protein (BCA assay) was applied to each lane, electrophoresed, transferred to nitrocellulose, and reacted with an anti–GP Ibα antibody. Shown is the resultant autoradiograph. Samples from hTgY605X show an increase in the amount of GP Ibα antigen compared with hTgWT, consistent with an increase in high-ploidy cells seen after a 5-day culture in the presence of TPO. However, as seen in Figures 1 and 2, gene expression levels for both transgenes were similar, but coincident with an increase in the percentage of high-ploidy megakaryocytes was an increase in GP Ibα antigen. The blot was subsequently reprobed with a pool of antibodies to confirm a similar protein load and is shown below for comparison.
In vitro analysis of cultured bone marrow cells. (A) Shown are images from the cultured bone marrow preparations illustrating the increased proliferation of hTgWT cells compared with hTgY605X cells (for comparison, see also Table 1). Images were captured with an Olympus IX71 inverted microscope equipped with a 40 ×/0.95 NA objective (Olympus, Tokyo, Japan). Images were photographed at 400 × original magnification with an Olympus DP70 CCD camera (Olympus) and acquired through Lumina Vision software (Mitani, Fukui, Japan). Cells were stained for acetylcholinesterase using 0.1 mol/L PBS containing 0.05% acetylthiocholine iodide, 0.1 mol/L sodium citrate, 30 mmol/L copper sulfate, and 5 mmol/L potasium ferricyanide (pH 6.0). (B) TUNEL assay on cultured bone marrow cells. Bone marrow cells were harvested and cultured for 5 days in the presence of TPO, and TUNEL assays were performed. Flow cytometry settings were used to gate and provide data for cells with greater than 4n ploidy. (C) Western blot from the same cultures depicted in the center panel was blotted for GP Ibα antigen, a marker of late-stage megakaryocytopoiesis. Ten micrograms protein (BCA assay) was applied to each lane, electrophoresed, transferred to nitrocellulose, and reacted with an anti–GP Ibα antibody. Shown is the resultant autoradiograph. Samples from hTgY605X show an increase in the amount of GP Ibα antigen compared with hTgWT, consistent with an increase in high-ploidy cells seen after a 5-day culture in the presence of TPO. However, as seen in Figures 1 and 2, gene expression levels for both transgenes were similar, but coincident with an increase in the percentage of high-ploidy megakaryocytes was an increase in GP Ibα antigen. The blot was subsequently reprobed with a pool of antibodies to confirm a similar protein load and is shown below for comparison.
Next, we considered whether the hTgY605X cells containing an increased percentage of high ploidy cells might also express increased levels of terminal differentiation markers. TUNEL assays were performed to monitor apoptosis as a function of DNA degradation. After 5 days the in vitro culture of hTgY605X bone marrow produced megakaryocytes with a higher occurrence of apoptosis (Figure 5B), a difference no longer apparent by day 8 of culture (not shown). Further evidence for the changes in the hTgY605X megakaryocyte population was apparent by the increased accumulation of GP Ibα antigen (Figure 5C). An increase in GP Ibα expression also supported the more differentiated state of the hTgY605X megakaryocytes after stimulation with TPO. Thus, the truncation of the GP Ibα tail limited the proliferative potential of megakaryocytic precursors (Table 1) while increasing their intrinsic ability to progress through a sequence of events leading to the presence of late-stage megakaryocytes.
Truncation of GP Ibα leads to increased phosphorylation of Akt
To explain the results observed in in vivo and in vitro assays, we considered the possibility that the PI3K/Akt axis of TPO stimulation is altered in the hTgY605X model.9 A physical link is reported among 14-3-3ξ, PI3K, and GP Ibα,33 and the relevance of PI3K for TPO stimulation of megakaryocytes is well documented.9,34 Our hypothesis suggested that ablation of the 14-3-3ξ interaction between GP Ibα and 14-3-3ξ has altered one of the major stimulatory pathways for megakaryocytopoiesis, the PI3K activation of Akt.9
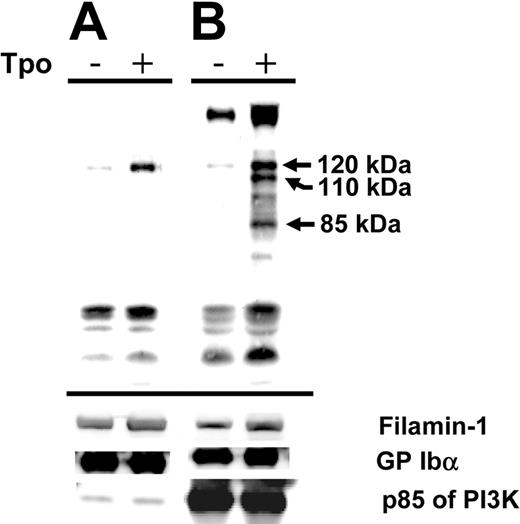
To address this problem and to determine how much PI3K was associated with GP Ibα, we stimulated human platelets with TPO, immunoprecipitated with either an anti–GP Ibα antibody or an anti-P13K antibody. We blotted the immunoprecipitated material with an antiphosphotyrosine antibody (4G10) and looked for links among GP Ibα, PI3K, and c-Mpl. Figure 6A shows a protein (120 kDa) that became increasingly phosphorylated in the presence of TPO and immunoprecipitated by an anti–GP Ibα antibody. As shown in the accompanying control, PI3K was immunoprecipitated by the anti–GP Ibα monoclonal antibody, but a phosphorylated form of the protein was not detected. A protein of similar mobility (120 kDa) was also immunoprecipitated by an anti-PI3K antibody along with major phosphorylated proteins of 110 and 85 kDa (Figure 6B). Thus, a 120-kDa protein appeared to be immunoprecipitated by anti-PI3K and anti–GP Ibα antibodies after stimulation with TPO. The results also illustrate that only a minor portion of PI3K is associated with GP Ibα. These results suggest that a complex exists of GP Ibα, PI3K, and an unidentified 120-kDa protein and that several of these proteins become phosphorylated in the presence of TPO.
TPO-induced phosphorylation. Purified human platelets were stimulated with TPO (200 ng/mL, 10 minutes, 37°C) and were immunoprecipitated with an anti–GP Ibα monoclonal (LJ-P3) (A) or an anti-PI3K antibody (B). Immunoprecipitation products were electrophoresed and blotted with an antiphosphotyrosine antibody (4G10). Phosphorylated proteins in the absence (–) or presence (+) of TPO are shown. Subsequent control blots were performed on the same filter for filamin-1, GP Ibα, and the p85 subunit of PI3K.
TPO-induced phosphorylation. Purified human platelets were stimulated with TPO (200 ng/mL, 10 minutes, 37°C) and were immunoprecipitated with an anti–GP Ibα monoclonal (LJ-P3) (A) or an anti-PI3K antibody (B). Immunoprecipitation products were electrophoresed and blotted with an antiphosphotyrosine antibody (4G10). Phosphorylated proteins in the absence (–) or presence (+) of TPO are shown. Subsequent control blots were performed on the same filter for filamin-1, GP Ibα, and the p85 subunit of PI3K.
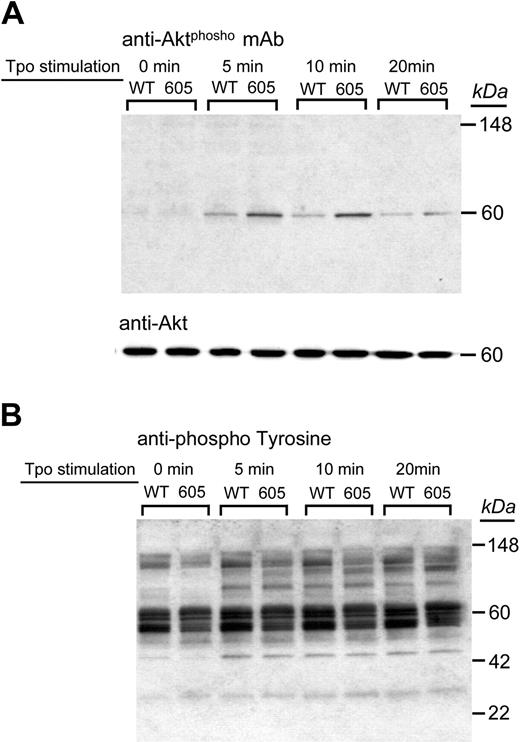
If the phosphorylation state of PI3K is relevant to the differences we observed between the hTgWT and hTgY605X colonies, we would expect to observe further downstream consequences, such as the phosphorylation of a PI3K target, Akt. We examined the TPO-induced phosphorylation of Akt in platelets from both models and found a heightened Akt phosphorylation in hTgY605X platelets (Figure 7A). In contrast, the overall phosphorylation pattern observed with an antiphosphotyrosine antibody revealed no dramatic differences in the 2 colonies (Figure 7B). These results confirm a shift in the PI3K/Akt pathway and establish an involvement of the GP Ibα/PI3K/14-3-3ξ interaction in the regulation of this signaling pathway.
TPO-induced Akt phosphorylation. Purified platelets from hTgWT and hTgY605X were stimulated with TPO (200 ng/mL) for the indicated times. Platelet lysates were subjected to Western blot analysis using either an antiphospho-Akt (Ser473) antibody (A) or an antiphosphotyrosine polyclonal antibody (B). The upper blot was reprobed using an anti-Akt polyclonal antibody to visualize protein loads and is shown for comparison (middle panel).
TPO-induced Akt phosphorylation. Purified platelets from hTgWT and hTgY605X were stimulated with TPO (200 ng/mL) for the indicated times. Platelet lysates were subjected to Western blot analysis using either an antiphospho-Akt (Ser473) antibody (A) or an antiphosphotyrosine polyclonal antibody (B). The upper blot was reprobed using an anti-Akt polyclonal antibody to visualize protein loads and is shown for comparison (middle panel).
Discussion
The presented results are consistent with a model in which the cytoplasmic tail of GP Ibα influences the temporal sequence of events necessary for the proliferation and maturation of megakaryocyte progenitors. Our hypothesis began with 14-3-3ξ and its sequestering by GP Ibα as a regulator of megakaryocytopoiesis. The sequestration of 14-3-3ξ by GP Ibα has implications for platelet function by down-regulating the activation of Cdc42 and Rac.35 Because our model with ablated interaction between GP Ibα and 14-3-3ξ displayed changes in megakaryocyte ploidy, we were led to consider the contributions of the major regulator of megakaryocyte development, TPO.6,7 The relevance of TPO to our results was evident by a decreased in vitro proliferation potential of hTgY605X megakaryocytic stem cells (Table 1) and an increased percentage of high ploidy hTgY605X cells (Figure 4). Of the TPO activation pathways, the PI3K/Akt pathway seemed to be the most likely involved because both the TPO receptor and the GP Ibα/14-3-3ξ complex sequester PI3K.34,36 Indeed, in the absence of a GPIbα/14-3-3ξ interaction, an increased phosphorylation of Akt in response to TPO was observed (Figures 7, 8A). Thus, the TPO response in megakaryocytes can be influenced by membrane receptors other than c-mpl, just as platelet activation can be primed by TPO and by the activation of a PI3K-dependent pathway.37
GP Ibα expression, megakaryocyte proliferation, and differentiation. (A) Results suggest a shift in the PI3K/Akt axis of TPO stimulation. A hypothesis is presented whereby the cytoplasmic tail of GP Ibα sequesters signaling proteins, such as 14-3-3ξ and PI3K, and down-regulates the Akt-dependent pathway. In the truncated GP Ibα variant, hTgY605X, a shift in the PI3K/Akt axis results in increased Akt activation and downstream consequences of increased endomitosis and accumulation of a greater percentage of high-ploidy megakaryocytes. (B) A schematic model is presented to illustrate how an increase in Akt phosphorylation results in a megakaryocyte population with increased ploidy. The precursor cell proliferation (2n → 4n → 2n) is more active in the hTgWT model (Table 1) and is depicted by larger arrows compared with proliferation in the hTgY605X model. However, Akt phosphorylation in response to TPO is increased in the hTgY605X model (Figure 6A) and corresponds to an increase in the accumulation of cells of higher ploidy (Figures 3B, 4). Thus, repeated endomitotic divisions in the hTgY605X marrow are depicted by larger arrows compared with a similar process in the hTgWT model. The increased proliferative potential of hTgWT cells and the potential for hTgY605X cells to generate megakaryocytes of a higher ploidy class provide mechanisms whereby both models produce similar levels of platelet. Results highlight the independence of ploidy and platelet release in response to stimuli. GP Ibα expression increases during megakaryocyte maturation and, as such, becomes a critical timing factor for normal megakaryocyte maturation.
GP Ibα expression, megakaryocyte proliferation, and differentiation. (A) Results suggest a shift in the PI3K/Akt axis of TPO stimulation. A hypothesis is presented whereby the cytoplasmic tail of GP Ibα sequesters signaling proteins, such as 14-3-3ξ and PI3K, and down-regulates the Akt-dependent pathway. In the truncated GP Ibα variant, hTgY605X, a shift in the PI3K/Akt axis results in increased Akt activation and downstream consequences of increased endomitosis and accumulation of a greater percentage of high-ploidy megakaryocytes. (B) A schematic model is presented to illustrate how an increase in Akt phosphorylation results in a megakaryocyte population with increased ploidy. The precursor cell proliferation (2n → 4n → 2n) is more active in the hTgWT model (Table 1) and is depicted by larger arrows compared with proliferation in the hTgY605X model. However, Akt phosphorylation in response to TPO is increased in the hTgY605X model (Figure 6A) and corresponds to an increase in the accumulation of cells of higher ploidy (Figures 3B, 4). Thus, repeated endomitotic divisions in the hTgY605X marrow are depicted by larger arrows compared with a similar process in the hTgWT model. The increased proliferative potential of hTgWT cells and the potential for hTgY605X cells to generate megakaryocytes of a higher ploidy class provide mechanisms whereby both models produce similar levels of platelet. Results highlight the independence of ploidy and platelet release in response to stimuli. GP Ibα expression increases during megakaryocyte maturation and, as such, becomes a critical timing factor for normal megakaryocyte maturation.
Although the target of activated Akt could be any number of proteins, activated Akt increases the posttranslational stability of cyclins.38 Cyclins are particularly relevant targets because they have been associated with promoting the endomitosis that leads to increased ploidy.39 Cyclin D3 is active in promoting passage through the G1 phase of mitosis,40 and its importance as a megakaryocytic gene product has been established.39,41 In addition, the overexpression of D1 or D3 cyclin in transgenic animals increases megakaryocyte ploidy.42,43 Phenotypically, the overexpression of D3 impairs formation of the mature megakaryocyte demarcation membrane system,43 similar to what occurs with a complete absence of a GP Ib-IX complex.11,12 Thus, the abnormal megakaryocyte cytoplasm associated with Bernard-Soulier syndrome may reflect an unregulated activation of cyclins in the absence of GP Ib-IX or, in the current study, by a short truncation in the cytoplasmic tail. Together, these results illustrate the requirement for a highly coordinated temporal sequence of events leading to the formation of a developing platelet field in the cytoplasm of a normal megakaryocyte.
Our results demonstrate increases in Akt phosphorylation and in markers of apoptosis (Figure 5B). This seems to be in direct conflict with the dogma establishing the PI3K/Akt pathway as a cell survival or an antiapoptotic pathway.44 Exceptions do exist, however, and they reflect the downstream targets of the activated Akt.45 Worth considering are the unique properties of the megakaryocyte. Increased apoptotic markers are not a deleterious result of maturation but are part of the normal physiology of the megakaryocyte proceeding along the pathway to maturation. Clearly, the PI3K/Akt pathway is crucial to a wide range of processes in the multicellular organism, and our results underscore the inability to identify a single unifying theme for activation through a PI3K/Akt pathway.
Originally, we believed the faster platelet recovery observed in the hTgY605X model (Figure 3) could be a consequence of the increased percentage of megakaryocytes of higher ploidy. However, this conclusion did not fit when we observed the in vivo administration of TPO-altered megakaryocyte ploidy in the 2 models without altering the rate of increase in the circulating platelet count. Thus, by what mechanism does antibody-induced thrombocytopenia occur, and, more important, what explains the different platelet recovery rates in the 2 animal models? We present data showing that the expressed levels of transgene on the platelet surface and within platelet lysates were similar in both animal models (Figure 1C-E). The levels of megakaryocytic GP Ibα were also similar before the induction of thrombocytopenia (not shown). However, the levels of GP Ibα antigen changed dramatically when the megakaryocytic population changed, as seen in Figure 5C. The mechanism of antibody-induced thrombocytopenia could have existed at several points. First, it could have had a direct effect on proplatelet production in the marrow. This would be analogous to the in vitro inhibition described by others.15,16 As such, proplatelet formation by megakaryocytes with increased GP Ibα antigen would be less inhibited, as would have occurred within the hTgY605X marrow after the induction of thrombocytopenia (Figure 3B). The net result would be an increased rate of circulating platelet recovery in the hTgY605X animal. Second, the antibody would have a direct effect on the circulating platelets. It is possible that by unlinking GP Ibα with 14-3-3ξ, we altered the clearance kinetics of the platelets in each model. In this case, the antibody produced a difference in platelet count that had nothing to do with the state of the megakaryocyte within the marrow.
In further support of the idea that the different platelet recovery rates observed in antibody-induced thrombocytopenia might be irrelevant to megakaryocyte proliferation and maturation, we observed that in vivo administration of TPO increased the hTgY605X ploidy (Figure 4) but did not increase the rate of platelet release. We suggest the similar increases in platelet count are explained by a level of compensation dependent on the increased cell proliferation in the hTgWT model (Table 1) balanced by the higher ploidy in the hTgY605X marrow (Figure 8B). In support of this idea is the similar percentage of αIIb-positive cells 4 days after TPO administration (2.50 ± 0.6 for hTgWT vs 2.66 ± 0.08 for hTgY605X; n = 6). These results highlight an independence of ploidy and platelet release in the megakaryocyte population.2,7,46
One noticeable aspect of the presented results is the requirement for a stressed system to identify differences between the hTgWT and the hTgY605X genotypes. The normal megakaryocyte ploidy profile and the circulating platelet counts are indistinguishable in the 2 models. However, changes are seen after induced severe thrombocytopenia or after in vivo administration of TPO. These results highlight the potential for compensation among signaling pathways in the unstressed situation. Indeed, the ability of hematopoietic cells to compensate in the absence of specific proteins or signaling pathways is not without precedence, and it highlights the complexity of normal hematopoiesis.47-49
In summary, we have found a mechanism by which the cytoplasmic tail of GP Ibα can modulate megakaryocyte ploidy and proliferation. The involved pathways most likely represent one defect contributing to the generation of the macrothrombocytopenic phenotype typical of Bernard-Soulier syndrome. Our results also identify factors capable of regulating the commitment of a normal megakaryocyte to become a polyploid cell. GP Ibα expression increases during megakaryocytic differentiation,50 but the low levels of GP Ibα expression during early megakaryocytopoiesis may influence the level of Akt phosphorylation and balance the proliferative potential of a precursor cell compared with the cell's commitment to a pathway of repeated endomitotic cycles. Our results illustrate a temporal sequencing of events that control the commitment of the megakaryocyte to undergo endomitosis. Expressed levels of GP Ibα and sequestration of signaling proteins become relevant in controlling the mechanisms associated with megakaryocytopoiesis and thrombopoiesis.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-03-0893.
Supported by grants HL50545 and HL31950 (J.W.) and HL30657, HL56264, and HL00903 (J.E.B.F.) from the Heart, Lung and Blood Institute of the National Institutes of Health. The authors are grateful for the support of the Sam and Rose Stein DNA Core Facility within the Department of Molecular and Experimental Medicine at The Scripps Research Institute.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal