Abstract
Glucose 6-phosphate dehydrogenase (G6PD) (EC 1.1.1.42) is an essential enzyme for the rapid production of NADPH, as required on exposure to oxidative stress. Mouse embryonic stem (ES) cells can produce all embryonic and fetal/adult cell types. By studying the in vitro differentiation of embryoid bodies produced from G6pdΔ ES cells that are totally unable to produce G6PD protein, we found that these cells are able to differentiate into mesodermal cells, cardiomyocytes, hepatocytes, and primitive erythroid cells. However, we show here that, after the hemoglobin switch has taken place, definitive erythrocytes die by apoptosis. This apoptotic death is delayed by reducing agents and by a caspase inhibitor, but it is prevented only by the restoration of G6PD activity. Thus, G6PD proves indispensable for definitive erythropoiesis.
Introduction
Erythropoiesis originates in the developing mouse embryo from blood islands in the extra-embryonic mesoderm in the yolk sac at embryonic day 7.5 (E7.5). Erythroid cells then migrate to the para-aortic splanchnopleura, shortly thereafter to the developing fetal liver and spleen, and eventually to the bone marrow.1,2 The transition from yolk sac to fetal liver is associated with the switch from a single-lineage primitive erythroid program to multilineage hematopoiesis that includes definitive erythropoiesis, myelopoiesis, and lymphopoiesis. Embryonic or primitive erythroid cells are large and nucleated, and they produce embryonic forms of globin. Definitive erythroid cells are small, they enucleate, and they produce the adult forms of globin.2 The production of red blood cells follows the sequential maturation of erythroid progenitors to proerythroblasts, then to basophilic, polychromatophilic, and orthochromatic normoblasts. In definitive erythropoiesis, orthochromatic normoblasts undergo terminal maturation and eventually lose their nuclei.3
In vitro differentiation of mouse embryonic stem (ES) cells carrying targeted gene deletion provides a powerful model to investigate the function of genes that play an important role in embryonic development. ES cells can generate various cell lineages: thus, one can obtain hematopoietic, endothelial, muscle, and neuronal lineages, bypassing the formation of the embryo itself.4 In addition, embryoid bodies (EBs) formed from ES cells recapitulate the differentiation program observed in the early mouse embryo (eg, erythropoiesis).5
The glucose-6-phosphate dehydrogenase (G6PD) gene is an X-linked housekeeping gene that encodes the first enzyme of the pentose phosphate pathway, an NADPH-producing dehydrogenase. NADPH is a critical modulator of intracellular redox potential. G6PD deficiency is the most common form of red blood cell (RBC) enzymopathy, affecting millions of persons worldwide. The distinctive phenotype of patients with G6PD deficiency is hemolytic anemia. In most patients, this is triggered by exposure to oxidative agents.6
We have generated mouse ES cells with a G6pd deletion (G6pdΔ ES), and we have previously shown that these cells are extremely sensitive to oxidative stress, in keeping with the notion that G6PD is essential for the production of high levels of NADPH required for the detoxification of reactive-oxygen species.7,8 In addition, it has recently been reported that severe G6PD deficiency is lethal to embryos. Severely G6PD-deficient hemizygous male embryos stop growing between E7.5 and E8.5 and show severe abnormalities,9 indicating that the role of G6PD is basic in mammalian development. Nevertheless, it is unclear which tissues are most directly affected by the absence of G6PD activity and which experience secondary damage. In addition, the early death of G6PD-deficient embryos has prevented the study in them of definitive erythropoiesis.
In this report we have dissected this question by investigating the in vitro differentiation of mouse G6pdΔ ES cells. We show that abrogation of G6PD activity does not significantly affect the production of mesodermal cells, cardiomyocytes, hepatocytes, or primitive erythroid cells. On the other hand, after the hemoglobin switch takes place, G6PD proves indispensable for the survival of definitive erythroid cells.
Materials and methods
Cell lines
Parental wild-type (WT) AK7 ES cell and 2 G6pdΔ ES cell lines8 were maintained in an undifferentiated state by culture on a monolayer of mitomycin-C–inactivated fibroblasts in the presence of leukemia-inhibiting factor (LIF).
Under these conditions, more than 95% of the cell population remained undifferentiated, as determined by visual inspection under phase-contrast microscopy. Two days before the initiation of differentiation, cells were passaged into gelatinized dishes.
Generation of G6pd- and BclXL-overexpressing ES cells
The 2 G6pdΔ ES cell lines and the WT ES cells were electroporated with 10 μg linearized plasmid vector Pallino β-actin (empty vector),10 Pallino β-actin G6pd, and Pallino β-actin BclXL. These plasmid vectors contain G6pd cDNA or BclXL cDNA driven by β-actin promoter and the puromycin resistance gene driven by the phospho-glycerokinase promoter. One week after selection with 1.2 μg/mL puromycin, the resistant colonies were again isolated and screened by polymerase chain reaction (PCR), enzymatic activity, and Western blotting. Positive clones were expanded and subjected to the described experiments.
In vitro differentiation
To induce differentiation, WT and G6pdΔ ES cell lines were dissociated by trypsinization and were suspended in Dulbecco modified Eagle medium (DMEM) containing 15% fetal bovine serum (FBS), 0.5 mM monothioglycerol (MTG; Sigma, St Louis, MO), 50 U/mL penicillin, and 50 μg/mL streptomycin. Cells were cultured for 2 days by the hanging-drop method (3 × 102 ES cell per 30 μL in each drop). EBs in hanging-drop were transferred to suspension culture in 60-mm dishes and were cultured for another 3 days. Resultant EBs were plated onto plastic 100-mm gelatin-coated dishes. Cultures were maintained in a humidified environment with 5% CO2 in air at a temperature of 37°C. Serum batches for ES differentiation were tested for their ability to support efficient EB development and for their ability to support the development of hematopoietic precursors. Rhythmic beating of the EBs, indicating cardiac muscle differentiation, and “red” or globinized EBs, indicating hematopoietic development, were monitored by daily inspection of the cultures using phase-contrast microscopy. In some differentiation experiments, the differentiation medium was further supplemented with erythropoietin (EPO; Janssen-Cilag) at 3 U/mL, reduced glutathione (GSH; Sigma-Aldrich, St Louis, MO) at 2.5 mM, N-acetyl-l-cysteine (NAC; Sigma-Aldrich) at 2.5 mM, and zVAD-fmk (Bachem, Bubendorf, Switzerland) at 100 μM. With the exception of EPO, all these reagents were added from day 11 of differentiation.
Methyl cellulose colony assay
To induce differentiation using the methylcellulose method, ES cells were plated in Iscove modified Dulbecco medium (IMDM) supplemented with 15% FBS, 50 U/mL penicillin, 50 mg/mL streptomycin, 500 μM MTG, 3 U/mL EPO, and 0.9% (wt/vol) methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada). ES cells were plated at a density of 3 × 103 cells/mL. After 8 days of incubation at 37°C in a fully humidified atmosphere supplemented with 5% CO2, the number of EBs formed was scored using an inverted microscope.
RNA isolation and RT-PCR
Total RNA was isolated by acid-phenol extraction (Trizol; Gibco/BRL, Gaithersburg, MD). Reverse transcription-PCR (RT-PCR) was performed with the Perkin-Elmer RT-PCR kit, as recommended by the manufacturer. cDNA was amplified by PCR using specific primers for β-major globin, β-H1, SCL, KL, c-vav11 ; hypoxanthine phosphoribosyl-transferase (Hprt)8 ; Brachyury (T), Nkx2.512 ; TDO13 ; MLC2v-specific primers 5′CTGTGGTTCAGGGCTCAGTCCTTC3′ and 5′GCCAAGAAGCGGATAGAAGGCGGG 3′), and α-myosin heavy chain (αMHC)–specific primers 5′GGAAGAGTGAGCGGCGCATCAAGG3′ and 5′CTGCTGGAGAGGTTATTCCTCG3′).
Semiquantitative RT-PCR was performed using the relative expression level of Hprt to normalize mRNA levels and to evaluate data from the exponential phase of PCR amplification (22 cycles). To correct for differences in RNA quantity among samples, data were normalized by using the ratio of the target cDNA concentration to that of Hprt samples.
Results
In vitro differentiation of G6pdΔ ES cells
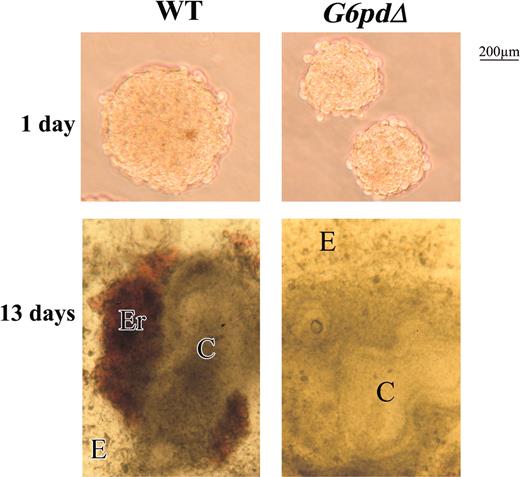
Mouse ES cells can be made to differentiate in vitro, under appropriate culture conditions, to a variety of cell types. In general, the first stage is the formation, once the feeder layer is removed, of cell aggregates called embryoid bodies (EBs). It was shown previously that the yield of EBs in a semisolid medium (methylcellulose) from severely G6PD-deficient ES cells is very low.9 We have confirmed this result (yield, less than 1%) with 2 independent clones of G6pdΔ ES cells. We then resorted to the “hanging-drop” culture method whereby, probably thanks to the effect of gravity bringing cells close together, we were able to obtain EBs from both WT and G6pdΔ ES cells (1 EB in each drop culture in which 300 cells had been inoculated). However, EBs derived from G6pdΔ ES cells were invariably smaller than those derived from WT cells (Figure 1). Larger EBs were obtained from G6PDΔ ES cells by introducing more cells (400-600) in each hanging drop, though these were still smaller than EBs from 400 to 600 WT ES cells (data not shown).
WT and G6pdΔ EB morphology. EBs formed from WT and G6pdΔ ES cells by hanging drops on days 1 and 13 of differentiation. Note that the smaller EBs derived from G6pdΔ cells at day 1 of differentiation, and the absence of red foci in EBs derived from G6pdΔ at 13 days of differentiation. Photomicrographs were obtained in culture media with a Zeiss (Jena, Germany) Stemi SV11 stereomicroscope at 5 × numerical aperture and 10 × magnification of the Zeiss objective lenses (Jena, Germany).
WT and G6pdΔ EB morphology. EBs formed from WT and G6pdΔ ES cells by hanging drops on days 1 and 13 of differentiation. Note that the smaller EBs derived from G6pdΔ cells at day 1 of differentiation, and the absence of red foci in EBs derived from G6pdΔ at 13 days of differentiation. Photomicrographs were obtained in culture media with a Zeiss (Jena, Germany) Stemi SV11 stereomicroscope at 5 × numerical aperture and 10 × magnification of the Zeiss objective lenses (Jena, Germany).
After 2 days, the EBs were transferred from hanging drop to suspension culture for another 3 days, and on day 5, they were transferred to gelatin-coated dishes. On day 9, we observed foci of contracting cells within both G6pdΔ EBs and WT EBs, indicating the presence of cardiac myocytes: this contractile activity lasted for at least 10 days (Figure 2B; data not shown).
Percentage of EBs with red foci and beating cardiomyocytes. (A) No red foci were observed in EBs derived from G6pdΔ ES cells. (B) No obvious differences were observed among EBs formed from WT (□) and G6pdΔ (▪) ES cells in cardiac differentiation. Data represent the average of 3 independent experiments (mean ± standard deviation [SD]).
Percentage of EBs with red foci and beating cardiomyocytes. (A) No red foci were observed in EBs derived from G6pdΔ ES cells. (B) No obvious differences were observed among EBs formed from WT (□) and G6pdΔ (▪) ES cells in cardiac differentiation. Data represent the average of 3 independent experiments (mean ± standard deviation [SD]).
On day 9, erythroid foci appeared regularly in WT EBs (Figures 1, 2A). However, erythroid foci never appeared in G6pdΔ EBs (Figures 1, 2A), even when 400 to 600 cells had been inoculated in each hanging drop. The failure of forming red foci could not be attributed to the EBs being too small, because these were as large as those obtained with a smaller inoculum from WT ES cells, which did produce red foci.
Assays for markers of differentiated tissues
To investigate further the extent to which tissue-specific differentiation takes place in EBs derived from G6pdΔ cells, we tested for the expression of tissue-specific genes at days 9, 11, 13, and 15. We found no difference between WT and G6pdΔ EBs in the expression patterns of genes sequentially involved in cardiac development. Among them were Brachyury (T), an early mesoderm marker, as well as Nkx2.5, a homeobox gene implicated in the early stages of heart formation and the gene encoding α-MHC and the cardiac-specific myosin light chain 2V (MLC2V). The later 2 are major contractile proteins of cardiomyocytes (Figure 3). A similar pattern of expression in WT and G6pdΔ EBs was also observed with respect to tryptophan 2,3-dioxygenase (TDO), regarded as a specific marker of hepatocytes.13
Analysis for markers of differentiated tissues. Ethidium bromide–stained gels of RT-PCR amplification using various marker genes of differentiation. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (G6pdΔ1 and G6pdΔ2). Number of days in culture is given at the top. Amplified Hprt is shown as a positive control.
Analysis for markers of differentiated tissues. Ethidium bromide–stained gels of RT-PCR amplification using various marker genes of differentiation. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (G6pdΔ1 and G6pdΔ2). Number of days in culture is given at the top. Amplified Hprt is shown as a positive control.
Assays for markers of hematopoiesis
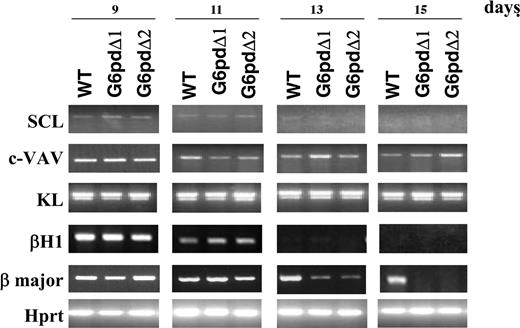
To characterize further the defect underlying the failure of G6pdΔ-derived EBs to form erythroid foci, we studied the expression patterns of several genes involved in hematopoiesis at days 9, 11, 13, and 15. We found no difference between WT and G6pdΔ EBs from day 9 to day 15 in the expression patterns of transcription factor SCL, growth factor KL, and proto-oncogene VAV. We next investigated the genes most characteristic of embryonic hematopoiesis (the globin gene β-H1), and of fetal/adult hematopoiesis (the β-major globin gene). The expression of β-H1 was normal in G6pdΔ EBs. In contrast, compared with WT EBs, at day 13 the expression of the adult globin β-major gene was much lower in G6pdΔ EBs, and by day 15 it became undetectable (Figure 4).
Analysis for markers of hematopoietic differentiation. Ethidium bromide–stained gels of RT-PCR amplification using various marker genes of hematopoietic differentiation. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (G6pdΔ1 and G6pdΔ2). Numbers referring to the days of EB development are shown at the top of the figure. Amplified Hprt is shown as a positive control.
Analysis for markers of hematopoietic differentiation. Ethidium bromide–stained gels of RT-PCR amplification using various marker genes of hematopoietic differentiation. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (G6pdΔ1 and G6pdΔ2). Numbers referring to the days of EB development are shown at the top of the figure. Amplified Hprt is shown as a positive control.
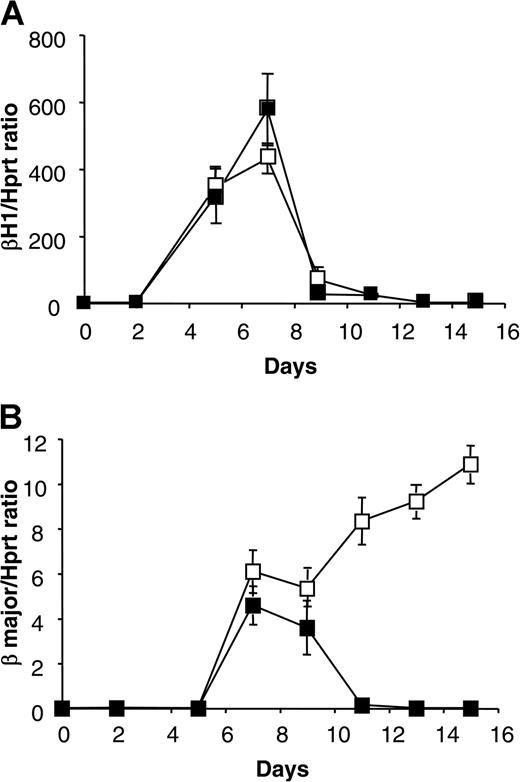
These data were confirmed by semiquantitative RT-PCR analysis (Figure 5). With respect to β-H1, there was no difference at any time. With respect to the adult globin gene β-major, we found no differences until day 9. Subsequently, the amount of adult globin β-major continues to increase in EBs derived from WT ES cells, whereas it decreases in EBs derived from G6pdΔ ES cells. These data suggest that G6PD is not indispensable for the development of hematopoietic progenitors of primitive erythropoiesis, but it is indispensable for the development of progenitors of definitive erythropoiesis.
Kinetics of β-H1 and β-major globin expression. Analysis of RNA extracted from EBs derived from WT (□) and G6pdΔ (▪) ES cells at different days of differentiation. (A) The ratio of bands specific for β-H1 globin to bands specific for Hprt, produced by semiquantitative RT-PCR, plotted against the days of differentiation. (B) Ratio of bands specific for adult globin β-major to bands specific for Hprt, produced by semiquantitative RT-PCR, plotted against days of differentiation. Data represent the average of three independent experiments (mean ± SD).
Kinetics of β-H1 and β-major globin expression. Analysis of RNA extracted from EBs derived from WT (□) and G6pdΔ (▪) ES cells at different days of differentiation. (A) The ratio of bands specific for β-H1 globin to bands specific for Hprt, produced by semiquantitative RT-PCR, plotted against the days of differentiation. (B) Ratio of bands specific for adult globin β-major to bands specific for Hprt, produced by semiquantitative RT-PCR, plotted against days of differentiation. Data represent the average of three independent experiments (mean ± SD).
Analysis of the mechanism of erythropoietic failure
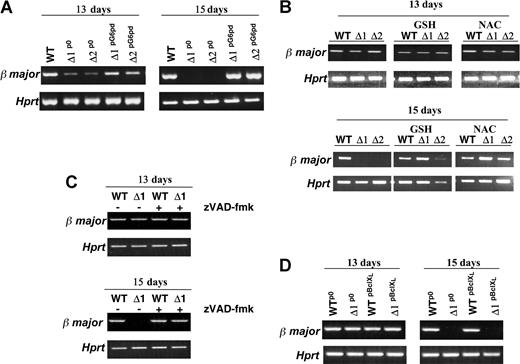
To confirm that the changes we observed were caused by inactivation of the G6pd gene (and not by some additional accidentally produced abnormality), we transfected 2 different G6pdΔ ES cell lines with an expression vector containing a puromycin resistance gene in which the expression of G6pd is driven by the β-actin promoter. Stable clones, from cells transfected with Pallino β-actin G6pd or with Pallino β-actin empty vector, were selected in puromycin, pooled, and tested for their capacity to form erythroid foci. The EBs derived from these cells, G6pdΔpG6pd, had reacquired the ability to form erythroid foci (data not shown). Moreover, in these EBs, we observed a normal level of expression of the adult globin (β-major) at day 13 and day 15 of differentiation (Figure 6A).
Relationship between β-major globin transcript and erythropoietic defect in G6pdΔ EBs. RT-PCR analysis of β-major RNA expression. (A) EBs derived from WT and G6pdΔ ES cell lines stably transfected with Pallino β-actin empty vector (WT and G6pdΔp0) or with Pallino β-actin G6pd expression vector (G6pdΔpG6pd) at days 13 and 15 of differentiation. RNA from WT ES cells (WT), 2 different cell lines of G6pdΔp0 (Δ1p0, Δ2p0) and G6pdΔpG6pd (Δ1pG6pd, Δ2pG6pd) were used in this experiment. (B) EB growth in the presence of 2.5 mM NAC or 2.5 mM GSH from day 11 of differentiation. EBs were harvested at 13 or 15 days of development. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (Δ1, Δ2). (C) EBs derived from WT ES cells (WT) and G6pdΔ ES cells (Δ1) were treated with 100 μM broad-range caspase inhibitor z-VAD-fmk from day 11 of differentiation. After 13 and 15 days of development, the EBs were harvested and assayed for β-major RNA expression. (D) EBs derived from WT and G6pdΔ ES cell lines stably transfected with Pallino β-actin empty vector (WTp0 and G6pdΔp0) or with Pallino β-actin BclXL expression vector (WTpBclXL and G6pdΔpBclXL). RNA used in this experiment was extracted from EBs derived from WTp0G6pdΔp0 (Δ1p0), WTpBclXL, and G6pdΔpBclXL (Δ1pBclXL) ES cells at days 13 and 15 of differentiation. Amplified Hprt is shown as a positive control.
Relationship between β-major globin transcript and erythropoietic defect in G6pdΔ EBs. RT-PCR analysis of β-major RNA expression. (A) EBs derived from WT and G6pdΔ ES cell lines stably transfected with Pallino β-actin empty vector (WT and G6pdΔp0) or with Pallino β-actin G6pd expression vector (G6pdΔpG6pd) at days 13 and 15 of differentiation. RNA from WT ES cells (WT), 2 different cell lines of G6pdΔp0 (Δ1p0, Δ2p0) and G6pdΔpG6pd (Δ1pG6pd, Δ2pG6pd) were used in this experiment. (B) EB growth in the presence of 2.5 mM NAC or 2.5 mM GSH from day 11 of differentiation. EBs were harvested at 13 or 15 days of development. RNA was extracted from EBs derived from WT ES cells (WT) and from 2 different G6pdΔ ES cell lines (Δ1, Δ2). (C) EBs derived from WT ES cells (WT) and G6pdΔ ES cells (Δ1) were treated with 100 μM broad-range caspase inhibitor z-VAD-fmk from day 11 of differentiation. After 13 and 15 days of development, the EBs were harvested and assayed for β-major RNA expression. (D) EBs derived from WT and G6pdΔ ES cell lines stably transfected with Pallino β-actin empty vector (WTp0 and G6pdΔp0) or with Pallino β-actin BclXL expression vector (WTpBclXL and G6pdΔpBclXL). RNA used in this experiment was extracted from EBs derived from WTp0G6pdΔp0 (Δ1p0), WTpBclXL, and G6pdΔpBclXL (Δ1pBclXL) ES cells at days 13 and 15 of differentiation. Amplified Hprt is shown as a positive control.
Reducing agents have an important role in erythroid differentiation14,15 and in RBCs. In human RBCs, the pentose phosphate pathway is the only pathway able to produce NADPH. We tested mouse RBCs for the other 2 enzymes able to produce NADPH, isocitrate dehydrogenase and malic enzyme, and we found that they are undetectable (data not shown). NADPH, by acting as the electron donor cofactor in the glutathione (GSH) reductase reaction, is critical in maintaining appropriate levels of GSH. By using 2.5 mm NAC (a GSH precursor) or 2.5 mM GSH in the culture medium, we were unable to recover erythroid foci formation in G6pdΔ EBs, though we did restore to normal the level of the β-major globin mRNA at day 13 and day 15 of differentiation (Figure 6B). A similar result was seen with 100 μm zVAD-fmk (Figure 6C), a pan-caspase inhibitor that blocks apoptosis, the major mechanism of demise of G6pdΔ ES cells when subjected to oxidative insults.16 Even the addition of 2.5 mM NAC and 100 μm zVAD-fmk was insufficient to restore the ability of G6pdΔ EBs to produce erythroid foci (data not shown), suggesting that simply adding these agents, at a certain stage of EB development, cannot surrogate the role of G6PD present all the time.
Mechanism of apoptosis
In most of the signal transduction pathways that lead to apoptotic cell death, an early marker of the engagement of the apoptotic machinery is mitochondrial dysfunction. Cells in which the apoptotic pathway is mediated by mitochondrial destabilization are called type 2 cells, whereas a mitochondria-independent apoptotic pathway is used by type 1 cells. BclXL is considered a general regulator of mitochondrial homeostasis, and it protects type 2 cells from apoptotic triggers.17 We have shown previously16 that G6pdΔ ES cells overexpressing BclXL are protected from oxidative stress-induced apoptosis. In contrast, EBs formed by G6pdΔ cells overexpressing BclXL were unable to form erythroid foci or even to maintain the expression of β-major globin mRNA at day 15 of differentiation (Figure 6D). These results suggest that erythroid cells are type 1 cells.
Discussion
G6PD null mutants have been isolated in unicellular organisms such as Escherichia coli18 and Saccharomyces cerevisiae.19 Human subjects with G6PD deficiency, who are asymptomatic in the steady state, experience hemolytic anemia under conditions of oxidative stress, and a subset of patients with severe G6PD deficiency has chronic nonspherocytic hemolytic anemia that is further exacerbated by oxidative stress.6 However, there is no known null mutant among the more than 130 spontaneous G6PD mutants found in human patients.20,21 In addition, we have previously shown that severe deficiency of G6PD activity is embryonic days lethal in mice: G6PD-deficient embryos initiate organogenesis, but they have severe abnormalities, and they die between embryonic days 10.5 and 11.5 because of oxidative damage to the embryo from the onset of blood circulation and impairment of placental function.9
Here we have investigated G6pdΔ ES cells, obtained by a strategy different from that previously described,9 that are not just severely G6PD deficient but are totally unable to produce G6PD. We have shown that these cells can proliferate in vitro. In the presence of oxidant agents, however, they become rapidly depleted of NADPH and GSH, and they die by apoptosis.8,16 Moreover, we have shown that G6PD is the only NADPH-producing enzyme rapidly activated in response to oxidative stress.8,16 We have used this new type of G6PD knockout cells to determine which tissues are most affected by the absence of G6PD and at which stage of development. Because severe G6PD deficiency is lethal for the mouse embryo, we have investigated these issues by studying in vitro differentiation of ES cells, which recapitulates embryonic development.2,4,5,11
Similar to ES cell lines with severe G6PD deficiency,9,22 cells with the deleted G6pd gene were unable to form EBs in methylcellulose. Nevertheless, by the hanging-drop method, and probably because of the effect of gravity in pulling the cells together, we were able to form EBs from G6pdΔ ES cells and WT ES cells, with similar efficiency.
Our experiments have proved the presence of mesodermal cells, cardiomyocytes, and hepatocytes in the embryoid bodies formed by G6pdΔ ES cells. Cells that totally lack G6PD can develop beating cardiomyocytes from mesodermal cells. In addition, we show that hepatocytes develop in the absence of G6PD. This was unknown because hemizygous mouse embryos with severe G6PD deficiency die before the liver develops.
To our knowledge, the hanging-drop method has never been used to study hematopoietic differentiation in vitro. By using this method, we obtained from WT ES cells embryoid bodies in which embryonic and adult erythroid cells differentiate, as demonstrated by the expression of β-H1 globin and, after the hemoglobin switch, by the expression of the adult β-major globin mRNA. G6pdΔ ES cells also develop embryonic and adult erythroid cells; but after the embryonic-adult hemoglobin switch, adult G6pdΔ erythroid cells die. The sharp difference in performance of G6pdΔ cells across the switch event is well illustrated by the perfectly normal β-H1 synthesis versus the rapid failure of β-major globin synthesis (Figure 5). Thus, G6PD is crucial for adult-type erythroid cells to reach the terminal stages of differentiation.
The simplest interpretation of these findings is that G6pdΔ adult erythroid cells are unable to cope with the high concentration of oxygen radicals generated by the hemoglobin within them, and they die by apoptosis. In fact, we knew already that G6pdΔ ES cells are more sensitive than WT ES cells to oxidative insults and undergo programmed cell death.16 During in vitro differentiation, we observed that the inhibition of apoptosis, through the use of a caspase inhibitor, causes a delay in the disappearance of the β-major globin mRNA. These data confirm that adult erythroid cells die by apoptosis. In addition, consistent with the role of oxidative stress in this phenomenon, a sulfhydryl group of reagents, such as NAC and GSH, exerts some protection, thus retarding the demise of the cells.
The cause for the apoptotic death of G6pdΔ adult erythroid cells might be that from the basophilic normoblast stage onward, there is massive synthesis of adult hemoglobin. Adult hemoglobin has a lower oxygen affinity than embryonic hemoglobin. Therefore, releasing oxygen from adult hemoglobin produces in the cytoplasm oxygen radicals that G6pdΔ adult erythroid cells are unable to eliminate. Consequently, the apoptotic pathway is triggered.
Adding reducing agents, apoptosis inhibitors, or both at day 11 did not rescue erythroid foci formation. Such rescue was achieved only by transducing the G6pd gene into G6pdΔ ES cells, indicating that the role of G6PD in protecting erythroid cells may be subtle, and it may be crucial that it be present at earlier stages of EB development.
In conclusion, although the phenotypic consequences of partial G6PD deficiency commonly observed in humans is limited to hemolytic anemia, the phenotype of complete G6PD deficiency is far more extensive. However, once again, one specific consequence is an erythroid phenotype. Once the hemoglobin switch has taken place, the formation, rather than the lifespan, of RBCs is compromised.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-03-0835.
Supported by the Italian Telethon Foundation, grant N. 324/bi, Progetto MURST-CNR Legge 488/92 (Cluster C02), Fondazione CARIGE (Genova, Italy), and FIRB (Ministero dell'Università e della Ricerca, Italy).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Rallo, M. Petrillo, and S. Cossu for their assistance with the computer work and M. Terracciano and R. Vito for their skillful laboratory assistance.

![Figure 2. Percentage of EBs with red foci and beating cardiomyocytes. (A) No red foci were observed in EBs derived from G6pdΔ ES cells. (B) No obvious differences were observed among EBs formed from WT (□) and G6pdΔ (▪) ES cells in cardiac differentiation. Data represent the average of 3 independent experiments (mean ± standard deviation [SD]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-0835/6/m_zh80220469170002.jpeg?Expires=1769106412&Signature=WPMiBus0mBBaZjIBhRFh5HOFARdsgDKXFtsFdDCr9Ag43vvR5LKyARa8aaxOCUTZegVv2DPe8-Tq09hRZBkJDKe9pfwZ8seSU~3CnQcaLNDurMkUYKuJqEO5tDchjRMT6Ba1nAR3bqLpGERvaWnxBUhB3KhSFH2I3pZ7IV58FiTr3xuTn5O7gTkOuy0cI~VqBn1mQK2sZX3l9wLw2agzjy~XAlBFOgztLuCqzlJE~gGzu1fpBVQfxtz-imGccKkrhHjsjbWpzfMAh2hZhPAmvRgpjBkYBYmDsxnXC-EaQIVeGL8j9Az7lcHdOnZEKLgV9zI0UnI2gxxu3Ra6v01LdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal