Abstract
Quantification of minimal residual disease (MRD) reveals significant prognostic information in patients treated for acute myeloid leukemia (AML). The application of multiparameter flow cytometry (MFC) for MRD assessment has resulted in significant prognostic information in selected cases in previous analyses. We analyzed MRD in unselected patients with AML in complete remission (CR) after induction (n = 58) and consolidation (n = 62) therapies. By using a comprehensive panel of monoclonal antibodies we identified at least one leukemia-associated aberrant immunophenotype (LAIP) in each patient. The degree of reduction between diagnosis and CR in LAIP-positive cells (log difference [LD]) as a continuous variable was significantly related to relapse-free survival (RFS) both after induction (P = .0001) and consolidation (P = .000 08) therapies, respectively. The LD determined after consolidation therapy was the only parameter related to overall survival (OS) (P = .005). Separation of patients based on the 75th percentile of LD after consolidation therapy resulted in groups with highly different RFS (83.3% versus 25.7%, P = .0034) and OS (87.5% versus 51.4%, P = .0507) at 2 years. Multivariate analysis identified LD as an independent prognostic factor for RFS at both checkpoints. MFC-based quantification of MRD reveals important prognostic information in unselected patients with AML in addition to cytogenetics and should be further evaluated and used in clinical trials.
Introduction
The management of acute myeloid leukemia (AML) is a therapeutic challenge due to resistant or relapsing disease in a significant number of patients despite intensive chemotherapeutic induction and postremission approaches as well as autologous and allogeneic stem cell transplantation.1-7 The occurrence of relapse is considered the result of persisting leukemic cells during complete remission giving rise to regrowth of the leukemic clone.8,9 This minimal residual disease (MRD) is not detectable by the standard method used to define complete remission (ie, cytomorphology), and therefore more sensitive methods such as quantitative polymerase chain reaction (PCR) and multiparameter flow cytometry are increasingly applied to quantify the degree of both response to therapy and MRD.10-14 As a consequence, new standards for the definition of remission have been proposed taking into consideration these novel applications.15
The proportion of AML cases in which PCR-based quantification of MRD is applicable may be significantly increased by targeting length mutations of the FLT3 gene and partial tandem duplications within the MLL gene in addition to the fusion transcripts AML1-ETO, CBFB-MYH11, and PML-RARA.11,12,16 However, still in one half of all cases the disease does not carry a leukemia-specific genetic alteration, and these patients therefore are not subject to PCR-based monitoring of MRD. In contrast, while early studies on the use of multiparameter flow cytometry focused on patients with significantly aberrant leukemia-associated immunophenotypes useful for MRD assessment,13,14,17 recent data indicate that immunologic monitoring may be applicable to virtually all patients with AML when pursuing a comprehensive approach.10,18 The present study followed this approach and correlated the flow cytometrically determined levels of MRD in unselected patients with AML to their prognosis.
Patients, materials, and methods
AML samples
Fresh bone marrow samples that were sent for reference diagnostics to the Laboratory for Leukemia Diagnostics from patients with newly diagnosed and untreated de novo or secondary AML following myelodysplastic syndrome (MDS) or chemotherapy for other malignancies were immunophenotyped as described in “Flow cytometry.” In all cases cytomorphology, cytochemistry, cytogenetics, and molecular genetics were also applied as detailed below.11,19,20 For inclusion into the study a follow-up bone marrow sample had to be sent to the Laboratory for Leukemia Diagnostics for assessment of complete remission and MRD status after regeneration of peripheral blood following double induction therapy or following thioguanine-arabinosylcytosine-daunorubicin (TAD9) consolidation therapy.
Antileukemic treatment
All patients were treated within the 1999 trial of the German AML Cooperative Group. Patients older than 16 years of age with newly diagnosed de novo or secondary AML were eligible for this trial. Patients with acute promyelocytic leukemia were treated in a separate trial.21 Patients with severe comorbidity precluding the initiation of intensive induction chemotherapy (ie, severe uncontrolled infections, coronary heart disease classified by the World Health Organization [WHO] as grade III/IV, congestive heart failure [WHO grade III/IV], severe hyperbilirubinemia [WHO grade III/IV], or severe creatinine elevation [WHO grade III/IV] unless due to leukemia) were excluded.
For remission induction, patients were treated according to the double induction strategy irrespective of response of the disease to the first course.1,2,22 The second course was applied to patients older than 60 years only if they had residual leukemic blasts of 5% or more in the bone marrow on day 16. After intensive consolidation therapy, patients underwent allogeneic or autologous peripheral blood stem cell transplantation or received 3 years of monthly maintenance therapy.23
Normal bone marrow samples
Normal bone marrow as a control was obtained from healthy volunteers and analyzed by flow cytometry as detailed in “Flow cytometry.”
Flow cytometry
All studies were performed on bone marrow samples. The samples were processed by a Ficoll densitiy gradient centrifugation to isolate mononuclear cells both at diagnosis and at the respective follow-up checkpoints.10,18,24 Applying triple stainings and isotype controls, monoclonal antibodies against 31 antigens were used in the following combinations as designed for the detection of leukemia-associated aberrant immunophenotypes (LAIPs) at diagnosis (conjugated with the fluorochromes fluorescein isothiocyanate [FITC], phycoerythrin [PE], and phycoerythrin-cyanin 5.1 [PC-5], respectively): CD11b/CD117/CD34, CD14/CD13/CD4, CD15/CD34/CD33, CD34/NG2(7.1)/CD33, CD34/CD116/CD33, CD34/CD13/CD19, CD34/CD135/CD117, CD34/CD15/CD33, CD34/CD19/CD13, CD34/CD2/CD33, CD34/CD56/CD33, CD36/CD235a/CD45, CD38/CD133/CD34, CD38/CD34/CD90, CD4/CD64/CD45, CD64/CD4/CD45, CD65/CD87/CD34, CD7/CD33/CD34, CD90/CD117/CD34, HLA-DR/CD33/CD34, MPO/LF/cCD15, TdT/cCD33/cCD45, TdT/cyCD22/cyCD3, TdT/cyCD79a/cyCD3. All antibodies were purchased from Immunotech (Marseilles, France) except for CD15, CD64, and cCD15 (Medarex, Annandale, NJ); CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany); and myeloperoxidase (MPO) and lactoferrin (LF) (Caltag, Burlingame, CA). After evaluation of the diagnostic samples, the combinations of antibodies were selected and applied to the follow-up samples that best covered the LAIP. The respective combinations of antibodies were added to 106 mononuclear cells (volume, 100 μL) and incubated for 10 minutes. After addition of 2 mL lysing solution (ammonium chloride–based; prepared at the pharmaceutical institute of the Ludwig-Maximilians University, Muenchen, Germany), the samples were incubated for an additional 10 minutes and were then washed twice in phosphate-buffered saline (PBS) and resuspended in 0.5 mL PBS. Multiparameter flow cytometry analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). For AML samples at diagnosis, 20 000 events were acquired; for follow-up samples and for normal bone marrow samples, 250 000 events were acquired. Life-gating was not applied. Analysis of list mode files was performed using CellQuest Pro Software (Becton Dickinson).
Gating strategy
Leukemia-associated immunophenotypes (LAIPs) were defined individually for each patient by gating on populations displaying an aberrant expression of surface or cytoplasmic antigens and by applying Boolean algebra.10,18 LAIPs were grouped into (1) asynchronous antigen expression, (2) cross-lineage antigen expression, (3) lack of antigen expression, and (4) antigen overexpression. The combination of gates obtained by this strategy was applied to the list mode files obtained during acquisition of follow-up samples as well as to the list mode files containing the measurements of normal bone marrow samples that had been performed using the same combinations of antibodies, respectively. In contrast to other diseases such as acute lymphoblastic leukemia that in general display homogeneous populations, in AML many cases present with several leukemic subpopulations within one sample. Because it is not possible in these cases to include all leukemic cells into one LAIP, the frequencies of LAIP-positive cells are relatively low in these cases (see “Results”).
Because the application of this approach results in the coverage only of subpopulations in some cases, the degree of reduction of the leukemic cell mass at the follow-up checkpoints rather than the crude percentage of cells carrying the LAIP was assessed for prognostic relevance. Thus, a “log difference (LD) diagnosis-to-checkpoint” was determined in each patient, which was defined as the logarithm of the ratio of the percentage of LAIP-positive cells at diagnosis per percentage of LAIP-positive cells at checkpoint. Accordingly, a reduction of LAIP-positive cells from 30% to 0.3% would result in an LD of 2.00.
To estimate the sensitivities of the respective LAIPs as well as the ranges in which a quantification of the LD is feasible, the percentages of LAIP-positive cells within normal bone marrow were determined for each of the applied LAIPs. Similar to the definition of the LD for AML blasts, a log difference to normal bone marrow was calculated that was defined as the logarithm of the ratio of the percentage of LAIP-positive cells in AML sample at diagnosis per median percentage of LAIP-positive cells in normal bone marrow. Thus, the log difference to normal bone marrow would equal 3.00 in a case in which LAIP-positive cells were present in 50.00% in the leukemic bone marrow and in a median of 0.05% in normal bone marrow. If more than one LAIP was present in a patient, analyses were performed using only the LAIP with the highest log difference to normal bone marrow in the respective patient.
In case of a median frequency of 0.00% of LAIP-positive cells in AML samples at day 16 or in normal bone marrow, this frequency was set to 0.004% to allow the calculation of the respective log difference (0.004% is the highest frequency displayed as 0.00% by the CellQuest Pro software and was chosen as worst case possible). If more than 1 LAIP was defined in one patient, the most sensitive LAIP was selected for the respective evaluations as indicated on the basis of the maximum log difference to normal bone marrow in comparison with other LAIPs in the same patient.
Cytomorphology, cytogenetics, and molecular genetics
Cytomorphologic assessment was based on May-Grünwald Giemsa stains, myeloperoxidase reaction, and nonspecific esterase using α-naphtyl acetate as described before.20,25,26 AML was diagnosed cytomorphologically according to the criteria defined in the French-American-British (FAB) classification and in the WHO classification.27-29 Cytogenetic analyses were performed centrally according to standard protocols. Cytogenetic data were classified according to the International System for Human Cytogenetic Nomenclature (ISCN).19,30-32 Patients were classified into 3 subgroups based on cytogenetics: The group associated with a favorable prognosis included AML with t(8;21), inv(16), or t(16;16); the unfavorable-prognosis group contained AML with aberrations of chromosomes 5 or 7, aberrations of 11q23 or 17p, inv(3), t(3;3), or with a complex aberrant karyotype (ie, 3 or more clonal chromosome aberrations); the group associated with an intermediate prognosis included AML with other karyotype aberrations as well as AML with a normal karyotype.
Study parameters
Follow-up bone marrow examinations were carried out after regeneration of peripheral blood following both double induction and consolidation therapies. Response to therapy was assessed according to standardized criteria.15,22 Patients not achieving a complete remission were not included in this analysis. Relapse, overall survival (OS), and relapse-free survival (RFS) were defined as described before.22
Statistics
Dichotomous variables were compared between different groups using the χ2 test and continuous variables by the Student t test. The time-dependent variables OS and RFS were estimated by the method of Kaplan and Meier,35 and differences between the respective groups were calculated using the log-rank test. Spearman rank correlation was used to analyze correlations between continuous parameters. Cox models (dependent variables: OS and RFS) were used for multivariate analyses. The covariates included in these models were age, white blood cell (WBC) count, bone marrow blasts at diagnosis, LAIP-positive bone marrow cells at follow-up checkpoint, and LD as continuous variables, as well as favorable cytogenetics, unfavorable cytogenetics, presence of secondary AML, FLT3-LM, and FLT3-TKD mutations as dichotomous variables, respectively. All calculations were performed using the software SPSS 11.0.1 (SPSS, Chicago, IL). All reported P values are 2 sided.
Study conduct
Prior to therapy all patients gave their informed consent for participation in the current evaluation after having been advised about the purpose and investigational nature of the study as well as potential risks. The study design adhered to the declaration of Helsinki and was approved by the ethics committees of the participating institutions prior to its initiation.
Results
Patients
Between March 2000 and June 2003, bone marrow samples of 1054 patients with newly diagnosed AML were flow cytometrically analyzed. Of these, 58 (MRD checkpoint: after induction therapy, group 1) and 62 (MRD checkpoint: after consolidation therapy, group 2) patients were treated within the German AML Cooperative Group (AMLCG) 1999 trial and were analyzed in addition at the respective follow-up checkpoints (Figure 1). Analyses at both checkpoints were performed in 27 patients. Samples were sent from 18 institutions by direct transport or overnight express mail. Shipment duration was 0 days or 1 day with the exception of 2 cases (shipment duration: 2 and 3 days). There was no impact of shipment time on sample quality. At diagnosis the complete panel of monoclonal antibodies as described in “Flow cytometry” was applied, while at follow-up only the selected combination of monoclonal antibodies was used, which allowed the best definition of an LAIP. The patients' characteristics are given in Table 1. The OS was 68% and 66% at 2 years, and the RFS was 37% and 44% at 2 years, respectively, in groups 1 and 2. An allogeneic stem cell transplantation was performed in 16 and 14 patients, respectively. In 11 of these patients MRD assessment was performed at least once after transplantation, which resulted in decreasing (n = 6), increasing (n = 2), increasing and then decreasing (n = 2), and constant levels (n = 1). The type of the applied postremission therapy had no impact on the outcome of the analyzed patients.
Flow chart of cases analyzed by flow cytometry at diagnosis of AML. After induction and consolidation therapies, 58 and 62 patients, respectively, were analyzed by flow cytometry; 27 patients were assessed at both checkpoints.
Flow chart of cases analyzed by flow cytometry at diagnosis of AML. After induction and consolidation therapies, 58 and 62 patients, respectively, were analyzed by flow cytometry; 27 patients were assessed at both checkpoints.
Normal bone marrow
A total of 26 normal bone marrow samples of healthy volunteers as controls was analyzed applying the complete panel of monoclonal antibodies as described in “Flow cytometry.”
Leukemia-associated aberrant immunophenotypes (LAIPs)
At least one LAIP has been identified in all of the patient samples that were sent to the Laboratory for Leukemia Diagnostics both at diagnosis and at the follow-up checkpoint (Table 1). The distribution of LAIPs between different classes were asynchronous antigen expression (group 1: n = 9; group 2: n = 11), cross-lineage expression of lymphoid antigens (n = 27; n = 18), lack of antigen expression (n = 7; n = 9), and antigen overexpression (n = 15; n = 24). The median portion of bone marrow cells within the diagnostic sample that was LAIP-positive amounted to 18.81% and 18.33% (range, 4.97% to 71.55% and 4.97% to 73.22%), respectively, in groups 1 and 2. The corresponding median portion of cells within the normal bone marrow samples that were LAIP-positive ranged from 0.00% to 0.64% and from 0.00% to 1.05% (median, 0.03% and 0.07%), respectively. The resulting log difference day 1 to normal bone marrow amounted to a median of 2.70 and 2.53 (range, 1.05 to 4.23 and 0.95 to 3.91), respectively. In most patients, it amounted to more than 2.
In 11 of the relapsing patients a full flow cytometric analysis has been performed applying the same panel of antibodies as at diagnosis. In 3 of these patients additional LAIPs have been detected at relapse. In 1 of these 3 patients the LAIP identified at diagnosis was not present at relapse.
Assessment of minimal residual disease after induction and consolidation therapies
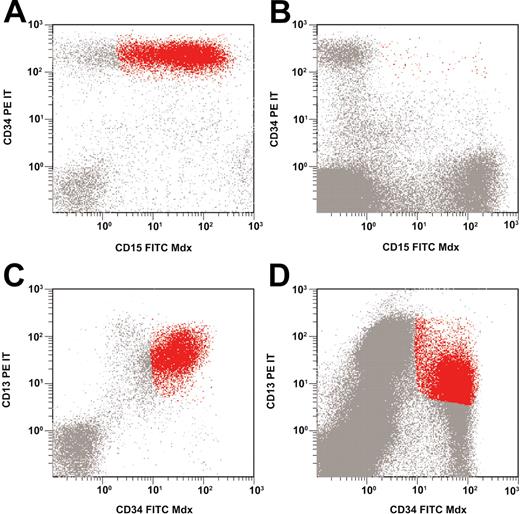
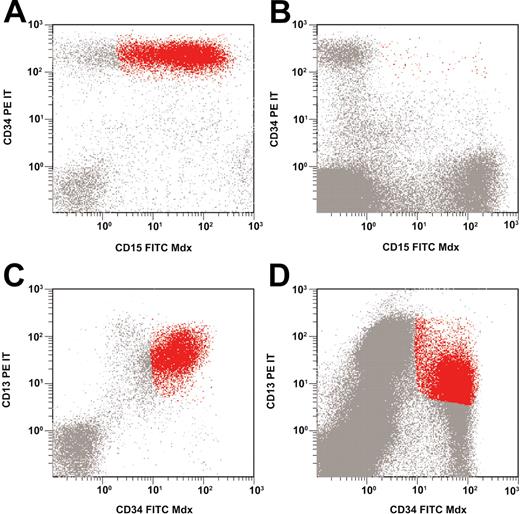
The median portion of LAIP-positive cells in bone marrow samples obtained after induction therapy amounted to 0.09% (range, 0.00% to 2.25%); the respective numbers after consolidation therapy were 0.08% (median) and 0.00% to 6.85% (range). This resulted in a median LD of 2.39 (range, 0.51 to 3.56) after induction therapy and 2.29 (range, –0.07 to 4.26) after consolidation therapy. Figure 2 shows examples of flow cytometric findings in patients with low-level and high-level minimal residual disease.
Low-level and high-level minimal residual disease. Asynchronous coexpression of CD34 and CD15 at diagnosis (A); low-level MRD at follow-up (B). Overexpression of CD34 and CD13 at diagnosis (C); high-level MRD at follow-up (D).
Low-level and high-level minimal residual disease. Asynchronous coexpression of CD34 and CD15 at diagnosis (A); low-level MRD at follow-up (B). Overexpression of CD34 and CD13 at diagnosis (C); high-level MRD at follow-up (D).
Prognostic impact of log difference after induction and consolidation therapies
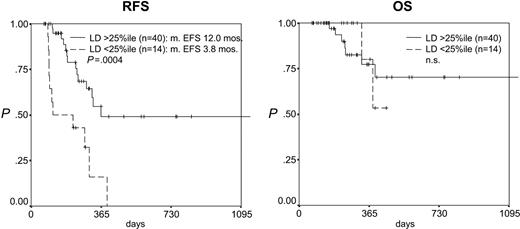
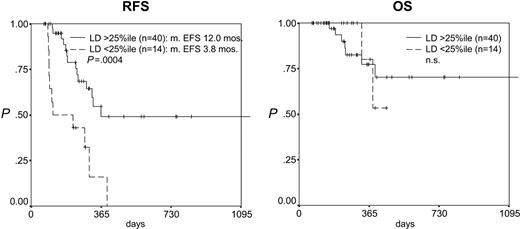
After induction therapy the LD as a continuous variable was correlated to RFS (P = .0001, risk ratio [RR] = 0.312) but not to OS (Table 2). After consolidation therapy the LD as a continuous variable was correlated to both RFS (P = .00008, RR = 0.418) and OS (P = .005, RR = 0.408; Table 2). Accordingly, the separation of the patients according to the 25th percentile of the LD after induction therapy (1.70) resulted in 2 groups with highly differing RFS but not OS (median RFS, 12.0 versus 3.8 months, P = .0004; OS, n.s.; Figure 3). Reflecting the stronger prognostic impact of MRD levels at the second checkpoint analyzed, the separation of the patients according to the 75th percentile of the LD after consolidation therapy (2.94) resulted in 2 groups with highly differing RFS and OS (RFS at 2 years, 83.3% versus 25.7%, P = .0034; OS at 2 years, 87.5% versus 51.4%, P = .0507; Figure 4). Importantly, in patients with a log difference day 1 to NBM of less than 2 (ie, the cases with a relatively low sensitivity), the 2 patients from both groups (after induction and after consolidation) with the lowest LD (ie, highest MRD levels) relapsed early while later relapses occurred in 2 of 6 and 3 of 11 of the remaining patients, respectively.
Prognostic impact of log difference (LD) after induction therapy. Patients with an LD higher than the 25th percentile (1.70) (ie, a stronger reduction in leukemic cell mass) have a significantly better RFS but not OS.
Prognostic impact of log difference (LD) after induction therapy. Patients with an LD higher than the 25th percentile (1.70) (ie, a stronger reduction in leukemic cell mass) have a significantly better RFS but not OS.
Prognostic impact of log difference (LD) after consolidation therapy. Patients with an LD higher than the 75th percentile (2.94) (ie, a stronger reduction in leukemic cell mass) have a significantly better RFS and OS.
Prognostic impact of log difference (LD) after consolidation therapy. Patients with an LD higher than the 75th percentile (2.94) (ie, a stronger reduction in leukemic cell mass) have a significantly better RFS and OS.
Prognostic impact of conventional parameters
The prognostic impact of favorable and unfavorable cytogenetics as dichotomous variables as well as of age, WBC count at diagnosis, and percentage of bone marrow blasts at diagnosis as continuous variables was analyzed using RFS and OS as dependent variables. For both analyses, after induction therapy and after consolidation therapy, unfavorable cytogenetics and WBC count were the only parameters besides the LD that were related to the prognosis (Table 2).
Multivariate analyses of prognostic parameters
All prognostic parameters that were identified in univariate analyses to carry significant prognostic impact were further evaluated in multivariate analyses. Cox regression models were used for the evaluation of RFS and OS (Table 3). The previously demonstrated high prognostic impact of an unfavorable karyotype is confirmed in this analysis with regard to RFS. The LD (ie, the flow cytometrically determined MRD levels) was prognostically highly relevant independent of cytogenetics with regard to RFS and, most important, the LD determined after consolidation therapy was the only parameter with significant impact on OS (Tables 2 and 3).
Prognostic impact of log difference in cytogenetically defined subgroups
To further prove the prognostic impact of the LD being independent of cytogenetics, the correlation of LD as a continuous variable with RFS and OS was analyzed in cytogenetically defined subgroups (favorable, intermediate, and unfavorable cytogenetics; Table 4). The median LD values and their ranges within these 3 groups were 2.63 (0.91 to 3.33), 2.35 (0.94 to 3.56), and 2.36 (0.51 to 3.16) after induction therapy and 2.44 (0.58 to 3.53), 2.26 (0.40 to 3.93), and 2.07 (–0.07 to 4.26) after consolidation therapy. With the exception of patients with favorable cytogenetics, there were significant associations between the LD (both after induction and consolidation therapies) and the RFS within all remaining cytogenetically defined subgroups. This association also was significant between the OS and the LD after consolidation therapy in patients with intermediate cytogenetics.
Assessment of LAIPs in genetically defined subgroups in an extended cohort
To estimate the sensitivity of the present approach more comprehensively, additional analyses (ie, comparisons of cells positive for each individual LAIP in leukemic versus normal bone marrow) have been performed in 521 samples with newly diagnosed AML for which a cytogenetic analysis also has been available. Six cases were excluded because of an insufficient flow cytometric assessment due to limited sample material. In all of the remaining 515 samples at least one LAIP has been identified that was present in a median of 15.90% of all leukemic cells (range, 3.08% to 83.61%; Table 5). The median percentages of LAIP-positive cells within the normal bone marrow samples ranged from 0.00% to 1.05% (median, 0.01%), resulting in a median log difference to normal bone marrow of 3.05 (range, 0.95 to 4.32). Only a minority of cases have a log difference to normal bone marrow of less than 2 (n = 24, 4.7% of all cases) while a log difference to normal bone marrow between 2 to 3 and higher than 3 was observed in 219 (42.5%) and 272 (52.8%) cases, respectively. The log difference to normal bone marrow was higher in cases with cross-lineage expression of lymphatic antigens (P = .001) and in those with lack of antigen expression (P = .057), respectively, as compared with cases with asynchronous antigen expression or antigen overexpression (Table 5). Overall, there were only slight differences in the log differences to normal bone marrow between cytogenetically defined subgroups with the exception of AML cases with t(8;21) in which higher values were observed (P = .012). Log differences to normal bone marrow in cases with length mutations of the FLT3 gene or partial tandem duplications of the MLL gene were not different from the values observed in other cases (Table 5).
Discussion
Monitoring of MRD is becoming increasingly important in the management of patients with AML. The approach of risk-adapted therapy essentially relies on powerful prognostic parameters allowing assignment of patients to a particularly high or low risk of treatment failure. However, despite the availability of pretherapeutically assessable prognostic parameters, among which cytogenetics is the most significant,36,37 many patients are assigned an intermediate risk and thus their prognosis in fact can hardly be estimated. This implies a strong need for treatment-dependent prognostic parameters, which are expected to add prognostic information to the pretreatment parameters.
While PCR-based methods for the quantification of MRD are available for less than half of all patients with AML,11,33,34 recent analyses have demonstrated that quantification of MRD by multiparameter flow cytometry may be applicable to virtually all patients with AML.9,10,18 Previous studies on the flow cytometric assessment of MRD in patients with AML in complete remission indicate a significant gain in prognostic information by MRD levels13,14,17 ; however, about 25% of the cases analyzed were considered not to express an aberrant immunophenotype and therefore were excluded from the analysis. The present study for the first time followed the approach of analyzing by multiparameter flow cytometry unselected patients with AML for MRD by the application of a comprehensive panel of monoclonal antibodies at diagnosis to optimize the identification of LAIPs.
In fact, none of the flow cytometrically analyzed patients was excluded from this study because of a lacking LAIP. To avoid a loss in validity of these analyses due to a decreased sensitivity, which must be taken into consideration when pursuing this approach for each of the defined LAIPs, the frequencies of LAIP-positive cells in normal bone marrow samples have been determined. The median values for these frequencies were 0.03% and 0.07%, respectively, for MRD analyses after induction and consolidation therapies, assuring a high degree of sensitivity. These data are in line with previous studies following the same approach of flow cytometric quantification of MRD in unselected patients with AML10,18 while the respective data have not been detailed in other studies.13,14,17
To further strengthen these findings a comprehensive series of 515 bone marrow samples of newly diagnosed AML was compared with normal bone marrow with regard to the log difference of LAIP-positive cells. The data obtained (median log difference, 3.05; range, 0.95 to 4.32) are completely in line with the findings discussed in the previous paragraph. Importantly, a log difference between 1 and 2 has been present in only 5% of the cases, and higher values were observed in all other cases. Thus, taking into account the methodic failures due to limited sample availability in overall about 1% and the clear trend for an association between a smaller log difference at follow-up and a higher risk of relapse, there is no indication for considering the results of MRD assessment in the latter cases not fitting in the present concept of flow cytometrically based MRD quantification. The results rather support the further evaluation of this approach always taking into consideration the assessment of potential drawbacks such as changes in the immunophenotype at relapse, the quantification of a clinically irrelevant subpopulation, or the interference of the population targeted on with normal bone marrow cells. In this regard it is important to apply samples of normal regenerating bone marrow as controls if MRD assessment is performed not in CR as in the present study but before full recovery of peripheral blood values.
Besides this methodologic validation and considerations, it is most important, of course, to perform a clinical validation of the measurements of MRD and thus to correlate the data to the outcome of the patients. Along this line, the present study demonstrated that the flow cytometrically determined levels of MRD were highly significantly and independently from other parameters related to the RFS at both checkpoints evaluated and that they were the only parameter related to OS in the analysis after consolidation therapy. Accordingly, 2 groups of patients with an almost 60% difference in the RFS at 2 years could be separated from each other based on the MRD levels after consolidation therapy. These results clearly validate the measurements of MRD levels and—particularly in light of the applicability of this approach to virtually all patients with AML—strongly suggest that the multiparameter flow cytometry–based assessment of MRD should be used as a stratification parameter in clinical trials to realize an optimization of risk-adapted therapy in patients with AML. One must consider, however, that during the follow-up of patients after allogeneic stem cell transplantation the reasons for treatment failure comprise not only relapse but also therapy-related issues; therefore, MRD monitoring in this context should be evaluated carefully. In the present analysis no deaths after allogeneic stem cell transplantation have occurred in the limited number of patients evaluated.
The present analysis may yield interesting data on the course of MRD during continued antileukemic treatment in patients with AML. While it has been shown repeatedly that at the earliest checkpoint day 16, (ie, 1 week after completion of the first induction course) highly significant information can be gained from MRD measurements,10,22 there are data on patients with core binding factor (CBF) leukemias indicating that MRD levels after induction therapy are less strongly associated with the prognosis as compared with MRD levels after consolidation therapy.11,38 This phenomenon also has been observed in the present study, suggesting that not only the very early efficacy of the first induction course but also the overall efficacy of the first 3 chemotherapy courses are prognostically highly relevant. However, there was only a partial overlap in the populations analyzed at the 2 checkpoints in the present analysis. Therefore, it is not possible to judge on the optimum and clinically most helpful checkpoint, which should be the focus of additional studies.
Overall, the results of the present study are in agreement with previous studies indicating a significant prognostic impact of flow cytometrically determined levels of MRD.13,14,17 However, while in these studies cytogenetic data were available for only a part of the analyzed patients or were totally lacking and thus interactions among both prognostic parameters, cytogenetics, and MRD levels could be estimated only, the present study has proven the prognostic importance of MRD levels in multivariate analyses taking into account cytogenetics as a covariate that was available in all patients. The results indicate an independency of both parameters from each other and show that—despite the limited patient numbers in subgroup analyses—MRD levels have prognostic impact even within cytogenetically defined risk groups. These analyses therefore underline the enormous relevance of combining pretherapeutic and treatment-dependent prognostic parameters to optimize the risk assignment of patients with AML. Further improvements of the presently described approach may be achieved by a vigorous analysis of disease subtypes and specific LAIPs in which an immunophenotypic switch, which may occur in about 10% of all cases, is observed more often than in others.
When putting these data into the practice of AML patient management, it is important with regard to the heterogeneity of the percentages of LAIP-positive cells at diagnosis not to rely on distinct percentages of LAIP-positive cells at follow-up checkpoints but rather to calculate the degree of reduction in leukemic cell mass achieved between diagnosis and checkpoint (ie, the log difference [LD]). The present data indicate that an LD in the range of 3 after consolidation therapy may be prognostically most favorable. These data must be reproduced and used in future clinical trials in patients with AML.
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2004-03-1036.
Supported by grants from the Else Kröner-Fresenius Stiftung and from the Wilhelm Sander-Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Karin Hecht, Rita Lapping, and Eva Goecke for their excellent technical assistance. The authors greatly acknowledge Th. Büchner (head of AMLCG 1999 trial) as well as more than 300 physicians for their confidence in our laboratory, sending the patient samples, and providing us with information on the clinical courses of the patients.